Published online Apr 6, 2021. doi: 10.12998/wjcc.v9.i10.2281
Peer-review started: September 20, 2020
First decision: October 17, 2020
Revised: December 30, 2020
Accepted: January 25, 2021
Article in press: January 25, 2021
Published online: April 6, 2021
Processing time: 190 Days and 17.9 Hours
Undifferentiated embryonal sarcoma of the liver (UESL) is a neoplasm that rarely develops in adults. The main treatments for UESL are upfront gross total surgical resection and adjuvant multiagent chemotherapy. Here, we report a case of recurrent UESL in an adult treated with pembrolizumab and discuss a method to identify proper candidates for antibody of programmed cell death protein 1 (anti-PD-1) treatment.
A 69-year-old woman was admitted for abdominal pain that developed for 1 wk. Computed tomography showed a 16 cm mass in the right lobe of the liver. Right hemihepatectomy and lymphadenectomy were performed, and histological diagnosis was UESL. Six months later, the patient suffered from painless obstructive jaundice, and positron emission tomography-computed tomography revealed multiple metastases. Then, percutaneous transhepatic cholangial drainage was applied to reduce jaundice, and radiofrequency ablation was used to control the lesion near the hepatic hilum. However, the patient suffered from a serious fever caused by the tumor. The patient received treatment with pembrolizumab, and the prescribed dosage was 2 mg/kg every 3 wk. After the seventh dose, positron emission tomography-computed tomography revealed that the multiple metastases had nearly disappeared. Radiologic exam was used to evaluate the disease state, and no new lesions were found. Next-generation sequencing and immunohistology were applied to determine the reason why the patient had such a favorable response to pembrolizumab. Tumor mutation burden, microsatellite instability, and programmed death ligand 1 expression can be combined to predict the effect of PD-1 antibodies. When every one of these biomarkers are detected in a tumor patient, the patient may be a proper candidate for PD-1 antibodies.
Anti-PD-1 treatment for tumors needs further research to identify indications and proper biomarkers.
Core Tip: Undifferentiated embryonal sarcoma of the liver is a neoplasm rarely diagnosed in adults, which was mainly treated by resection and adjuvant multiagent chemotherapy. Immunotherapy, such as antibody of programmed cell death protein 1, has not been reported to be used to treat this disease. Here we reported a recurrent undifferentiated embryonal sarcoma of the liver case in an adult that was treated by pembrolizumab and discussed the way to find the proper candidates for antibody of programmed cell death protein 1 treatment.
- Citation: Yu XH, Huang J, Ge NJ, Yang YF, Zhao JY. Recurrent undifferentiated embryonal sarcoma of the liver in adult patient treated by pembrolizumab: A case report. World J Clin Cases 2021; 9(10): 2281-2288
- URL: https://www.wjgnet.com/2307-8960/full/v9/i10/2281.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i10.2281
Undifferentiated embryonal sarcoma of the liver (UESL) is a mesenchymal tumor that was first described in 1978[1]. UESL is an aggressive pediatric tumor that is classically diagnosed in adolescents and rarely diagnosed in adults[2]. The main treatments for UESL are upfront gross total surgical resection and adjuvant multiagent chemo-therapy, which have led to improved long-term survival[3]. However, immunotherapy, such programmed cell death protein 1 (anti-PD-1) antibodies, has not been reported to be used to treat this disease. Here, we report a case of recurrent UESL in an adult treated with pembrolizumab (Keytruda, Merck Sharp & Dohme Ltd) and discuss a method to identify proper candidates for anti-PD-1 treatment.
A 69-year-old woman was admitted for abdominal pain that developed for 1 wk (Figure 1).
Right hemihepatectomy and lymphadenectomy were performed in Oct 2016.
She had no history of a previously diagnosed malignancy.
No abnormalities.
Serum levels of tumor markers (carcinoembryonic antigen, CA-19-9, alpha-fetoprotein and prostate-specific antigen) were all within normal limits, and she was negative for hepatic viral markers (hepatitis B surface antigen and anti-hepatitis C virus).
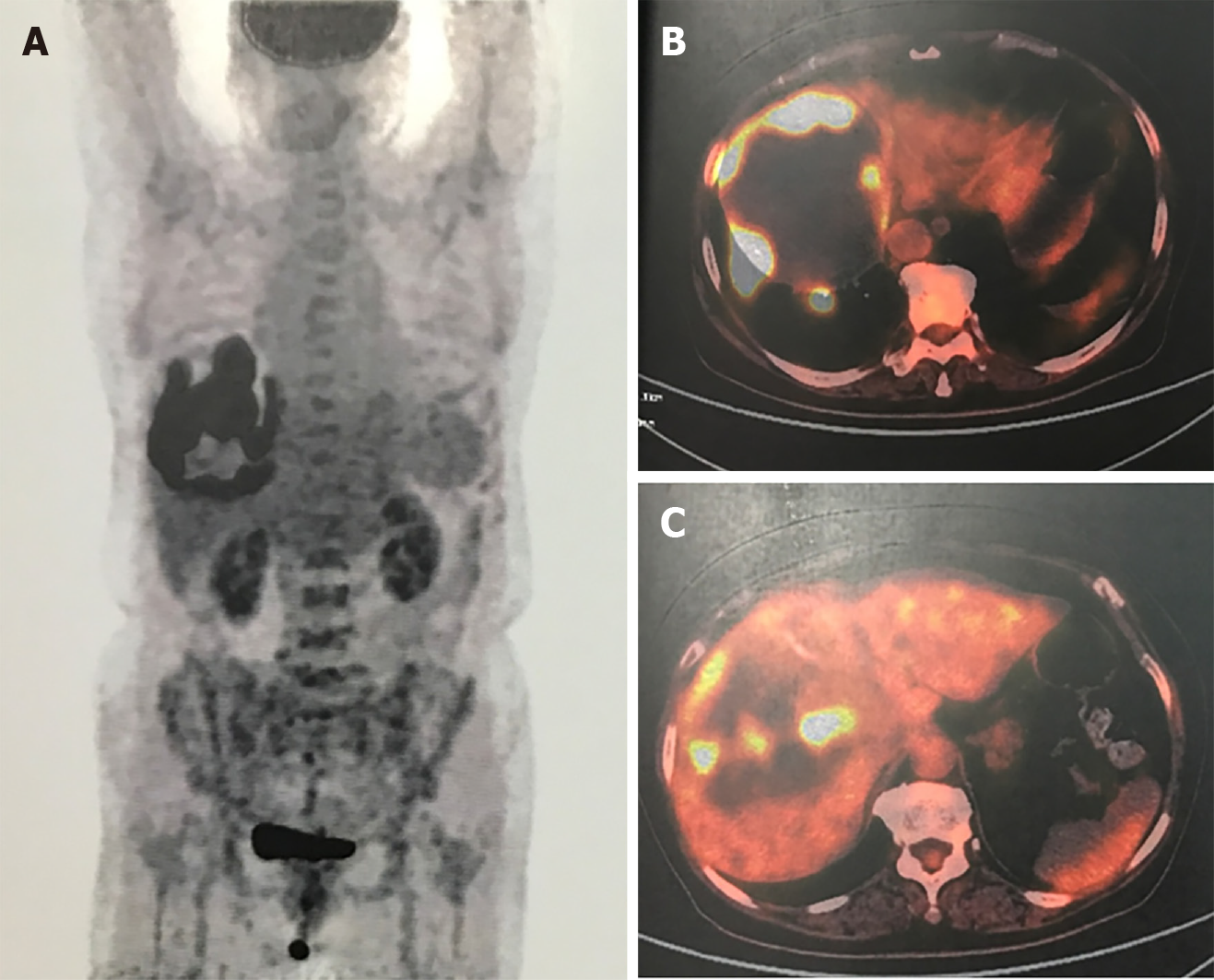
Abdominal contrast-enhanced computed tomography (CT) showed a 16 cm mass in the right lobe of the liver, and positron emission tomography-CT showed radioactive concentration of 18F-fluorodeoxyglucose on the tumor rim with no radioactive concentration in other areas of the body (Figure 2).
The histological report showed a solitary 16.0 cm × 12.5 cm × 10.5 cm well-defined and encapsulated tumor. Tumor cells were found in some dilated lymph vessels. The immunohistological stain was positive for vimentin, S-100 protein, A1AT, LCA, CD68 and Ki67. The staining for CD20, CD45R0, CK, CAM5.2, smooth muscle actin, CK8/18, CK20, CK19, villin, AFP, HSA, HMB45, A103, TTF-1, CA125, caldesmon, MyoD1, CD34, CD21 and CD57 demonstrated negative findings. The diagnosis of UESL was made using the examinations mentioned above.
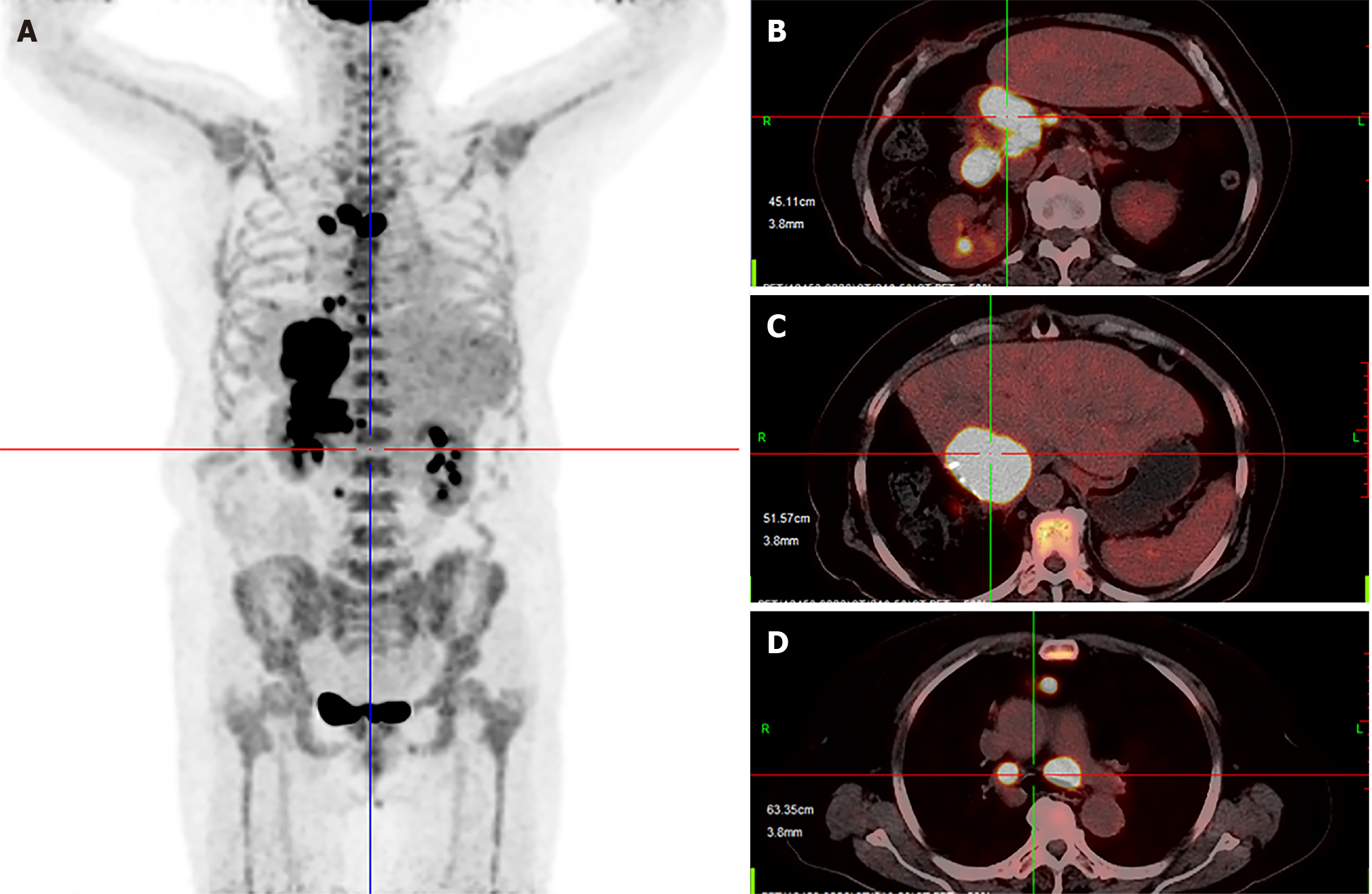
Six months after surgery, the patient suffered from painless obstructive jaundice. Liver ultrasonography revealed a new lesion near the hepatic hilum and intrahepatic bile duct dilation. Then, positron emission tomography-CT revealed multiple metastases located in the liver, mediastinum and peritoneum (Figure 3). Next, percutaneous transhepatic cholangial drainage and stenting were applied to reduce jaundice. After that, radiofrequency ablation with intraductal chilled saline perfusion to prevent bile duct injury through percutaneous transhepatic cholangial drainage was used to control the lesion near the hepatic hilum[4]. However, the patient suffered from a serious fever caused by the tumor. Due to the fever, the patient became asthenic and was afraid to undergo chemotherapy or targeted therapy. Therefore, the patient was received pembrolizumab for treatment, and the prescribed dosage was 2 mg/kg once every 3 wk.
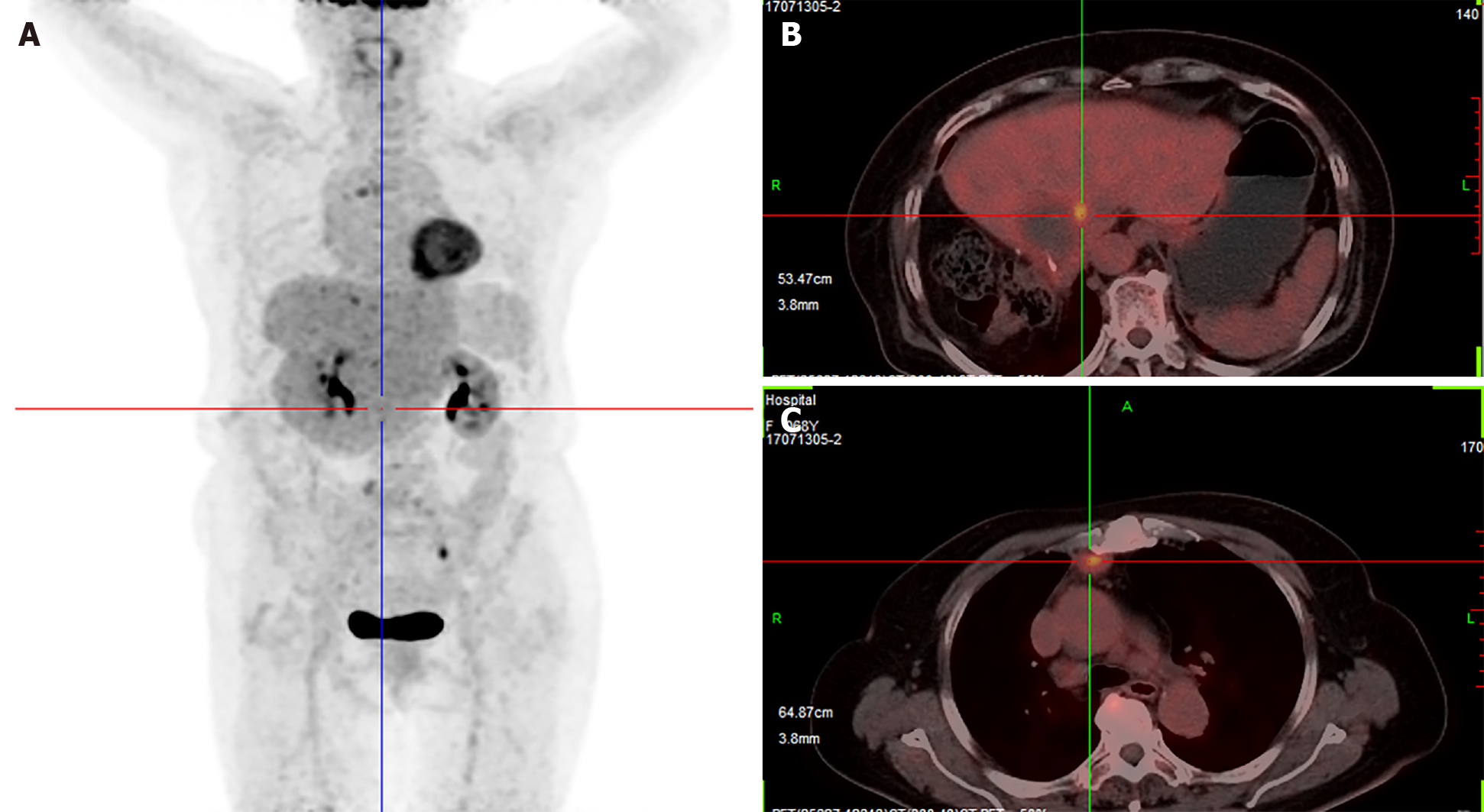
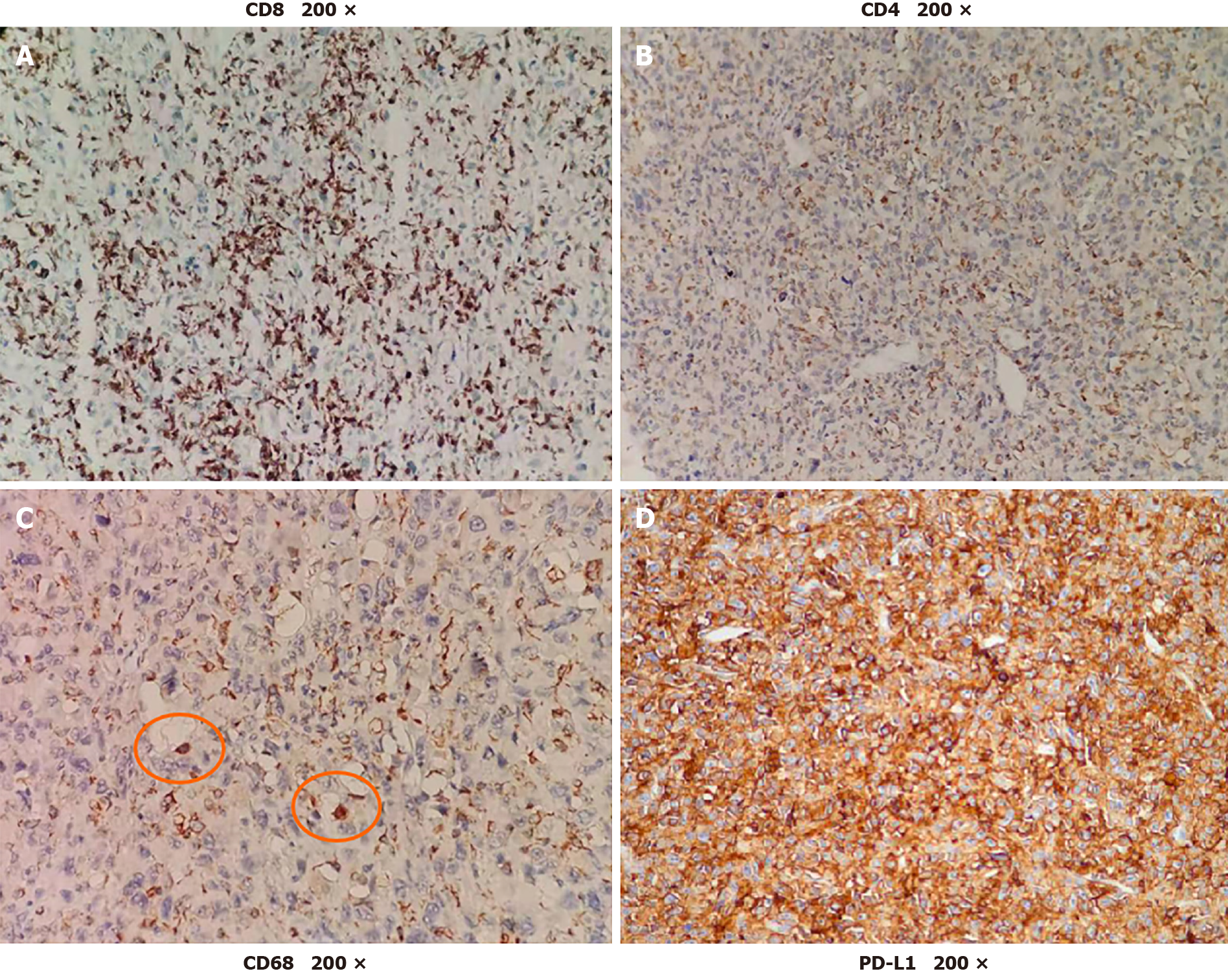
Two days after the first dose, the fever was relieved. After the seventh dose, new positron emission tomography-CT was conducted revealing that the multiple metastases had nearly disappeared (Figure 4). The patient received six more doses of pembrolizumab. Every 6 mo, chest CT and abdominal contrast-enhanced CT were used to evaluate the disease state, and no new lesions were found. To determine the reason why the patient had such a favorable response to pembrolizumab, biopsy and peripheral blood were used for next-generation sequencing (NGS). NGS revealed PTK2 gene amplification, FGF10 gene amplification and USP34/XPO1 gene rearrangement. The tumor mutation burden (TMB) was 3.2 muts/Mb (< 75%), and the tumor showed microsatellite stability. There was no strong association between the favorable response and NGS result, as previously reported[5]. Then, further immunohistological staining of CD68, CD8 and CD4 were used to detect macrophage and T cell distribution in the tumor, and immunohistological staining of programmed death ligand 1 (PD-L1) was used to detect the expression of PD-L1 in the tumor cell. Immunohistological staining showed that there was high expression of CD8, low expression of CD4 and little expression of CD68 in the tumor, and up to 90% of tumor cells expressed PD-L1 (Figure 5).
Anti-PD-1 treatment, as a new immunotherapy, has the advantages of few side effects, limited pain and long-lasting effects[6]. Therefore, an increasing number of researchers have attempted to use anti-PD-1 to treat multiple types of tumors[7,8]. For the first time in this case, pembrolizumab was used to treat UESL, and the result was encouraging. Pembrolizumab may be a new method to treat UESL.
In our case, radiofrequency ablation was applied to control the lesion near the hepatic hilum. Although ablation does not provide a cure, it may assist pem-brolizumab in destroying tumor cells. Local ablation can destroy tumor cells, and then tumor antigens are released into the microenvironment and blood. Recent research has revealed that immunotherapy combined with local treatment can prolong the survival time of liver cancer[9]. Other research has revealed that local treatment, such as radiotherapy, can release antigenic peptides from tumors, cause the activation and migration of dendritic cells and enhance antigen presentation by dendritic cells leading to enhanced antitumor T-cell recognition and activity[10].
We were curious why the patient had a good response to pembrolizumab and wanted to determine how other appropriate candidates for anti-PD-1 treatment could be identified. In our case, biopsy and peripheral blood were used to perform NGS. However, the gene mutations found in this patient were not associated with a favorable response to pembrolizumab. High TMB and microsatellite instability in tumors are associated with a favorable response to pembrolizumab, and the Food and Drug Administration has authorized pembrolizumab to be used to treat multiple types of tumors with high TMB and microsatellite instability[11]. However, in this case, NGS showed that the tumor had low TMB and microsatellite stability, which was contradictory to other responsive tumors[5]. Therefore, low TMB and microsatellite stability were not contraindications for anti-PD-1 treatment.
Then, immunohistological staining showed that up to 90% of the tumor cells expressed PD-L1. This may be the reason why the patient had a favorable response to pembrolizumab. Many researchers have found that PD-L1 is expressed in many types of tumors, and high-level expression of PD-L1 is always associated with poor tumor prognosis[12]. In some types of tumors, such as lung cancer, high-level expression of PD-L1 is associated with a good response to anti-PD-1 treatment[13]. However, in other types of tumors, the expression of PD-L1 is not associated with responses to anti-PD-1 treatment[14]. Defining the high-level expression of PD-L1 is also controversial[15]. Moreover, the location of PD-L1 expression also needs further research[16]. Therefore, PD-L1 expression, as a biomarker for predicting the effect of PD-1 antibodies, also needs further research.
In our opinion, TMB, microsatellite instability and PD-L1 expression can be combined to predict the effect of PD-1 antibodies. When every one of these biomarkers is detected in a tumor patient, the patient may be a proper candidate for PD-1 antibodies. However, anti-PD-1, as a new treatment for tumors, needs further research to identify the indications and proper biomarkers.
We reported a case of recurrent UESL in an adult patient treated with pembrolizumab, and UESL had a nearly complete response to pembrolizumab. Immunohistological staining of PD-L1 was highlighted and corresponded to the effect of pembrolizumab. Therefore, PD-L1 expression may be combined with TMB and microsatellite instability to predict the effect of PD-1 antibody treatment.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kurokawa T S-Editor: Gao CC L-Editor: Filipodia P-Editor: Li X
| 1. | Stocker JT, Ishak KG. Undifferentiated (embryonal) sarcoma of the liver: report of 31 cases. Cancer. 1978;42:336-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 2. | Esteban SG, Emilio CU, Emmanuel AF, Oscar SJ, Paulina CE, Angel MM. Undifferentiated embryonal sarcoma of the liver in adult patient: A report of two cases. Ann Hepatobiliary Pancreat Surg. 2018;22:269-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Techavichit P, Masand PM, Himes RW, Abbas R, Goss JA, Vasudevan SA, Finegold MJ, Heczey A. Undifferentiated Embryonal Sarcoma of the Liver (UESL): A Single-Center Experience and Review of the Literature. J Pediatr Hematol Oncol. 2016;38:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Ohnishi T, Yasuda I, Nishigaki Y, Hayashi H, Otsuji K, Mukai T, Enya M, Omar S, Soehendra N, Tomita E, Moriwaki H. Intraductal chilled saline perfusion to prevent bile duct injury during percutaneous radiofrequency ablation for hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:e410-e415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3799] [Cited by in RCA: 4953] [Article Influence: 619.1] [Reference Citation Analysis (0)] |
| 6. | Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8900] [Cited by in RCA: 9912] [Article Influence: 762.5] [Reference Citation Analysis (0)] |
| 7. | Mehnert JM, Varga A, Brose MS, Aggarwal RR, Lin CC, Prawira A, de Braud F, Tamura K, Doi T, Piha-Paul SA, Gilbert J, Saraf S, Thanigaimani P, Cheng JD, Keam B. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced, PD-L1-positive papillary or follicular thyroid cancer. BMC Cancer. 2019;19:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 8. | Plimack ER, Bellmunt J, Gupta S, Berger R, Chow LQ, Juco J, Lunceford J, Saraf S, Perini RF, O'Donnell PH. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol. 2017;18:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 278] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 9. | Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, Uppala A, Korangy F, Kleiner DE, Figg WD, Venzon D, Steinberg SM, Venkatesan AM, Krishnasamy V, Abi-Jaoudeh N, Levy E, Wood BJ, Greten TF. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 640] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 10. | Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, Garon EB, Lee P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18:895-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 833] [Article Influence: 104.1] [Reference Citation Analysis (0)] |
| 11. | Freshwater T, Kondic A, Ahamadi M, Li CH, de Greef R, de Alwis D, Stone JA. Evaluation of dosing strategy for pembrolizumab for oncology indications. J Immunother Cancer. 2017;5:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 198] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 12. | Wang X, Bao Z, Zhang X, Li F, Lai T, Cao C, Chen Z, Li W, Shen H, Ying S. Effectiveness and safety of PD-1/PD-L1 inhibitors in the treatment of solid tumors: a systematic review and meta-analysis. Oncotarget. 2017;8:59901-59914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, Poddubskaya E, Borghaei H, Felip E, Paz-Ares L, Pluzanski A, Reckamp KL, Burgio MA, Kohlhäeufl M, Waterhouse D, Barlesi F, Antonia S, Arrieta O, Fayette J, Crinò L, Rizvi N, Reck M, Hellmann MD, Geese WJ, Li A, Blackwood-Chirchir A, Healey D, Brahmer J, Eberhardt WEE. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35:3924-3933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 688] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 14. | Spaas M, Lievens Y. Is the Combination of Immunotherapy and Radiotherapy in Non-small Cell Lung Cancer a Feasible and Effective Approach? Front Med (Lausanne). 2019;6:244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Jing W, Wang S, Ding X, Guo H, Li J, Wang H, Kong L, Yu J, Zhu H. High level of programmed death ligand 1 (PD-L1) predicts longer survival in patients with resectable small cell lung cancer. Int J Clin Exp Pathol. 2018;11:2675-2682. [PubMed] |
| 16. | Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064-5074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1633] [Cited by in RCA: 1971] [Article Influence: 179.2] [Reference Citation Analysis (0)] |