Published online Apr 6, 2021. doi: 10.12998/wjcc.v9.i10.2259
Peer-review started: August 29, 2020
First decision: December 28, 2020
Revised: January 10, 2021
Accepted: January 27, 2021
Article in press: January 27, 2021
Published online: April 6, 2021
Processing time: 212 Days and 19.4 Hours
Co-morbidity of SRY gene turner syndrome (TS) with positive SRY gene and non-classical congenital adrenal hyperplasia (NCAH) is extremely rare and has never been reported to date.
In this article, we present a 14-year-old girl who was referred to our hospital with short stature (weight of 43 kg and height of 143 cm, < -2 SD) with no secondary sexual characteristics (labia minora dysplasia). Laboratory tests indicated hypergonadotropic hypogonadism with significantly increased androstenedione and 17-hydroxyprogesterone (17-OHP) levels. This was accompanied by the thickening of the extremity of the left adrenal medial limb. The patient’s karyotype was 45,X/46,X, +mar, and cytogenetic analysis using multiplex ligation-dependent probe amplification and high-throughput sequencing indicated that the SRY gene was positive with compound heterozygous mutations in CYP21A2 as the causative gene for congenital adrenal hyperplasia. The sites of the suspected candidate mutations were amplified and verified using Sanger sequencing. The patient was finally diagnosed as having SRY positive TS with NCAH. The patient and her family initially refused medical treatment. At her most recent follow-up visit (age = 15 years old), the patient presented facial hair, height increase to 148 cm, and weight of 52 kg, while androstenedione and 17-OHP levels remained high. The patient was finally willing to take small doses of hydrocortisone (10 mg/d).
In conclusion, upon evaluation of the patient mentioned in the report, we feel that 17-OHP measurement and cytogenetic analysis are necessary for TS patients even in the absence of significant virilization signs. This will play a significant role in guiding diagnosis and treatment.
Core Tip: This paper reports a 14-year-old girl who was diagnosed with non-classical congenital adrenal hyperplasia (NCAH) and turner syndrome (TS) with a positive SRY gene concurrently. The combination of these three factors increases the difficulty of diagnosis and treatment but provides insights into the understanding of TS. At the same time, in the diagnosis of TS, in addition to karyotype analysis, physical examination of virilization characteristics, 17-hydroxyprogesterone detection, and even cytogenetic analysis are of vital importance, which are helpful for clinicians to identify the diagnosis and treatment of the disease and avoid missed diagnosis and misdiagnosis. This report expands clinicians' understanding of TS and NCAH.
- Citation: He MN, Zhao SC, Li JM, Tong LL, Fan XZ, Xue YM, Lin XH, Cao Y. Turner syndrome with positive SRY gene and non-classical congenital adrenal hyperplasia: A case report. World J Clin Cases 2021; 9(10): 2259-2267
- URL: https://www.wjgnet.com/2307-8960/full/v9/i10/2259.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i10.2259
Turner syndrome (TS) is the most common chromosomal abnormality in females. It has a prevalence of 1:2500, which approximates to 3% of live female births and approximately 15% of miscarriages[1,2]. TS patients are characterized by short stature, broad chest, low posterior hairline, prominent ears, narrow and acutely arched palate, lack of pubertal onset at adolescence, and presence of streak ovaries with normal intelligence[3]. Monosomy X is the most common karyotype observed in patients with TS (50%-60%), in addition to other structural abnormalities in the X chromosome or mosaicism karyotype reported[4-6]. Additionally, the presence of Y chromosome material has been reported in about 10%-18% of patients. The role of the Y chromosome in human tumorigenesis remains controversial. However, the risk of virilization during puberty, gonadoblastoma, and malignant transformation increases by 7%-10% when Y chromosome material is present in gonadal tissue or peripheral blood in TS patients[4,7,8].
Non-classical congenital adrenal hyperplasia (NCAH) is a common autosomal recessive disorder that manifests due to P450c21 (21-hydroxylase) deficiency. NCAH is caused by mutations in the CYP21A2 gene or microconversions between CYP21 pseudogenes and active genes[9,10]. The disorder is a mild form of congenital adrenal hyperplasia (CAH) and may result in infertility, miscarriages, oligomenorrhea, hirsutism, acne, advanced bone age, and clitoromegaly in females. 17-hydroxyprogesterone (17-OHP) levels could be used for diagnosis, but the gold standard is the adrenocorticotropic hormone (ACTH) stimulation test and 17-OHP measurement based on several studies and guidelines. However, the optimal cutoff for baseline 17-OHP levels or post-ACTH peak 17-OHP levels for NCAH diagnosis remains controversial[11-14]. Based on a large data set in combination with genetic diagnosis, NCAH could be diagnosed using baseline 17-OHP levels > 6 nmol/L or > 30 nmol/L after ACTH stimulation[13,14]. The diagnosis could be substantiated using CYP21A2 mutation analysis. In contrast to CAH patients, the majority of patients with NCAH are never diagnosed due to very mild symptoms. In addition, compared to the majority of patients with classic CAH requiring life-long glucocorticoid treatment for survival, NCAH patients are seldom diagnosed and treated only when symptomatic[15].
The diagnosis of CAH is difficult in females with TS. This is because of the shared common clinical features like short stature, amenorrhea, and infertility and the rare reports of the condition. Moreover, patients with a combination of TS with a positive SRY gene and NCAH have never been reported. In this article, we present a 14-year-old girl who was diagnosed with NCAH and TS with a positive SRY gene concurrently. The combination of these three factors increases the difficulty of diagnosis and treatment but provides insights into the understanding of TS. It also suggests that 17-OHP together with genetic screening is necessary for TS patients.
A 14-year-old girl was referred to our hospital with growth and developmental retardation.
Based on her medical history, the patient was born after a normal full-term delivery and her parents were not genetically related. She appeared to be in good health. Her height and weight were 51 cm and 3.2 kg, respectively, at birth. However, her growth rate was slow (1 cm/year) and her height was 143 cm at age 12 years (< -2 SD), which was significantly lower than normal when she visited our hospital.
On physical examination, the patient’s general status was good, her vital signs were stable, and no masculine features such as hirsutism, acne, and clitoromegaly were observed. Axillary and pubic hair was absent. Breast and labia minora dysplasia was observed.
Biochemistry and electrolyte analysis showed no abnormalities (Table 1). Hormone profiles indicated hypergonadotropic hypogonadism, and karyotype analysis indicated 45,X/46,X, +mar, and mosaicism with suspected Y chromosome material (Figure 1).
| Parameter | First visit | Follow-up (after 1 yr) | Reference range |
| Serum sodium, mmol/L | 138 | 140 | 135-145 |
| Serum potassium, mmol/L | 3.82 | 4.38 | 3.50-5.30 |
| Serum chloride, mmol/L | 102.9 | 103.9 | 137-147 |
| Serum calcium, mmol/L | 2.40 | 2.40 | 2.20-2.65 |
| Serum phosphorus, mmol/L | 1.36 | 1.40 | 0.81-1.45 |
| Serum magnesium, mmol/L | 0.78 | 0.80 | 0.77-1.03 |
| Follicle stimulating hormone, mIU/mL | 60.86 | 60.83 | 3.50-12.50 |
| Luteinizing hormone, mIU/mL | 19.98 | 23.88 | 2.40-12.60 |
| Estradiol, pg/mL | < 5.00 | 11.96 | 12.40-233.00 |
| Progesterone, ng/mL | 0.101 | 0.722 | 0.00-0.89 |
| Testosterone, ng/mL | 0.200 | 0.310 | 0.025-0.481 |
| Free testosterone, pg/mL | 3.48 | 4.12 | 0.00-4.20 |
| 17Hydroxyprogesterone, ng/mL (first time) | 24.70 | 27.26 | 0.05-1.02 |
| 17Hydroxyprogesterone, ng/mL (second time) | 12.10 | - | 0.05-1.02 |
| Dehydroepiandrosterone, μg/dL | 4.15 | 4.77 | 4.30-22.40 |
| Androstenedione, ng/mL | 7.12 | 8.18 | 0.30-3.30 |
| Adrenocorticotropic hormone, pg/mL | 39.74 | 51.06 | 7.20-63.30 |
| Cortisol (8:00), nmol/L | 15.32 | 13.20 | 1.65-9.23 |
| Cortisol (16:00), nmol/L | 4.96 | - | 3.44-16.76 |
| Cortisol (0:00), nmol/L | 1.83 | - | - |
| AMH, ng/mL | < 0.06 | - | - |
Ultrasonic gynecopathy examinations and pelvic magnetic resonance showed no development of the uterus or ovaries (Figure 2).
The patient’s final diagnosis was SRY positive TS with NCAH.
The patient initially refused medical treatment. At the most recent follow-up visit (one year later), the patient finally accepted low doses of hydrocortisone (10 mg/d).
At the most recent follow-up visit (patient’s age was 15 years old), the electrolytes were normal. However, the patient had facial hair, her height increased to 148 cm, and her weight was 52 kg, with androstenedione and 17-OHP levels remaining high (Table 1). Human chorionic gonadotropin (HCG) stimulation test showed absence of Leydig cell function (Table 2). We will continue to follow the patient for therapeutic effects.
| Parameter | 0 min | 24 h | 48 h | 72 h |
| Testosterone, ng/mL | 0.250 | 0.300 | 0.260 | 0.290 |
| Androstenedione, mmol/L | 8.18 | - | - | 4.86 |
| Dihydrotestosterone | 35.59 | - | - | 28.90 |
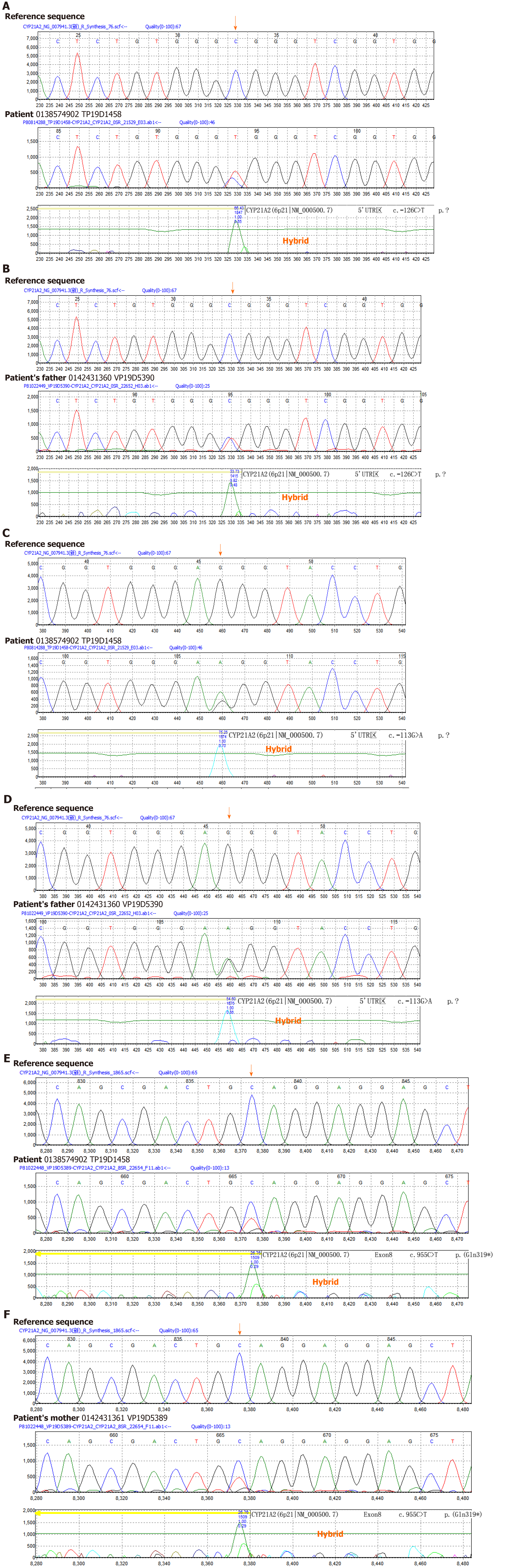
As mentioned previously, CAH diagnosis is difficult in females with TS. The majority of previously reported cases with concomitant TS and CAH were diagnosed as TS. They had ambiguous genitalia or their NCAH was misdiagnosed as TS[9,16-26]. Although our patient was of short stature with no virilization signs and ambiguous genitalia, Larizza et al[21] reported that the frequencies of both abnormal 17-OHP response to ACTH stimulation test and CYP21 gene mutation carriers were prominently higher in patients with TS than in healthy controls. Next, Linglart et al[27] found that the proportion of 21-hydroxylase deficiency carriers in TS patients was up to 21.6%, which was significantly higher than that in the general Italian population[27]. Based on previous studies and the labia minora dysplasia observed in our patient, we measured 17-OHP levels and performed karyotyping and cytogenetic analysis. Our results demonstrated that 17-OHP levels were significantly increased and karyotype and cytogenetic analysis demonstrated 45,X/46,X, +mar with a positive SRY gene with compound heterozygous mutations in CYP21A2, suggesting it to be the causative gene for CAH.
The reason for the absence of typical clinical manifestations despite the presence of the causative gene mutation for CAH is the wide phenotypic variability of the non-classical form. This is due to different enzymatic activity levels induced by several gene mutations in the CYP21A2, as well as microconversions between CYP21 pseudogenes and active genes[25]. To-date, > 200 CYP21A2 inactivating mutations have been recorded in the Human Gene Mutation Database (www.hgmd.org). New et al[26] extensively investigated the genotype-phenotype correlation in 1507 patients with CAH and demonstrated the presence of genotype-phenotype discordance. Moura-Massari et al[28] investigated the correlation between genotypes and the severity of hyperandrogenic phenotype in a cohort of 114 NCAH patients. Their results demonstrated that CYP21A2 genotypes do not predict the severity of hyperandrogenic manifestation in the non-classical form of CAH[28].
Gonadal dysgenesis observed in TS patients is associated with gonadoblastomas and malignant transformation when Y-chromosome-derived genetic material is present in the genome[4]. If the presence of Y chromosome fragments in TS patients could be detected at an early stage, the incidence of gonadoblastomas could be reduced or even prevented. Treatment of TS could be guided with prophylactic gonadectomy considered[29].
Regrettably, our patient refused laparoscopic exploration to further determine the presence and nature of gonadal tissue and determine whether she was at risk for gonadoblastoma. HCG stimulation test showed the absence of Leydig cell function. We will assess disease progression during follow-up.
In conclusion, upon evaluation of the patient mentioned in the report, we feel that 17-OHP measurement and cytogenetic analysis are necessary for TS patients even in the absence of significant virilization signs. This will play a significant role in guiding diagnosis and treatment.
The authors are grateful to Professors Ying Cao, Shan-Chao Zhao, and Yao-Ming Xue for their assistance in providing insights to the diagnosis and reviewing this manuscript. In addition, we are thankful to Jin-Min Li, Lu-Lu Tong, and Xin-Zhao Fang who were involved in the patient’s care and cytogenetic analysis. We also thank the patient and her family for their cooperation and permission.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Reyes TME S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Kurnaz E, Çetinkaya S, Savaş-Erdeve Ş, Aycan Z. Detection of the SRY gene in patients with Turner Syndrome. J Gynecol Obstet Hum Reprod. 2019;48:265-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Ibrahim MN, Laghari TM, Hanif MI, Khoso ZA, Riaz M, Raza J. Comparison of Classical and Non-Classical Turner Syndrome at NICH Karachi. J Coll Physicians Surg Pak. 2018;28:840-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Kavoussi SK, Christman GM, Smith YR. Healthcare for adolescents with Turner syndrome. J Pediatr Adolesc Gynecol. 2006;19:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Baer TG, Freeman CE, Cujar C, Mansukhani M, Singh B, Chen X, Abellar R, Oberfield SE, Levy B. Prevalence and Physical Distribution of SRY in the Gonads of a Woman with Turner Syndrome: Phenotypic Presentation, Tubal Formation, and Malignancy Risk. Horm Res Paediatr. 2017;88:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Bispo AV, Dos Santos LO, Burégio-Frota P, Galdino MB, Duarte AR, Leal GF, Araújo J, Gomes B, Soares-Ventura EM, Muniz MT, Santos N. Effect of chromosome constitution variations on the expression of Turner phenotype. Genet Mol Res. 2013;12:4243-4250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Saenger P, Wikland KA, Conway GS, Davenport M, Gravholt CH, Hintz R, Hovatta O, Hultcrantz M, Landin-Wilhelmsen K, Lin A, Lippe B, Pasquino AM, Ranke MB, Rosenfeld R, Silberbach M; Fifth International Symposium on Turner Syndrome. Recommendations for the diagnosis and management of Turner syndrome. J Clin Endocrinol Metab. 2001;86:3061-3069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Gravholt CH, Fedder J, Naeraa RW, Müller J. Occurrence of gonadoblastoma in females with Turner syndrome and Y chromosome material: a population study. J Clin Endocrinol Metab. 2000;85:3199-3202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Freriks K, Timmers HJ, Netea-Maier RT, Beerendonk CC, Otten BJ, van Alfen-van der Velden JA, Traas MA, Mieloo H, van de Zande GW, Hoefsloot LH, Hermus AR, Smeets DF. Buccal cell FISH and blood PCR-Y detect high rates of X chromosomal mosaicism and Y chromosomal derivatives in patients with Turner syndrome. Eur J Med Genet. 2013;56:497-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Mishra VV, Pritti K, Aggarwal R, Choudhary S. Nonclassic congenital adrenal hyperplasia misdiagnosed as Turner syndrome. J Hum Reprod Sci. 2015;8:239-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Araújo RS, Mendonca BB, Barbosa AS, Lin CJ, Marcondes JA, Billerbeck AE, Bachega TA. Microconversion between CYP21A2 and CYP21A1P promoter regions causes the nonclassical form of 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2007;92:4028-4034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Trapp CM, Oberfield SE. Recommendations for treatment of nonclassic congenital adrenal hyperplasia (NCCAH): an update. Steroids. 2012;77:342-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, Meyer-Bahlburg HF, Miller WL, Montori VM, Oberfield SE, Ritzen M, White PC; Endocrine Society. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4133-4160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 675] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 13. | Livadas S, Dracopoulou M, Dastamani A, Sertedaki A, Maniati-Christidi M, Magiakou AM, Kanaka-Gantenbein C, Chrousos GP, Dacou-Voutetakis C. The spectrum of clinical, hormonal and molecular findings in 280 individuals with nonclassical congenital adrenal hyperplasia caused by mutations of the CYP21A2 gene. Clin Endocrinol (Oxf). 2015;82:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Savaş-Erdeve Ş, Çetinkaya S, Abalı ZY, Poyrazoğlu Ş, Baş F, Berberoğlu M, Sıklar Z, Korkmaz Ö, Buluş D, Akbaş ED, Güran T, Böber E, Akın O, Yılmaz GC, Aycan Z. Clinical, biochemical and genetic features with nonclassical 21-hydroxylase deficiency and final height. J Pediatr Endocrinol Metab. 2017;30:759-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Falhammar H, Nordenström A. Nonclassic congenital adrenal hyperplasia due to 21-hydroxylase deficiency: clinical presentation, diagnosis, treatment, and outcome. Endocrine. 2015;50:32-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Maciel-Guerra AT, Guerra G Jr, Marini SH, Matias Baptista MT, Marques-de-Faria AP. Female pseudohermaphroditism due to classical 21-hydroxylase deficiency in a girl with Turner syndrome. Clin Genet. 1997;51:351-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Atabek ME, Kurtoğlu S, Keskin M. Female pseudohermaphroditism due to classical 21-hydroxylase deficiency and insulin resistance in a girl with Turner syndrome. Turk J Pediatr. 2005;47:176-179. [PubMed] |
| 18. | del Arbol JL, Soto Más JA, Fernández-Abril JA, Raya Muñoz J, Martínez Tormo F, Gómez Rodríguez J, Gómez Capilla JA, Peña Yáñez A. [Turner syndrome caused by deletion of the long arm of the X chromosome associated with adrenogenital syndrome caused by partial deficiency of 21-hydroxylase]. Rev Clin Esp. 1983;171:67-71. [PubMed] |
| 19. | Montemayor-Jauregui MC, Ulloa-Gregori AO, Flores-Briseño GA. Associated adrenogenital and Turner's syndrome mosaicism. Plast Reconstr Surg. 1985;75:877-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Cohen MA, Sauer MV, Lindheim SR. 21-hydroxylase deficiency and Turner's syndrome: a reason for diminished endometrial receptivity. Fertil Steril. 1999;72:937-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Larizza D, Cuccia M, Martinetti M, Maghnie M, Dondi E, Salvaneschi L, Severi F. Adrenocorticotrophin stimulation and HLA polymorphisms suggest a high frequency of heterozygosity for steroid 21-hydroxylase deficiency in patients with Turner's syndrome and their families. Clin Endocrinol (Oxf). 1994;40:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Jia HY, Sun SY, Chen YH, Yang ZW, Zhang J, Qi Y, Zhang YW, Wang WQ, Ning G. A case of 21-hydroxylase deficiency in Turner's syndrome and literature review. Zhonghua Neifenmi Daixie Zazhi. 2017;33:760-764. [DOI] [Full Text] |
| 23. | Kendirci HN, Aycan Z, Çetinkaya S, Baş VN, Ağladıoğlu SY, Önder A. A rare combination: congenital adrenal hyperplasia due to 21 hydroxylase deficiency and Turner syndrome. J Clin Res Pediatr Endocrinol. 2012;4:213-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Lee KF, Chan AO, Fok JM, Mak MW, Yu KC, Lee KM, Shek CC. Late presentation of simple virilising 21-hydroxylase deficiency in a Chinese woman with Turner's syndrome. Hong Kong Med J. 2013;19:268-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Fu R, Lu L, Jiang J, Nie M, Wang X, Lu Z. A case report of pedigree of a homozygous mutation of the steroidogenic acute regulatory protein causing lipoid congenital adrenal hyperplasia. Medicine (Baltimore). 2017;96:e6994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | New MI, Abraham M, Gonzalez B, Dumic M, Razzaghy-Azar M, Chitayat D, Sun L, Zaidi M, Wilson RC, Yuen T. Genotype-phenotype correlation in 1,507 families with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Proc Natl Acad Sci USA. 2013;110:2611-2616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 240] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 27. | Linglart A, Cabrol S, Berlier P, Stuckens C, Wagner K, de Kerdanet M, Limoni C, Carel JC, Chaussain JL; French Collaborative Young Turner Study Group. Growth hormone treatment before the age of 4 years prevents short stature in young girls with Turner syndrome. Eur J Endocrinol. 2011;164:891-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Moura-Massari VO, Bugano DD, Marcondes JA, Gomes LG, Mendonca BB, Bachega TA. CYP21A2 genotypes do not predict the severity of hyperandrogenic manifestations in the nonclassical form of congenital adrenal hyperplasia. Horm Metab Res. 2013;45:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Hersmus R, Stoop H, Turbitt E, Oosterhuis JW, Drop SL, Sinclair AH, White SJ, Looijenga LH. SRY mutation analysis by next generation (deep) sequencing in a cohort of chromosomal Disorders of Sex Development (DSD) patients with a mosaic karyotype. BMC Med Genet. 2012;13:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |