Published online Apr 6, 2021. doi: 10.12998/wjcc.v9.i10.2218
Peer-review started: December 21, 2020
First decision: December 31, 2020
Revised: January 12, 2021
Accepted: February 11, 2021
Article in press: February 11, 2021
Published online: April 6, 2021
Processing time: 98 Days and 14.5 Hours
Neurogenic tumors are rare but represent an important consideration in the differential diagnosis of abdominal mesenchymal tumors. Reports on their incidence, pathological features and clinical characteristics are scarce.
To advance the overall knowledge on the histologic, immunohistochemical, clinical and radiologic characteristics of neurogenic tumors through this case series.
An established database of a nationwide tertiary referral center, covering a 15-year period (2005 and 2020), was retrospectively re-evaluated. Diagnoses of neurogenic tumor cases were confirmed by two experts following review of the macroscopic, histological and immunohistochemical records along with findings from analysis of archived tissue sections for each included patient. Tissue microarrays were constructed for cases lacking necessary immunohistochemical studies. Clinical data and follow-up information were collected from the hospital records and the patients themselves, when available.
The study included 19 cases of intraabdominal neurogenic tumors, representing 12 women and 7 men, between 18 and 86 years of age (median: 51 years). Final confirmed diagnoses were 12 schwannomas, 2 diffuse submucosal neuro-fibromatoses, 2 ganglioneuromas, 2 malignant peripheral sheath nerve tumors, and 1 mucosal Schwann cell hamartoma. Sizes of the tumors were variable, with a median diameter of 4 cm; the two largest (> 10 cm) were schwannomas. The majority of cases were asymptomatic at presentation, but the most frequent symptom was abdominal pain. Gastrointestinal tract lesions were detected with endoscopy and extra-luminal lesions were detected with cross-sectional imaging. All cases were S100-positive and CD117-negative; most cases were negative for desmin, epithelial membrane antigen, smooth muscle actin and CD34. In all but 5 cases, the Ki67 proliferation index was ≤ 1%.
Re-evaluation of 19 cases of abdominal neurogenic tumors demonstrated con-siderable variability in clinicopathologic characteristics depending on location, dimension and histological features.
Core Tip: This is the largest single institution study to re-assess abdominal neurogenic tumors listed in an established database of a national tertiary referral center to improve the overall knowledge on the histologic, immunohistochemical, clinical and radiologic characteristics of such tumors. In total, 19 cases of intraabdominal neurogenic tumors with complete clinical, pathologic and immunohistochemical data were included in the analysis. We believe the new insights on clinicopathologic characteristics, related to location, dimension and histological features, will provide guidance to pathologists, gastroenterologists, surgeons and oncologists involved in the diagnosis and management of future cases.
- Citation: Simsek C, Uner M, Ozkara F, Akman O, Akyol A, Kav T, Sokmensuer C, Gedikoglu G. Comprehensive clinicopathologic characteristics of intraabdominal neurogenic tumors: Single institution experience. World J Clin Cases 2021; 9(10): 2218-2227
- URL: https://www.wjgnet.com/2307-8960/full/v9/i10/2218.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i10.2218
Intraabdominal neurogenic lesions encompass a heterogenous group including schwannomas, neurofibromas, mucosal Schwann cell hamartomas, gang-lioneuromas/ganglioneuromatosis, perineuromas, granular cell tumors, malignant peripheral nerve sheath tumors (MPNSTs) as well as their hybrids and variants, all of which are characterized by Schwann cell differentiation[1]. These tumors can occur in virtually any region of the body that includes neural structures. As such, when they develop in the abdomen, they are most frequently situated in the retroperitoneum and gastrointestinal tract, having originated from vertebral and myenteric plexuses respectively. Nerve sheath tumors are frequent among patients with neu-rofibromatosis 1 (NF1), and these cases appear to be mostly non-hereditary (sporadic)[2]. While their overall prevalence is not definitively established, especially for those involving the abdomen, an increased detection rate has been realized with the widespread use of cross-sectional imaging and endoscopic screening examinations. The most common subtype, schwannoma, has a prevalence of 6%-8% among all reported mesenchymal tumors[3]. The clinical presentation is diverse, depending on location and origin of the tumor(s). Retroperitoneal tumors can present with neurologic signs or symptoms related to the space-occupying presence of the mass itself, whereas gastrointestinal tumors can manifest signs and symptoms according to their physical obstruction of the region or bleeding events[4]. The management approach depends on the clinical context and histological findings but, generally, surgical or endoscopic resection is performed if feasible.
The distinction of MPNSTs from their benign counterparts is relatively str-aightforward, following the high mitotic activity and presence of cytological atypia and necrosis for the former. However, the classification and diagnosis of benign peripheral nerve sheath tumors is still controversial, owing to the great variability that exists among them and the significant overlap of histologic features among the numerous subgroups. Another complicating feature is that the benign forms are not uncommon among intraabdominal mesenchymal tumors; certainly, they should be taken into consideration in the differential diagnosis of any intraabdominal soft tissue neoplasm.
In this study, we aimed to expand the existing knowledge on the histological, immunohistochemical, clinical and radiological characteristics of peripheral nerve sheath tumors by performing a retrospective analysis of cases listed in the database of a nationwide tertiary referral center[5].
We reviewed cases reported in the electronic database of Hacettepe University’s Pathology Department from 2005 to 2020. The criteria for intraabdominal neurogenic tumor case selection were: (1) Abdominal location and proximity to the gas-trointestinal system; (2) Morphological resemblance to peripheric nerve cell origin; and (3) Immunohistochemical proof of diagnosis [e.g., S100 positivity, CD117 and neurofilament protein (NFP) negativity]. A total of 19 patients were identified and selected for assessment according to their records of histological and immu-nohistochemical features and for confirmation of the diagnoses by two path-ologists (Gedikoglu G, Uner MB).
Archived specimens (paraffin-embedded) for each case were re-evaluated mac-roscopically, histologically and immunohistochemically. Twelve of them had been obtained in Hacettepe University, and the remaining seven were brought from another health center.
Then, a tissue microarray was constructed for any cases that lacked findings for the necessary immunohistochemical studies. Macroscopic findings (i.e., tumor border, size, gastrointestinal stroma contact, and location) were recorded from available pathology reports. All cases were reviewed from a histological perspective to identify the rates of mitosis and nuclear atypia, as well as cellularity, hemorrhage, de-generation and tumoral necrosis. Mitoses were counted for 50 consecutive high power fields. A comprehensive immunohistochemical panel was applied for classification, which included immunoreactive staining for CD117 (c-kit) (1:200, EP10; Novacastra Laboratories Ltd, Newcastle upon Tyne, United Kingdom), S100 (1:200, NCL-L-S100p; Novacastra Laboratories Ltd), desmin (1:100, DE-R-11; Novacastra Laboratories Ltd), CD34 (1:100, QBend/10; Leica Biosystems, Buffalo Grove, IL, United States), smooth muscle actin (SMA) (1:800, 1A4:asm-1; Thermo Fisher Scientific, Waltham, MA, United States), epithelial membrane antigen (EMA) (1:300, GP1.4; Novacastra Laboratories Ltd), and NFP (1:200, FNP7, DA2, RMdO20.11; Invitrogen, Carlsbad, CA, United States); the Ki-67 (1:200, Ki-67P; Dianova, Barcelona, Spain) proliferation index was also calculated (> 2% high cell proliferation). EMA and S100 positivity helped to determine the peripheral nerve sheath or perineurium ori-gin/differentiation. CD117 positivity helped to exclude gastrointestinal stromal tumor (GIST). NFP positivity indicated neurogenic derivation. Desmin and SMA were used to exclude smooth muscle origin.
Clinical data and follow-up information were collected from hospital records and from patients themselves when available. Follow-up data were available for the 19 selected patients, with duration ranging from 1 mo to 12 years. At the time of original diagnosis, the tumors had been visualized with abdominal ultrasonography (commonly known as USG), computerized tomography (CT), magnetic resonance imaging (MRI), endoscopy and colonoscopy.
We identified 19 cases of intraabdominal neurogenic tumor after reviewing 15 consecutive years of data in our center’s pathology database. These cases represented 12 women and 7 men, of ages ranging from 18 years to 86 years (median: 51 years).
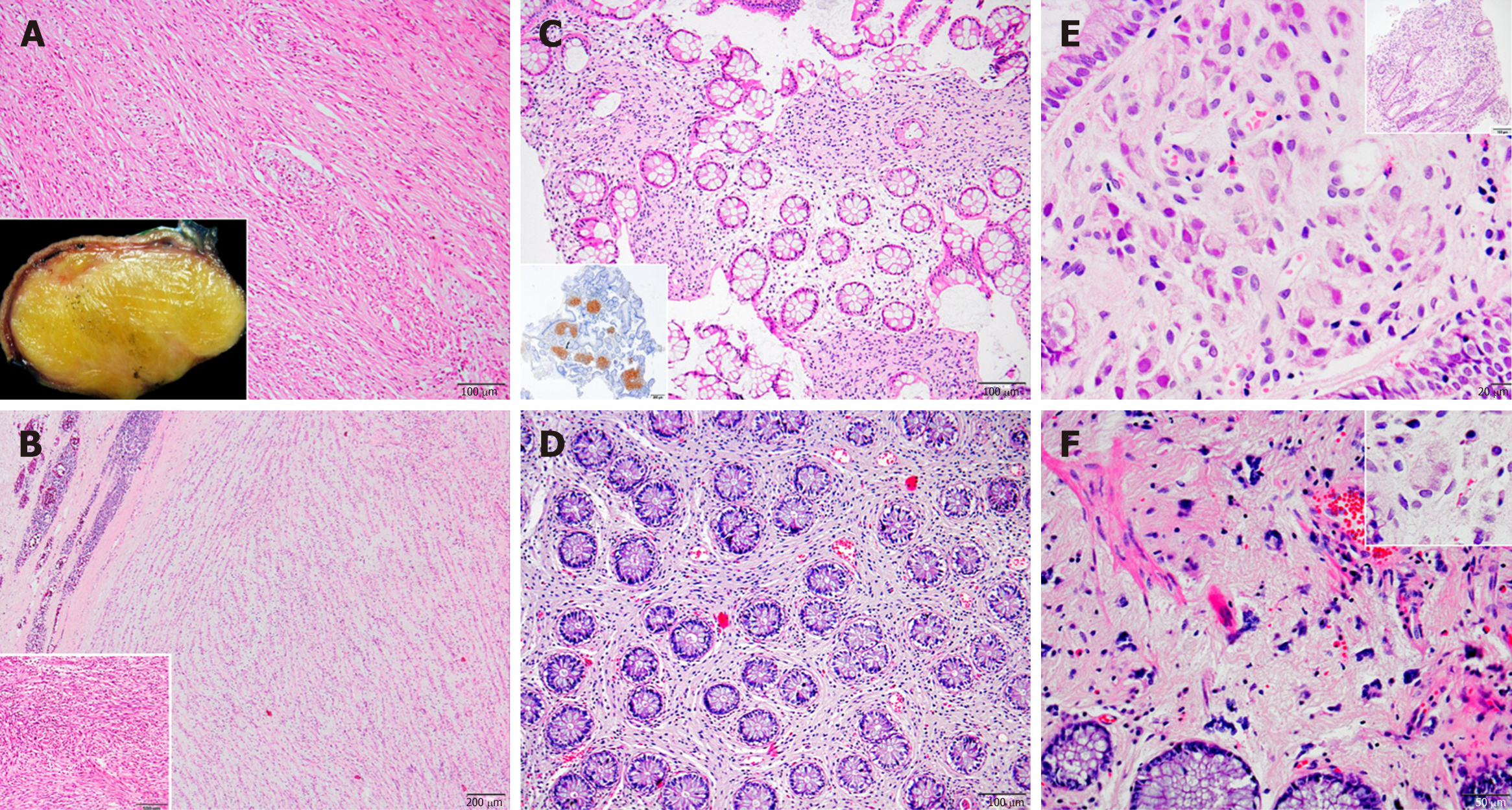
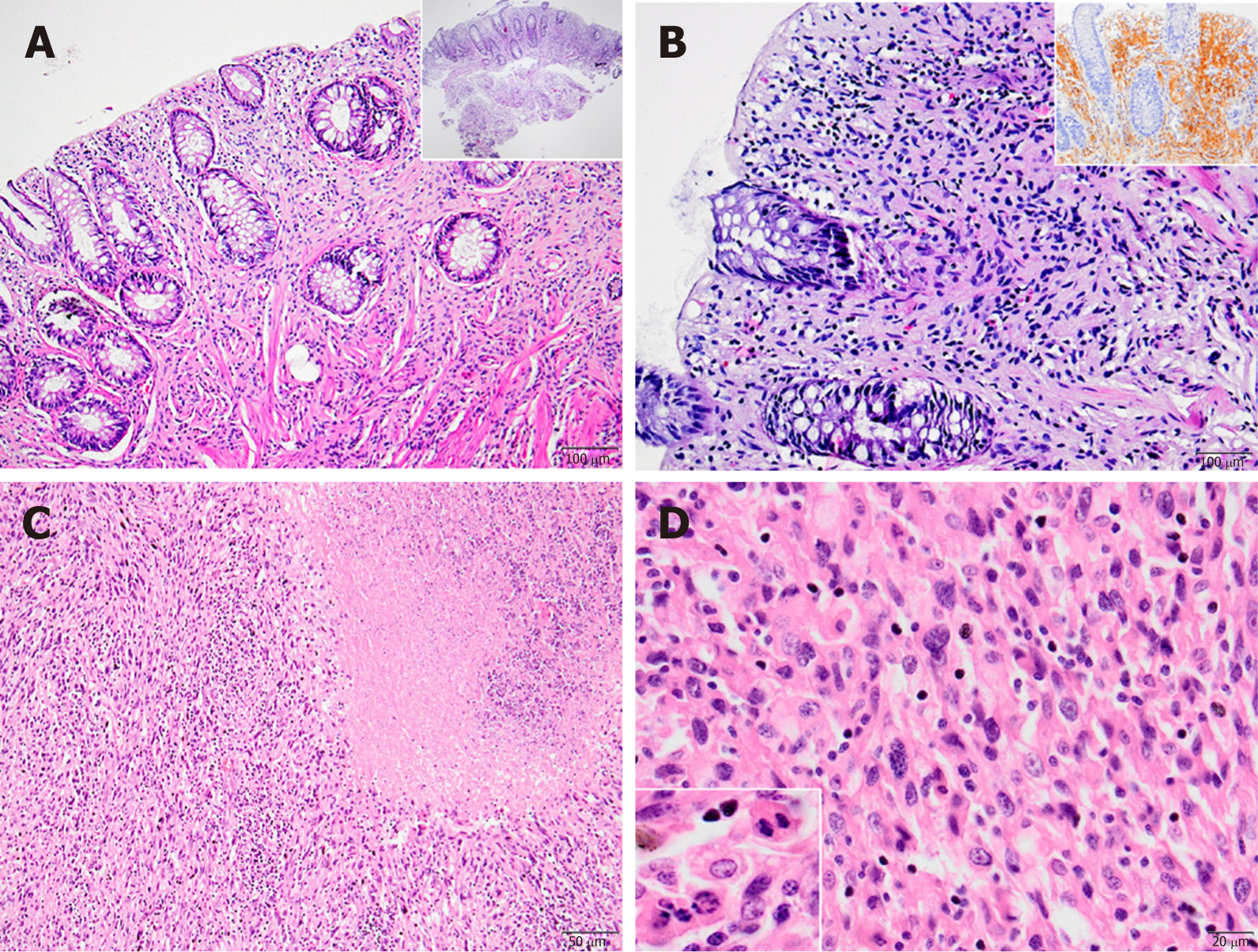
When we classified these cases according to their location, immunohistochemical and histopathological features, 12 were identified as schwannoma (Figure 1A and B), 2 as diffuse submucosal neurofibromatosis (Figure 1C and D), 2 as ganglioneuroma (Figure 1E and F), 1 as mucosal Schwann cell hamartoma (Figure 2A and B) and 2 as MPNST (Figure 2C and D).
The schwannomas were located most frequently in stomach, with 6 in the distal stomach-antrum and 2 in the proximal stomach. The second most frequent location was retroperitoneum, including 1 in the posterior rectum, 1 in the rectosigmoid junction, 1 in the posterior of the second part of the duodenum, and 1 in the retroperitoneum/intraabdominal region. The 2 diffuse submucosal neurofibromatosis cases involved NF1 patients, with one located throughout duodenal to transverse colonic walls and the other in the sigmoid colon wall. The 2 cases of ganglioneuroma were both located in the transverse and sigmoid colon regions. The 2 MPNSTs were located, respectively, in the stomach and in close contact with the colon. The 1 mucosal Schwann cell hamartoma was located in the sigmoid colon.
The colonic ganglioneuromas and mucosal Schwann cell hamartoma appeared as polypoid lesions. Other than polyps and diffuse submucosal neurofibromatosis, the remaining tumors predominantly showed as masses.
The tumors varied in size. Two were larger than 10 cm-both cellular schwannomas, with the largest measuring 13 cm × 7 cm × 7 cm and the other measuring 12 cm × 7 cm × 5 cm. The mean diameter of the other tumors was approximately 4 cm, except for polypectomies in 2 cases and diffuse submucosal neurofibromatosis in 2 cases.
Most of the cases were asymptomatic at presentation. The most common presentation for symptomatic patients was abdominal pain, which was reported by 3 patients who represented the largest case of cellular schwannoma, a colonic ganglioneuroma, and a gastric schwannoma. Other symptoms reported were nausea and vomiting (in colonic ganglioneuroma and colonic schwannoma cases), dyspepsia (in 2 cases of gastric schwannoma), diarrhea (in 1 of the cases of diffuse submucosal neurofibromatosis), rectal pain (in 1 of the cases of retroperitoneal schwannoma, which was located in the posterior rectum) and weight loss (in 1 of the cases of retroperitoneal schwannoma, which was situated neighboring the duodenum).
As expected, lesions in the gastrointestinal tract had been detected by endoscopic examinations and extra-visceral lesions had been detected by cross-sectional imaging methods, such as CT, MRI, and USG. Notably, 1 of the diffuse submucosal neu-rofibromatosis cases had a family history of colon cancer; that lesion had been detected during a colon cancer screening with routine colonoscopy.
In the majority of cases, the methods used for diagnosis and treatment had been the same and had been performed simultaneously, with the exception being cases of colonic schwannoma, colonic ganglioneuroma, and diffuse submucosal neuro-fibromatosis. The colonic ganglioneuroma and schwannoma cases had been diagnosed by polypectomy, while the diffuse submucosal neurofibromatosis cases and mucosal Schwann cell hamartoma cases had been diagnosed by endoscopic biopsy. Excisional biopsy had been performed on all retroperitoneal schwannomas and the MPNST. Gastric schwannomas located in the distal gastric region (n = 4) were addressed by partial gastrectomy, while those located in the proximal gastric region (n = 3) were addressed by total gastrectomy and the 1 originating from the minor curvature by wedge resection.
All 19 cases showed S100 positivity, indicating peripheral nerve sheath origin; most were also negative for CD117, with the notable exception of the GIST cases. Most of the cases were negative for desmin, EMA, SMA and CD34 (Table 1); exceptions were a MPNST case that showed focal S100 positivity, and 3 cases which showed focal desmin positivity (1 each for gastric schwannoma, cellular schwannoma located in rectum posterior, and ganglioneuroma in the colon). Similar to the desmin findings, there was focal CD34 positivity in 3 cases, 1 being the cellular schwannoma located in the rectum posterior, 1 being the gastric schwannoma, and 1 being the diffuse submucosal neurofibromatosis throughout the duodenum to transverse colon. NFP positivity was seen in 1 of the ganglioneuroma cases. EMA was focally positive in 2 of the cellular schwannomas and 1 conventional gastric schwannoma. There was focal SMA positivity in 6 cases total, representing 5 schwannomas (including the 2 cellular cases) and 1 ganglioneuroma in the sigmoid colon.
| Case | Age | Sex | Diagnosis | Localization | S100 | CD117 (c-kit) | Desmin | CD34 | Ki-67 | SMA | EMA | NFP |
| 1 | 41 | Female | Schwannoma | Duodenum and retroperitone | Positive | Negative | Negative | Negative | < 1% | Negative | Negative | Negative |
| 2 | 64 | Female | Schwannoma | Stomach | Positive | Negative | Negative | Negative | 2% | Focal positive | Negative | Negative |
| 3 | 36 | Female | Schwannoma | Stomach | Positive | Negative | Negative | Negative | 1% | Negative | Negative | Negative |
| 4 | 47 | Female | Schwannoma | Stomach | Diffuse positive | Negative | Focal positive | Negative | 1% | Focal positive | Negative | Negative |
| 5 | 44 | Male | Ganglioneuroma/Ganglioneuromatosis | Transvers colon | Positive | Negative | Negative | Negative | < 1% | Negative | Negative | Positive |
| 6 | 51 | Female | Schwannoma | Stomach | Diffuse positive | Negative | Negative | Negative | 1% | Negative | Negative | Negative |
| 7 | 50 | Female | Cellular schwannoma | Rectum posterior | Diffuse positive | Negative | Focal positive | Focal positive | 2% | Focal positive | Focal positive | Negative |
| 8 | 51 | Male | Cellular schwannoma | Retroperitoneum (descending colon-sigmoid colon junction, rectosigmoid posterior) | Diffuse positive | Negative | Negative | Negative | < 1% | Focal positive | Focal positive | Negative |
| 9 | 30 | Female | Schwannoma | Retroperitoneum (duodenum posterior) | Diffuse positive | Negative | Negative | Negative | < 1% | Negative | Negative | Negative |
| 10 | 71 | Male | Schwannoma | Stomach | Diffuse positive | Negative | Negative | Negative | < 1% | Negative | Negative | Negative |
| 11 | 82 | Male | Malignant peripheral nerve sheath tumor | Around colon | Focal positive | Negative | Negative | Negative | 5% | Negative | Negative | Negative |
| 12 | 72 | Male | Schwannoma | Stomach | Positive | Negative | Negative | Negative | < 1% | Negative | Negative | Negative |
| 13 | 52 | Female | Schwannoma | Stomach | Diffuse positive | Negative | Negative | Focal positive | 2% | Focal positive | Focal positive | Negative |
| 14 | 57 | Female | Schwannoma | Stomach | Diffuse positive | Negative | Negative | Negative | 2% | Negative | Negative | Negative |
| 15 | 18 | Male | Neurofibromatosis | Duodenum -> transverse colon | Diffuse positive | Negative | Negative | Focal positive | < 1% | Negative | Negative | Negative |
| 16 | 36 | Male | Ganglioneuroma | Sigmoid colon | Positive | Negative | Focal positive | Negative | < 1% | Focal positive | Negative | Negative |
| 17 | 35 | Female | Neurofibromatosis | Sigmoid colon | Positive | Negative | Negative | Negative | < 1% | Negative | Negative | Negative |
| 18 | 79 | Female | Mucosal Schwann cell hamartoma | Sigmoid colon | Positive | Negative | Negative | Negative | < 1% | Negative | Negative | Negative |
| 19 | 86 | Female | Malignant peripheral nerve sheath tumor | Stomach | Focal positive | Negative | Negative | Negative | 30% | Focal positive | Negative | Negative |
In all but 5 cases, the Ki67 proliferation index was equal to or less than 1%. The MPNST case had a Ki67 proliferation index ranging broadly, from 5% to 30% in the various high power fields examined. A Ki67 proliferation index of 2% was found in 4 cases, including the retroperitoneal cellular schwannoma located in the posterior of rectum and 3 of the gastric schwannomas (Table 1).
One of the interesting diagnoses among our cases was the rare mucosal Schwann cell hamartoma, located in the sigmoid colon (Figure 2A and B). It was composed of benign mucosal proliferative Schwann cells, with no whorls, no palisading, and no fasciculation, filling the lamina propria and having poorly circumscribed margins. The cells in the lesion showed generally small, bland and elongated nuclei with spindle-shaped, fuzzy cytoplasm. No necrosis, increased mitotic rate or pleomorphism was identified.
Neurogenic tumors are a relatively rare group but have important implication for the differential diagnosis of abdominal mesenchymal tumors. Unfortunately, reports of their incidence and pathological and clinical characteristics are scarce.
Abdominal neurogenic tumors constitute a heterogenous group, including the peripheral nerve sheath tumors and ganglionic tumors. The sine qua non mutual characteristics are S100 positivity along with c-kit negativity (the latter of which excludes GIST). Schwannomas are the most common among this group[6], which agreed with our findings from this study; yet, their incidence is still low compared to other tumors. They are mostly found in older adults but can also develop in the younger population[7]. Beyond the strong S100 positivity, glial fibrillary acidic protein and CD34 positivity is variable, and, as expected, other mesenchymal or neural markers, such as desmin, cytokeratin, NFP or synaptophysin, are negative. Similar to other neurogenic tumors, they can present as gastrointestinal submucosal tumors originating from the myenteric plexus or retroperitoneal masses. Although the stomach is the most common location, they can be found anywhere in the gastrointestinal tract, including esophagus, small intestine and colon[3]. They can also be found in retroperitoneum originating from the vertebral plexus or peripheral nerves[8]. Schwannomas appear as solid, whitish-tan, well demarcated lesions, with occasional central ulceration[9]. Histologic examination reveals mature Schwann cells that are spindle-shaped and taper-ended, with occasional lymphocytic cuffing.
Neurofibromas are another type of benign peripheral nerve sheath tumor originating from Schwann cells[10]. They are commonly found in NF1, being the most frequently encountered tumor in these patients[11]. Grossly, they can show a well-demarcated perineural lesion or diffuse infiltrative masses in different regions. The tumor itself is composed of a neoplastic Schwann cell component, along with myxoid stroma and a mixture of other perineural and inflammatory cell types in a myxoid stroma.
Ganglioneuromas and ganglionic tumors differ from benign peripheral nerve sheath tumors in several aspects. First, they are composed of ganglionic and stromal cells, along with mature Schwann cells; thus, the tumor stains with both S100 and neural markers. Second, they are more commonly found in younger ages[12]. Third, apart from NF1, ganglioneuromas are also known to be frequent among patients with Cowden syndrome and MEN1 syndrome, both as a solitary tumor or as a diffuse form of ganglioneuromatosis[13]. None of our cases were diagnosed with either of these syndromes.
MPNSTs represent the malignant counterpart of neural sheath tumors. They are thought to originate from Schwann cells. Opposite to benign neurogenic tumors, nearly half of MPNST cases arise in NF1 patients, with a reported 10% of cases having a history of prior radiation exposure; approximately one-third are considered sporadic, mostly in the older age group[14]. They are mostly located in peripheral nerves or neural plexuses though the intraabdominal location has been reported, albeit very rarely. MPNSTs can occur along the gastrointestinal tract or in other abdominal viscera, like liver and pancreas[15,16]. Histologically, MPNSTs are composed of monomorphic, spindle-shaped cells, with occasional palisades and necrosis, along with mitotic activity and cytologic atypia. S100 staining is characteristically patchy but not diffuse[17].
NF1 patients are predisposed to neurogenic tumors of many kinds, including but not limited to schwannomas, neurofibromas, perineuriomas, ganglioneuromas, granular cell tumors, gangliocytic paragangliomas, gastrointestinal neuroectodermal tumors and MPNSTs[18]. Theoretically, these tumors can be encountered in any neural structure in the body but are most commonly found in peripheral nerves and the plexuses. Intraabdominal lesions are reported in 25% of patients, with the gastrointestinal tract being the most common location and neurofibroma being the most frequent lesion type[19]. As described above, only 2 of our patients had a NFI diagnosis.
In conclusion, herein, we have reported 19 cases of abdominal neurogenic tumors, along with their clinical presentation, histological characteristics and comprehensive immunohistochemical profiles. The strengths of this study are 3-fold. First, the cases were all evaluated and followed-up in a single institution, which is a nationwide tertiary referral center. Second, the extensive follow-up periods facilitated an accurate assessment of each case’s prognosis. Third, all cases underwent re-review by two expert pathologists (Gedikoglu G, Uner MB) to confirm the diagnoses and provide an updated concurrent description of the histopathologic characteristics. There are some weaknesses to our study, which should be kept in mind when interpreting our results. The first among these is that our evaluated cases were collected retrospectively, and missing data points were unavoidable, as mentioned above. Second, the limited number of cases for each of the separate neoplasm types precluded our ability to deduce a generalized profile. Third, some of the cases had been referred from other institutions and their gross pathologic examinations had been performed by other pathologists outside of our institution. Fourth, although the immunohistochemical analyses were comprehensive, the profiles could not provide a clear-cut distinction among the different tumor types examined; thus, the profile itself needs further elaboration in future prospective studies that include molecular or immuno-histochemical biomarkers, which may also be predictive of prognosis and clinical outcome.
Abdominal neurogenic tumors constitute an extremely rare yet important group of intraabdominal soft tissue lesions.
The current knowledge regarding abdominal neurogenic tumors is limited. A more detailed understanding of their clinical and pathological characteristics will benefit diagnosis as well as management of these tumors and their disease manifestations.
We aimed to delineate the comprehensive clinical, radiologic and histopathological characteristics of intraabdominal neurogenic tumors.
We reviewed a nationwide referral center’s 15 years’ worth of biobank data, collecting clinical, radiologic and clinical variables of all patients during that period. In addition, we obtained the archived paraffin-embedded specimens for each of the 19 cases identified and re-evaluated the histopathologic and immunohistochemical features.
Nineteen cases of tumors were identified from our database for the 15-year period. The most common lesion was schwannoma (n = 12), followed by diffuse submucosal neurofibromatosis, ganglioneuromas, and malignant peripheral sheath nerve tumors (n = 2 each), and mucosal Schwann cell hamartoma (n = 1).
Intraabdominal neurogenic tumors have excellent clinical outcome. However, there are nuances in their radiologic, endoscopic and histologic diagnoses.
Multi-institutional studies with larger study populations are merited.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang XJ S-Editor: Zhang H L-Editor: A P-Editor: Zhang YL
| 1. | Rodriguez FJ, Folpe AL, Giannini C, Perry A. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123:295-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 438] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 2. | Baehring JM, Betensky RA, Batchelor TT. Malignant peripheral nerve sheath tumor: the clinical spectrum and outcome of treatment. Neurology. 2003;61:696-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 120] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Prévot S, Bienvenu L, Vaillant JC, de Saint-Maur PP. Benign schwannoma of the digestive tract: a clinicopathologic and immunohistochemical study of five cases, including a case of esophageal tumor. Am J Surg Pathol. 1999;23:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 98] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Bohlok A, El Khoury M, Bormans A, Galdon MG, Vouche M, El Nakadi I, Donckier V, Liberale G. Schwannoma of the colon and rectum: a systematic literature review. World J Surg Oncol. 2018;16:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Karakaş Y, Dizdar Ö, Kösemehmetoğlu K, Bozbulut Ub, Demir M, Gedikoğlu G, Söylemezoğlu F, Türker A, Kars A. Multimodality treatment in malignant peripheral nerve sheath tumors. J Oncol Sci. 2020;6:23-28. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Kwon MS, Lee SS, Ahn GH. Schwannomas of the gastrointestinal tract: clinicopathological features of 12 cases including a case of esophageal tumor compared with those of gastrointestinal stromal tumors and leiomyomas of the gastrointestinal tract. Pathol Res Pract. 2002;198:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Agaimy A, Märkl B, Kitz J, Wünsch PH, Arnholdt H, Füzesi L, Hartmann A, Chetty R. Peripheral nerve sheath tumors of the gastrointestinal tract: a multicenter study of 58 patients including NF1-associated gastric schwannoma and unusual morphologic variants. Virchows Arch. 2010;456:411-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Tao LP, Huang EJ, Li P, Lu YY. Schwannoma of stomach: a clinicopathologic study of 12 cases. Int J Clin Exp Pathol. 2018;11:1679-1683. [PubMed] |
| 9. | Voltaggio L, Murray R, Lasota J, Miettinen M. Gastric schwannoma: a clinicopathologic study of 51 cases and critical review of the literature. Hum Pathol. 2012;43:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Perry A, Roth KA, Banerjee R, Fuller CE, Gutmann DH. NF1 deletions in S-100 protein-positive and negative cells of sporadic and neurofibromatosis 1 (NF1)-associated plexiform neurofibromas and malignant peripheral nerve sheath tumors. Am J Pathol. 2001;159:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 92] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Garrouche N, Ben Abdallah A, Arifa N, Hasni I, Ben Cheikh Y, Ben Farhat W, Ben Amor S, Jemni H. Spectrum of gastrointestinal lesions of neurofibromatosis type 1: a pictorial review. Insights Imaging. 2018;9:661-671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Okamatsu C, London WB, Naranjo A, Hogarty MD, Gastier-Foster JM, Look AT, LaQuaglia M, Maris JM, Cohn SL, Matthay KK, Seeger RC, Saji T, Shimada H. Clinicopathological characteristics of ganglioneuroma and ganglioneuroblastoma: a report from the CCG and COG. Pediatr Blood Cancer. 2009;53:563-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Chan OT, Haghighi P. Hamartomatous polyps of the colon: ganglioneuromatous, stromal, and lipomatous. Arch Pathol Lab Med 2006; 130: 1561-1566 [PMID: 17090203 DOI: 10. 1043/1543-2165(2006)130. |
| 14. | Manger T, Pross M, Haeckel C, Lippert H. Malignant peripheral nerve sheath tumor of the esophagus. Dig Surg. 2000;17:627-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Hirose T, Scheithauer BW, Sano T. Perineurial malignant peripheral nerve sheath tumor (MPNST): a clinicopathologic, immunohistochemical, and ultrastructural study of seven cases. Am J Surg Pathol. 1998;22:1368-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 130] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Iddings DM, Wright BE, Bilchik A. A rare cause of primary hepatic neoplasm: malignant peripheral nerve sheath tumor in the age of modern liver surgery. Am Surg. 2008;74:47-50. [PubMed] |
| 17. | Farid M, Demicco EG, Garcia R, Ahn L, Merola PR, Cioffi A, Maki RG. Malignant peripheral nerve sheath tumors. Oncologist. 2014;19:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 248] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 18. | McCarthy AJ, Karamchandani DM, Chetty R. Neural and neurogenic tumours of the gastroenteropancreaticobiliary tract. J Clin Pathol. 2018;71:565-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Basile U, Cavallaro G, Polistena A, Giustini S, Orlando G, Cotesta D, Petramala L, Letizia C, Calvieri S, De Toma G. Gastrointestinal and retroperitoneal manifestations of type 1 neurofibromatosis. J Gastrointest Surg. 2010;14:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |