Published online Apr 6, 2021. doi: 10.12998/wjcc.v9.i10.2192
Peer-review started: August 7, 2020
First decision: December 3, 2020
Revised: December 16, 2020
Accepted: January 25, 2021
Article in press: January 25, 2021
Published online: April 6, 2021
Processing time: 235 Days and 5.2 Hours
Controversy exists about the benefit of additional surgery after endoscopic submucosal dissection (ESD) for early gastric cancer (EGC).
To examine risk factors for overall survival (OS) after additional surgery in patients with EGC who initially underwent ESD.
This was a retrospective analysis of patients with EGC who underwent additional surgery after ESD at the Beijing Friendship Hospital affiliated to Capital Medical University between August 2012 and August 2019. OS was the primary outcome. Lymph node metastasis and residual tumor were secondary outcomes. Logistic regression models and Kaplan-Meier curves were used for further analysis.
Forty-two patients were evaluated, including 35 (83.3%) males and 7(16.7%) females. The mean age was 62 (range, 32-82) years. Male sex [hazard ratio (HR) = 21.906, 95% confidence interval (CI): 3.762-229.250; P = 0.039), T1b invasion (HR = 3.965, 95%CI: 1.109-17.432; P = 0.047), undifferentiated tumor (HR = 9.455, 95%CI: 0.946-29.482; P = 0.049), lymph node metastasis (HR = 2.126, 95%CI: 0.002-13.266; P = 0.031), and residual tumor (HR = 4.275, 95%CI: 1.049-27.420; P = 0.043) were independently associated with OS. The follow-up duration was 4-81 mo (median: 50.7 mo). OS was 77.0 ± 12.1 mo (95%CI: 53.3-100.7 mo). The 3-year and 5-year OS rates were 94.1% and 85%, respectively.
Male sex, T1b invasion, undifferentiated tumor, lymph node metastasis, and residual tumor are independently associated with OS in patients with EGC who underwent additional surgery after ESD.
Core Tip: This was a retrospective study examining risk factors for overall survival after additional surgery in patients with early gastric cancer who initially underwent endoscopic submucosal dissection, especially the effects of lymph node metastasis and residual tumor. The results indicated that male sex, T1b invasion, undifferentiated tumor, lymph node metastasis, and residual tumor were independently associated with overall survival in patients with early gastric cancer who underwent additional surgery after endoscopic submucosal dissection.
- Citation: Zheng Z, Bu FD, Chen H, Yin J, Xu R, Cai J, Zhang J, Yao HW, Zhang ZT. Factors associated with overall survival in early gastric cancer patients who underwent additional surgery after endoscopic submucosal dissection. World J Clin Cases 2021; 9(10): 2192-2204
- URL: https://www.wjgnet.com/2307-8960/full/v9/i10/2192.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i10.2192
The incidence of gastric cancer (GC) is highest in Eastern Asia, Eastern Europe, and South America[1,2]. GC affects men more than women[1]. The direct cause of GC is unclear, but Helicobacter pylori infection and some hereditary cancer predisposition syndromes may play a role[1]. Patients often present with nonspecific symptoms, which may include anorexia, weight loss, abdominal pain, dyspepsia, vomiting, and early satiety[1,2]. With the improvement of public health awareness and the development of endoscopic and imaging technologies, the detection rate of early GC (EGC) among the overall GCs is increasing[3,4]. Statistics from the China Gastrointestinal Cancer Surgery Union show that EGC accounts for 19.5% of all GCs in China[5]. Therefore, the management of EGC warrants further study.
Current treatments for EGC include endoscopic mucosal resection/endoscopic submucosal dissection (EMR/ESD) and standard radical gastrectomy[6,7]. Standard radical gastrectomy can achieve a good radical effect; however, due to the low lymph node metastasis rate of EGC, most patients undergo unnecessary or excessive lymph node dissection, which increases the surgical trauma[6,7]. On the other hand, although EMR/ESD can completely remove the lesion, it cannot evaluate for possible metastasis in the gastric peripheral lymph node, which may lead to treatment failure[6,7]. With the refinement of ESD indications, an increasing number of patients with EGC who meet the expanded indications undergo ESD[8-11].
Nevertheless, whether patients not meeting the criteria for curative resection after ESD need further surgery remains largely controversial[12-15]. A large-scale non-inferiority study[16] found that endoscopic treatment can achieve the same results as surgery in the long run, but other studies have suggested that additional surgery is recommended in patients who underwent endoscopic non-curative resection[17,18].
Therefore, factors associated with prognosis upon further surgery following ESD should be urgently explored, which would provide predictive value for clinical decisions. In this setting, the present study aimed to mainly examine risk factors for overall survival (OS) upon additional surgery in patients with EGC who initially underwent ESD, especially the impacts of lymph node metastasis and residual tumor. The results could provide a reference for clinicians to better select treatments for EGC patients.
This was a retrospective analysis of patients with EGC who underwent additional surgery after ESD at the General Surgery Center of Beijing Friendship Hospital affiliated to Capital Medical University between August 2012 and August 2019. This study was approved by the Ethics Committee of Beijing Friendship Hospital, Capital Medical University (No. 2018-P2-015-02). Because this was a retrospective study only analyzing clinical data, informed consent was waived. All patient data were analyzed after anonymization.
The inclusion criteria were: (1) Diagnosis of EGC[7,19,20]; (2) Underwent additional radical surgery after ESD; and (3) Complete clinical data. The exclusion criteria were: (1) Gastric stump cancer, recurrent GC, multiple primary malignant tumors of the abdominopelvic cavity, or a history of other malignant tumors within 5 years; (2) Postoperative pathologically confirmed advanced GC (pT2N0-3M0-1); or (3) Uncontrollable internal medicine diseases, including unstable angina pectoris, myocardial infarction, or cerebrovascular accident, within 6 mo.
All ESD procedures were performed by experienced endoscopic surgeons, who had completed at least 100 ESD surgeries independently. The surgical equipment included an Olympus GIF-Q260J single-channel therapeutic endoscope, a dual knife, an IT-knife, a hook knife, and a flex knife. ESD resection mainly comprised of three steps. First, a dual knife or an IT-knife was used to generate a circular mark along the edge of the lesion at least 5 mm away. Fluid was injected into the submucosa to fully separate the tumor lesion from the muscularis propria. Second, a dual knife or an IT-knife was used to circularly cut the mucosa around the lesion. Finally, the tumor lesion and submucosal connective tissue were completely dissected from the circular incision, and hemostasis of the wound was performed. The detailed surgical procedures have been thoroughly described in previous research reports[21,22]. The collected specimens were fixed and examined by experienced pathologists.
The criteria for curative resection of ESD were: (1) Undifferentiated intramucosal carcinoma with no ulcer and a tumor size ≤ 2 cm; (2) Differentiated submucosal carcinoma T1b-SM1 with a tumor size ≤ 3 cm (submucosal invasion depth < 500 μm); (3) Ulcerative, differentiated intramucosal carcinoma with a tumor size < 3 cm, accompanied by undifferentiated components; (4) Differentiated intramucosal carcinoma with no ulcer and a tumor size > 2 cm; and (5) Differentiated intramucosal carcinoma with no ulcer and a tumor size < 2 cm[7,19]. After complete resection of the lesions, the horizontal and vertical resection margins were negative, with no blood vessels or lymphatic invasion. Otherwise, ESD was considered non-curative.
In patients with EGC whose pathology did not meet the criteria for curative resection after ESD, radical surgery for GC was additionally performed. The interval between the two surgeries depended on the patient's physical conditions. Radical distal, proximal, or total gastrectomy was selected based on tumor location. All patients underwent standard D1 or D2 lymph node dissection, according to the specific conditions of the tumor. The specific procedures and range of lymph node dissection were based on the Japanese Gastric Cancer Treatment Guidelines 2018 (5th edition)[7].
OS was the primary outcome of this study. OS was defined as the length of time from additional surgery to death from any cause. In patients undergoing non-curative resection, the treatment focused on two aspects: (1) Risk of lymph node metastasis; and (2) Risk of residual tumor (positive resection margin or local recurrence). Therefore, the above two aspects were considered secondary outcomes.
Clinical data were extracted from medical charts, including sex, age, number of tumor lesions, tumor location, general type, tumor size, histological type, Lauren classification, depth of invasion, pattern of invasion, horizontal resection margin, vertical resection margin, nerve invasion, vascular invasion, eCura score[23], degree of radical endoscopic treatment (eCura), ulcer, surgical methods, range of lymph node dissection, and cancerous nodules.
The patients were assigned to two age groups (< 60 years vs ≥ 60 years) according to the age bracket proposed by the World Health Organization. The general type included the protruded, flat, and pitting types according to the Japanese Gastric Cancer Treatment Guidelines 2018 (5th edition)[7]. Tumor size was determined as the largest tumor diameter. Histological types were divided into differentiated (including highly and moderately differentiated adenocarcinoma, tubular adenocarcinoma, and papillary adenocarcinoma) and undifferentiated (poorly differentiated adeno-carcinoma, signet-ring cell carcinoma, and mucinous adenocarcinoma) types. Based on the depth of invasion, the tumors were divided into intramucosal carcinoma (T1a) and submucosal carcinoma (T1b). Among them, submucosal carcinoma was further classified into three subtypes according to the depth of tumor invasion: T1b-SM1 (submucosal invasion depth < 500 μm), T1b-SM2 (depth of 500-1000 μm), and T1b-SM3 (depth of 1000-1500 μm). The invasion patterns included INFa (tumor lesion grew expansively, showing a clear boundary with the surrounding tissues), INFb (growing status of tumor lesion between INFa and INFc), and INFc (tumor lesion grew invasively, showing an unclear boundary with the surrounding tissues). The eCura score included tumor size (1 point), invasion depth (1 point), lymphatic invasion (3 points), venous invasion (1 point), and positive vertical margin (1 point)[23]. Based on eCura scores, the patients were divided into three groups: Low-risk (0-1 points), moderate-risk (2-4 points), and high-risk (5-7 points) groups. Based on the degree of radical endoscopic treatment (eCura), cases were assigned to the eCura-A (tumor completely resected, meeting the absolute indication for ESD), eCura-B (tumor completely resected for one time, meeting the relatively expanded indication for ESD resection), eCura-C1 (in differentiated carcinoma, other conditions of eCura A or B were met, but complete tumor resection or positive horizontal margin was not achieved), and eCura-C2 (none of the above conditions were met) groups.
The patients were followed every 6 mo after surgery. After discharge, follow-up was performed via outpatient visits, inpatient reexaminations, telephone calls, or mails. During the follow-up period, physical examination, laboratory tests (including routine blood tests, biochemical examinations, and gastrointestinal tumor markers), chest computed tomography (CT), abdominal and pelvic CT scans, and gastroscopy were performed annually. The follow-up period ended on December 31, 2019.
SPSS 21.0 (IBM Corp., Armonk, NY, United States) was used for all statistical analyses. Categorical data are presented as frequencies and percentages, and were compared by the chi-square test, the corrected chi-square test, or the Fisher's exact test. Ranked data are expressed by frequencies and percentages, and were compared by the rank-sum test. Continuous data were tested for normal distribution using the Kolmogorov-Smirnov test. Those with a normal distribution are presented as the mean ± SD, and were analyzed using the Student t-test; those with a skewed distribution are presented as medians (interquartile ranges) and were analyzed using the Mann-Whitney U-test. Univariable and multivariable analyses were performed by Cox regression to determine the independent risk factors for OS. The stepwise method was used for multivariable Cox regression analysis. Survival analysis was performed using the Kaplan-Meier method and the log-rank test. P < 0.05 was considered statistically significant.
A total of 51 patients with EGC were eligible for this study. Nine patients were excluded: One with gastric stump cancer, one with ascending colon cancer 2 years ago, six with postoperatively pathologically confirmed advanced GC, and one elderly male patient with a history of acute cerebral infarction 5 mo before enrollment. Finally, 42 patients with EGC were eventually included in the study (Table 1). Among them, there were 35 (83.3%) males and 7 (16.7%) females, indicating a male to female ratio of 5:1. The mean age was 62 (range, 32-82) years. The degree of radical endoscopic treatment was evaluated after ESD in all patients, including one with eCura A, one with eCura B, 11 with eCura C1, and 29 with eCura C2. All patients underwent standard radical gastrectomy or celiac lymph node dissection. Postoperative pathology revealed that there were 19 (45.2%) patients with tumor invasion to T1a, 6 (14.3%) to T1b-SM1, 15 (35.7%) to T1b-SM2, and 2 (4.8%) to T1b-SM3. There were 19 (45.2%) patients with highly differentiated adenocarcinoma, 8 (19.1%) with moderately differentiated adenocarcinoma, and 15 (35.7%) with poorly differentiated adenocarcinoma; 27 (64.3%) and 15 (35.7%) had differentiated and undifferentiated tumors, respectively. Five (11.9%) cases had vascular invasion and 37 (88.1%) had no vascular invasion. There were 4 (9.5%) patients with nerve invasion and 38 (90.5%) without nerve invasion.
| Clinicopathological factor | Total (n = 42) | Lymph node metastasis | Residual tumor | ||||
| No (n = 37) | Yes (n = 5) | P value | No (n = 29) | Yes (n = 13) | P value | ||
| Age (yr), n (%) | 0.926 | 0.513 | |||||
| < 60 | 16 (38.1) | 14 (37.8) | 2 (40.0) | 12 (41.4) | 4 (30.8) | ||
| ≥ 60 | 26 (61.9) | 23 (62.2) | 3 (60.0) | 17 (58.6) | 9 (69.2) | ||
| Sex (male), n (%) | 35 (83.3) | 34 (91.9) | 1 (20.0) | < 0.001 | 26 (89.7) | 9 (69.2) | 0.101 |
| Tumor location, n (%) | 0.316 | 0.360 | |||||
| Upper stomach | 12 (28.6) | 12 (32.4) | 0 (0) | 9 (31.0) | 3 (23.1) | ||
| Middle stomach | 17 (40.5) | 14 (37.9) | 3 (60.0) | 13 (44.8) | 4 (30.8) | ||
| Lower stomach | 13 (30.9) | 11 (29.7) | 2 (40.0) | 7 (24.2) | 6 (46.1) | ||
| Tumor size (cm), n (%) | 0.281 | 0.862 | |||||
| < 2 | 9 (21.4) | 7 (18.9) | 2 (40.0) | 6 (20.7) | 3 (23.1) | ||
| ≥ 2 | 33 (78.6) | 30 (81.1) | 3 (60.0) | 23 (79.3) | 10 (76.9) | ||
| Gross type, n (%) | 0.207 | 0.106 | |||||
| Protruded | 8 (19) | 8 (21.6) | 0 (0) | 6 (20.7) | 2 (15.4) | ||
| Flat | 7 (16.7) | 7 (18.9) | 0 (0) | 7 (24.1) | 0 (0) | ||
| Pitting | 27 (64.3) | 22 (59.5) | 5 (100.0) | 16 (55.2) | 11 (84.6) | ||
| Surgical method, n (%) | 0.322 | 0.152 | |||||
| Distal subtotal gastrectomy | 26 (61.9) | 21 (56.8) | 5 (100.0) | 15 (51.7) | 11 (84.6) | ||
| Proximal subtotal gastrectomy | 8 (19) | 8 (21.6) | 0 (0) | 6 (20.7) | 2 (15.4) | ||
| Total gastrectomy | 3 (7.2) | 3 (8.1) | 0 (0) | 3 (10.3) | 0 (0) | ||
| Celiac lymph node dissection | 5 (11.9) | 5 (13.5) | 0 (0) | 5 (17.3) | 0 (0) | ||
| Invasion depth, n (%) | 0.018 | 0.307 | |||||
| T1a | 19 (45.2) | 19 (51.4) | 0 (0) | 13 (44.8) | 6 (46.2) | ||
| T1b | 23 (54.8) | 18 (48.6) | 5 (100) | 16 (55.2) | 7 (53.8) | ||
| Histological type, n (%) | 0.028 | 0.654 | |||||
| Differentiated | 27 (64.3) | 26 (70.3) | 1 (20) | 18 (62.1) | 9 (69.2) | ||
| Undifferentiated | 15 (35.7) | 11 (29.7) | 4 (80) | 11 (37.9) | 4 (30.8) | ||
| Number of tumor lesions, n (%) | 0.710 | 0.498 | |||||
| Solitary | 41 (97.6) | 36 (97.3) | 5 (100.0) | 28 (96.6) | 13 (100.0) | ||
| Multiple | 1 (2.4) | 1 (2.7) | 0 (0) | 1 (3.4) | 0 (0) | ||
| Positive vascular invasion, n (%) | 5 (11.9) | 0 (0) | 5 (100.0) | < 0.001 | 0 (0) | 5 (38.5) | < 0.001 |
| Lauren classification, n (%) | 0.039 | 0.532 | |||||
| Intestinal type | 22 (52.4) | 21 (56.8) | 1 (20.0) | 15 (51.7) | 7 (53.8) | ||
| Diffuse | 13 (31.0) | 9 (24.3) | 4 (80.0) | 8 (27.6) | 5 (38.5) | ||
| Mixed | 7 (16.6) | 7 (18.9) | 0 (0) | 6 (20.7) | 1 (7.7) | ||
| Pattern of invasion, n (%) | < 0.001 | 0.001 | |||||
| IFN-A | 8 (19.0) | 8 (21.6) | 0 (0) | 6 (20.7) | 2 (15.4) | ||
| IFN-B | 26 (62.0) | 26 (70.3) | 0 (0) | 22 (75.9) | 4 (30.8) | ||
| IFN-C | 8 (19.0) | 3 (8.1) | 5 (100.0) | 1 (3.4) | 7 (53.8) | ||
| Positive horizontal resection margin, n (%) | 19 (45.2) | 18 (48.6) | 1 (20.0) | 0.227 | 10 (34.5) | 9 (69.2) | 0.036 |
| Positive vertical resection margin, n (%) | 21 (50.0) | 16 (43.2) | 5 (100.0) | 0.017 | 14 (48.3) | 7 (53.8) | 0.739 |
| Positive nerve invasion, n (%) | 4 (9.5) | 1 (2.7) | 3 (60.0) | < 0.001 | 0 (0) | 4 (30.8) | 0.002 |
| eCura score, n (%) | < 0.001 | 0.007 | |||||
| Low-risk group | 24 (57.1) | 24 (64.9) | 0 (0) | 18 (62.1) | 6 (46.2) | ||
| Moderate-risk group | 14 (33.3) | 13 (35.1) | 1 (20.0) | 11 (37.9) | 3 (23.1) | ||
| High-risk group | 4 (9.6) | 0 (0) | 4 (80.0) | 0 (0) | 4 (30.7) | ||
| Degree of radical endoscopic treatment (eCura), n (%) | 0.467 | 0.790 | |||||
| eCura-A | 1 (2.4) | 1 (2.7) | 0 (0) | 1 (3.5) | 0 (0) | ||
| eCura-B | 1 (2.4) | 1 (2.7) | 0 (0) | 1 (3.5) | 0 (0) | ||
| eCura-C1 | 11 (26.2) | 11 (29.7) | 0 (0) | 7 (24.1) | 4 (30.8) | ||
| eCura-C2 | 29 (69.0) | 24 (64.9) | 5 (100.0) | 20 (68.9) | 9 (69.2) | ||
| Ulcer, n (%) | 26 (61.9) | 22 (59.5) | 4 (80.0) | 0.375 | 16 (55.2) | 10 (76.9) | 0.180 |
| Range of lymph node dissection, n (%) | 0.409 | 0.101 | |||||
| D1 | 24 (57.1) | 22 (59.5) | 2 (40.0) | 19 (65.5) | 5 (38.5) | ||
| D2 | 18 (42.9) | 15 (40.5) | 3 (60.0) | 10 (34.5) | 8 (61.5) | ||
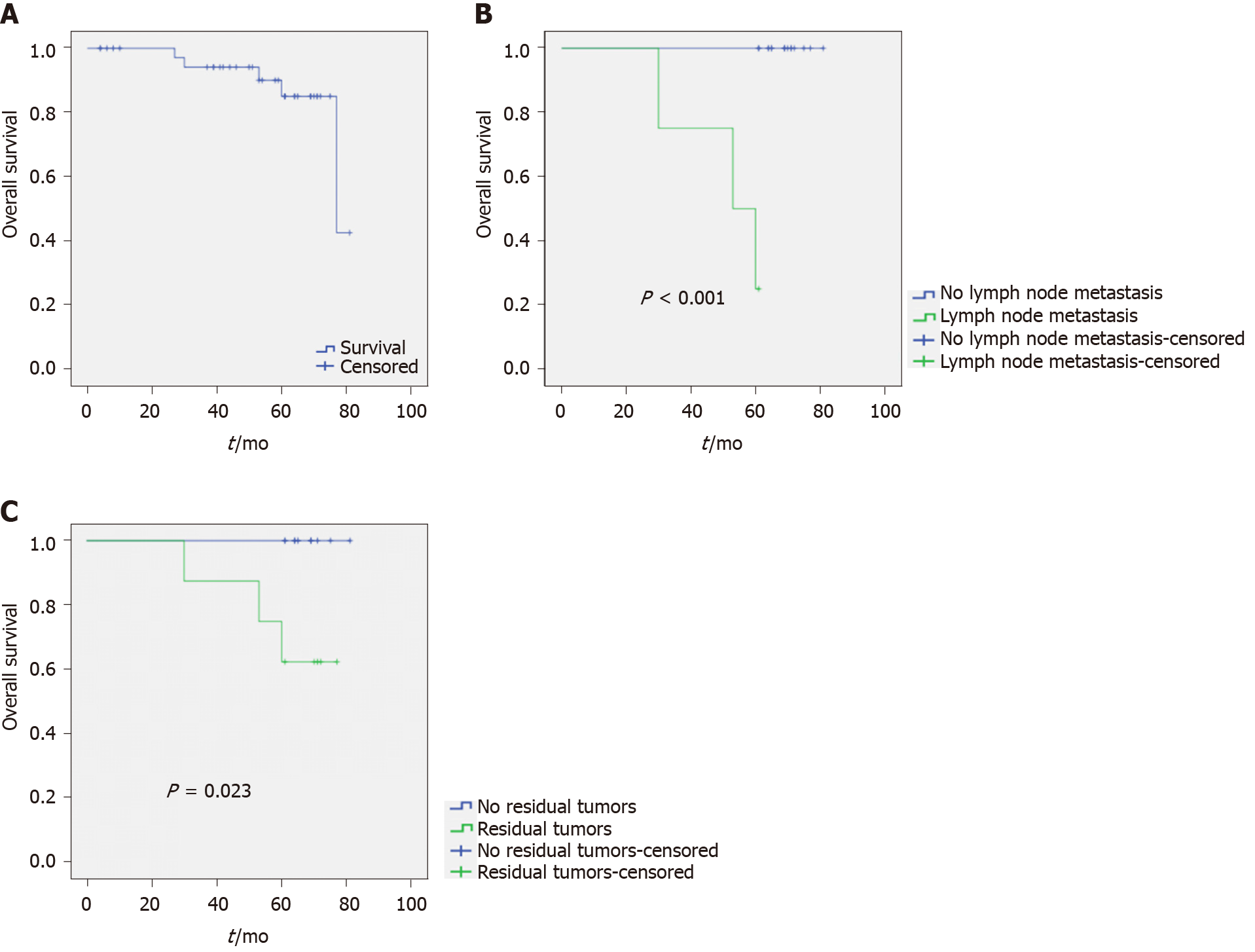
Among the 42 patients with EGC, the follow-up period was 4-81 mo, with a median duration of 50.7 mo. During follow-up, three (7.1%) patients were lost to follow-up. A total of five deaths occurred among all patients. One individual died of advanced age complicated with organ failure, with no tumor recurrence at death; the remaining four died of tumor recurrence or liver metastasis. OS was 77.0 ± 12.1 mo (95% confidence interval (CI): 53.3-100.7 mo). The 3-year and 5-year OS rates were 94.1% and 85%, respectively (Figure 1A).
The three patients that were lost to follow-up were not included in survival analysis; therefore, 39 patients were assessed for risk factors for OS. As shown by the multivariable analysis, male sex [hazard ratio (HR) = 21.906, 95%CI: 3.762-229.250; P = 0.039), T1b invasion (HR = 3.965, 95%CI: 1.109-17.432; P = 0.047), undifferentiated tumor (HR = 9.455, 95%CI: 0.946-29.482; P = 0.049), lymph node metastasis (HR = 2.126, 95%CI: 0.002-13.266; P = 0.031), and residual tumor (HR = 4.275, 95%CI: 1.049-27.420; P = 0.043) were independently associated with OS (Table 2).
| Characteristic | Univariable analysis | Multivariable analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age (yr) | ||||||
| < 60 | Reference | - | - | Reference | - | - |
| ≥ 60 | 1.152 | 0.119-11.174 | 0.902 | |||
| Sex (male) | 13.550 | 1.407-130.515 | 0.024 | 21.906 | 3.762-229.250 | 0.039 |
| Tumor location | ||||||
| Upper stomach | Reference | - | - | Reference | - | - |
| Middle stomach | 2.740 | 0.563-13.340 | 0.212 | |||
| Lower stomach | 2.124 | 0.417-13.101 | 0.197 | |||
| Tumor size (cm) | ||||||
| < 2 | Reference | - | - | Reference | - | - |
| ≥ 2 | 0.222 | 0.031-18.579 | 0.133 | |||
| Gross type | ||||||
| Protruded | Reference | - | - | Reference | - | - |
| Flat | 0.000 | 0.000 | 0.984 | |||
| Pitting | 1.440 | 0.149-27.959 | 0.753 | |||
| Invasion depth | ||||||
| T1a | Reference | - | - | Reference | - | - |
| T1b | 2.011 | 0.621-6.213 | 0.018 | 3.965 | 1.109-17.432 | 0.047 |
| Histological type | ||||||
| Differentiated | Reference | - | - | Reference | - | - |
| Undifferentiated | 13.155 | 0.991-25.014 | 0.028 | 9.455 | 0.946-29.482 | 0.049 |
| Number of tumor lesions | ||||||
| Solitary | Reference | - | - | Reference | - | - |
| Multiple | 0.912 | 0.301-9.761 | 0.781 | |||
| Positive vascular invasion | 0.818 | 0.169-8.965 | 0.803 | |||
| Lauren classification | ||||||
| Intestinal type | Reference | - | - | Reference | - | - |
| Diffuse | 1.683 | 0.518-15.468 | 0.386 | |||
| Mixed | 0.968 | 0.512-14.129 | 0.571 | |||
| Pattern of invasion | ||||||
| IFN-A | Reference | - | - | Reference | - | - |
| IFN-B | 0.545 | 0.080-33.730 | 0.537 | |||
| IFN-C | 9.899 | 1.159-19.526 | 0.036 | |||
| Positive horizontal resection margin | 0.989 | 0.164-45.987 | 0.990 | |||
| Positive vertical resection margin | 29.109 | 0.172-353.649 | 0.353 | |||
| Positive nerve invasion | 1.000 | 0.10-102.816 | > 0.999 | |||
| eCura score | ||||||
| Low-risk group | Reference | - | - | Reference | - | - |
| Moderate-risk group | 0.938 | 0.126-27.910 | 0.810 | |||
| High-risk group | 0.994 | 0.108-10.192 | 0.715 | |||
| Degree of radical endoscopic treatment (eCura) | ||||||
| eCura-A | Reference | - | - | Reference | - | - |
| eCura-B | 0.997 | 0.000 | > 0.999 | |||
| eCura-C1 | 0.812 | 0.101-15.171 | 0.969 | |||
| eCura-C2 | 1.745 | 0.257-11.846 | 0.569 | |||
| Ulcer | 0.829 | 0.134-13.122 | 0.840 | |||
| Range of lymph node dissection | ||||||
| D1 | Reference | - | - | Reference | - | - |
| D2 | 4.066 | 0.421-39.278 | 0.225 | |||
| Lymph node metastasis | 1.813 | 0.390-3.871 | 0.003 | 2.126 | 0.002-13.266 | 0.031 |
| Residual tumor | 5.160 | 2.391-9.107 | 0.021 | 4.275 | 1.049-27.420 | 0.043 |
The OS of patients with lymph node metastasis was 69.0 ± 2.7 mo (95%CI: 63.7-74.3), which was statistically different from that of patients without lymph node metastasis (P < 0.001) (Figure 1B). A total of 34 patients were followed for 3 years. The 3-year OS rate of those with lymph node metastasis was 60%, while individuals without lymph node metastasis all survived (OS rate of 100%). A total of 20 patients were followed for 5 years. The 5-year OS rate of patients without lymph node metastasis was significantly higher than that of patients with lymph node metastasis (100% vs 25%).
The OS of patients with residual tumor did not reach the median survival time, but there was a statistically significant difference in the survival vs those without residual tumor (P = 0.023) (Figure 1C). A total of 34 patients were followed for 3 years. The 3-year OS rate of patients with residual tumor was 80%, and that of patients without residual tumor was 100%. A total of 20 patients were followed for 5 years. The 5-year OS rate of patients without residual tumor was significantly higher than that of patients with residual tumor.
At present, for EGC patients who underwent non-curative ESD, surgeons need to comprehensively assess the risks of lymph node metastasis and residual tumor to determine the potential benefits of additional surgery[24] in order to keep surgical trauma as low as possible while maximizing prognosis. Achieving the best balance between radical surgery for EGC and surgical trauma remains an unresolved issue. This study aimed to examine the impacts of lymph node metastasis and residual tumor on long-term prognosis, as well as risk factors for OS after additional surgery in patients with EGC who initially underwent ESD. The results indicated that male sex, T1b invasion, undifferentiated tumor, lymph node metastasis, and residual tumor were independently associated with OS in patients with EGC who underwent additional surgery after ESD.
Lymph node metastasis is one of the most important factors affecting the prognosis of patients with cancer in general, including EGC, and has a direct impact on the selection of the treatment method[6,7]. Due to the low positive lymph node rate in EGC, many patients undergo unnecessary lymph node dissection or radical surgery, resulting in increased morbidity. Therefore, in order to effectively evaluate the risk of lymph node metastasis in patients who underwent non-curative ESD, multiple studies performed in Japan, South Korea, and Western countries reported rates of lymph node metastasis in EGC patients undergoing additional surgery after ESD of 4.3%-12.7%[25-28]. In the present study, lymph node metastasis occurred in five of the 42 patients with EGC, reflecting a metastasis rate of 11.9%, corroborating previous studies. Ryu et al[29] showed that in 1076 patients with EGC, the rate of lymph node metastasis was lower in male patients than in females, i.e., 7.8% vs 12.9%. In the latter report, multivariable analysis revealed that in female patients, invasion depth and vascular invasion were independent risk factors for lymph node metastasis. Compared with male patients, females were prone to developing lymph node metastasis, which may be due to the differences of molecular biological characteristics of EGC in different genders. Second, it may be related to differences in the hormonal regulatory system in vivo, but the specific mechanism needs further investigation. Meanwhile, large-scale studies of EGC patients with tumor invasion to T1b-SM2 showed that undifferentiated tumor is an independent prognostic factor of lymph node metastasis in case of tumor invasion into the submucosa[30,31]. Previous findings also suggested that vascular invasion, nerve invasion, tumor size, positive margin, and general type are high-risk factors for lymph node metastasis in EGC[15,23,25,32-34]. In the present study, the number of patients with lymph node metastasis was too small to determine the exact factors associated. However, lymph node metastasis was independently associated with OS in patients with EGC in this study. In addition, male sex, deeper tumor invasion, and undifferentiated histological type were all independent risk factors for OS.
Nevertheless, in patients undergoing non-curative resection, the rate of lymph node metastasis was not high; in such cases, a simple follow-up could be considered instead of additional surgery. Indeed, additional surgery after ESD increases the risk of surgical complications and morbidity, and there is also the possibility of no residual cancer in pathological tissue specimens after radical surgery[35]. Therefore, developing an accurate model for assessing lymph node metastasis and tumor recurrence to reduce unnecessary surgery remains a hot topic. Hatta et al[23] evaluated 1101 EGC patients who underwent additional surgery after ESD and proposed the “eCura scoring system”, demonstrating lymph node metastasis rates in the low-risk, moderate-risk, and high-risk groups of 2.5%, 6.7%, 22.7%, respectively. In a subsequent multicenter study[36], authors found that in high-risk group patients according to the “eCura scoring system”, the simple observation group had a higher tumor recurrence rate compared with the additional surgery group (HR = 3.13, P = 0.024). In the present study, the 3- and 5-year OS rates in patients without lymph node metastasis were significantly higher than those of patients with lymph node metastasis, partly confirming that lymph node metastasis affects the long-term prognosis of patients with EGC. Another study by Hatta et al[37] showed that in patients with EGC and lymph node metastasis or vascular invasion, even if additional radical surgery is performed after ESD, there is still a high risk of recurrence, and adjuvant chemotherapy might be considered. Therefore, in patients with EGC and lymph node metastasis, adjuvant chemotherapy should be performed at the same time according to each patient's specific conditions to improve the prognosis while performing additional radical surgery.
In addition, the depth of invasion was independently associated with OS. Deeper tumors have a higher likelihood of leaving cancer cells in place and subsequently increase the risk of residual tumor, adversely affecting prognosis. Sekiguchi et al[38] conducted a postoperative follow-up of 77 patients after ESD with a positive horizontal resection margin, and found that 11.9% had tumor recurrence after surgery, with a 5-year OS rate of 94.2%. Suzuki et al[26] reported that in patients who underwent non-curative resection after ESD, the 5-year OS rates in the additional surgery and the observation groups were 91.0% and 75.5%, respectively (P < 0.0001), and the disease-specific survival rates were 99% and 96.8%, respectively (P = 0.01). Yano et al[20] found that the prognosis of patients with additional surgery after non-curative ESD was significantly better than that of patients without, and the 5-year OS rates were 96% and 73.3%, respectively. In this study, the 3- and 5-year OS rates of patients with residual tumor were 80% and 62.5%, respectively, which were significantly lower than those of cases without residual tumor (P < 0.05). Therefore, in patients with potential residual tumor or non-curative resection, additional surgery could benefit survival to a certain extent. In addition, consistent with the above results, an undifferentiated tumor is also associated with OS[8,12,14,18,39].
This study had some limitations, mainly as a retrospective, single-center study. Although the proportion of EGC among all GCs has increased in recent years in China compared with Japan, South Korea, and other countries, the detection rate of EGC in the Chinese population remains low, leading to a small sample size for patients undergoing additional radical surgery after ESD. Thus, biases may be present in the analysis of clinical data. Consequently, large-sample, multicenter clinical studies are still needed for confirmation.
In conclusion, male sex, T1b invasion, undifferentiated tumor, lymph node metastasis, and residual tumor were independently associated with OS in patients with EGC who underwent additional surgery after ESD. The long-term survival rate of patients with EGC but without lymph node metastasis and no residual tumor is significantly higher than that of those with lymph node metastasis and residual tumor. Therefore, in some patients with EGC, ESD could be considered a safe and effective treatment. Nevertheless, additional radical surgery must be considered on a case-by-case basis in order to maximize the radical resection of the tumor and improve long-term prognosis.
Controversy exists about the benefit of additional surgery after endoscopic submucosal dissection (ESD) for early gastric cancer (EGC).
Whether patients who do not meet the criteria for curative resection after ESD need further surgery remains largely controversial. Therefore, factors associated with patient prognosis should be identified, which would provide predictive tools for clinical decisions.
This study aimed to assess the risk factors for overall survival (OS) upon additional surgery in patients with EGC who initially underwent ESD, especially the impacts of lymph node metastasis and residual tumor.
Patients were retrospectively assessed, evaluating OS as the primary outcome, and lymph node metastasis and residual tumor as secondary outcomes. Logistic regression models and Kaplan-Meier curves were used for further analysis.
Male sex, T1b invasion, undifferentiated tumor, lymph node metastasis, and residual tumor were independently associated with OS. In the 4-81-mo follow-up period, OS was 77.0 ± 12.1 mo, and the 3-year and 5-year OS rates were 94.1% and 85%, respectively.
Male sex, T1b invasion, undifferentiated tumor, lymph node metastasis, and residual tumor are independently associated with OS in patients with EGC undergoing additional surgery after ESD.
Compared with surgery, ESD could be a safe and effective treatment for some EGC patients to some extent. Nevertheless, additional radical surgery must be considered on a case-by-case basis in order to maximize the radical resection of the tumor and improve long-term prognosis.
We thank the Clinical Data and Biobank Resource of Beijing Friendship Hospital, Capital Medical University, for their help in data processing.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zambrano ÁR S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1282] [Cited by in RCA: 1468] [Article Influence: 163.1] [Reference Citation Analysis (0)] |
| 2. | Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v38-v49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1118] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 3. | Anderson WF, Camargo MC, Fraumeni JF Jr, Correa P, Rosenberg PS, Rabkin CS. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA. 2010;303:1723-1728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 354] [Cited by in RCA: 356] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 4. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55826] [Article Influence: 7975.1] [Reference Citation Analysis (132)] |
| 5. | Wang Y, Li Z, Shan F, Miao R, Xue K, Li Z, Gao C, Chen N, Gao X, Li S, Ji J. [Current status of diagnosis and treatment of early gastric cancer in China--Data from China Gastrointestinal Cancer Surgery Union]. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21:168-174. [PubMed] |
| 6. | NCCN E. NCCN Clinical Practice Guidelines in Oncology. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2007: 1. Available from: http://www.nccn.org/professionals/physican_gls/default.aspx. |
| 7. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1337] [Article Influence: 334.3] [Reference Citation Analysis (2)] |
| 8. | Bang CS, Park JM, Baik GH, Park JJ, Joo MK, Jang JY, Jeon SW, Choi SC, Sung JK, Cho KB. Therapeutic Outcomes of Endoscopic Resection of Early Gastric Cancer with Undifferentiated-Type Histology: A Korean ESD Registry Database Analysis. Clin Endosc. 2017;50:569-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Nishizawa T, Yahagi N. Long-Term Outcomes of Using Endoscopic Submucosal Dissection to Treat Early Gastric Cancer. Gut Liver. 2018;12:119-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 10. | Tanabe S, Ishido K, Matsumoto T, Kosaka T, Oda I, Suzuki H, Fujisaki J, Ono H, Kawata N, Oyama T, Takahashi A, Doyama H, Kobayashi M, Uedo N, Hamada K, Toyonaga T, Kawara F, Tanaka S, Yoshifuku Y. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a multicenter collaborative study. Gastric Cancer. 2017;20:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 11. | Rong L, Cai Y, Nian W, Wang X, Liang J, He Y, Zhang J. [Efficacy comparison between surgical resection and endoscopic submucosal dissection of early gastric cancer in a domestic single center]. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21:190-195. [PubMed] |
| 12. | Zhu ZL, Shi HP, Beeharry MK, Feng TN, Yan M, Yuan F, Zheng-Gang Zhu, Zhang BY, Wu W. Expanding the indication of endoscopic submucosal dissection for undifferentiated early gastric cancer is safe or not? Asian J Surg. 2020;43:526-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Hatta W, Gotoda T, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, Hoteya S, Nakamura K, Hirano M, Esaki M, Matsuda M, Ohnita K, Shimoda R, Yoshida M, Dohi O, Takada J, Tanaka K, Yamada S, Tsuji T, Ito H, Hayashi Y, Nakamura T, Shimosegawa T. Is radical surgery necessary in all patients who do not meet the curative criteria for endoscopic submucosal dissection in early gastric cancer? J Gastroenterol. 2017;52:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 14. | Kim HW, Kim JH, Park JC, Jeon MY, Lee YC, Lee SK, Shin SK, Chung HS, Noh SH, Kim JW, Choi SH, Park JJ, Youn YH, Park H. Additive endoscopic resection may be sufficient for patients with a positive lateral margin after endoscopic resection of early gastric cancer. Gastrointest Endosc. 2017;86:849-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Akaike H, Kawaguchi Y, Shiraishi K, Shimizu H, Furuya S, Hosomura N, Amemiya H, Kawaida H, Sudoh M, Inoue S, Kohno H, Ichikawa D. Validity of additional surgical resection by comparing the operative risk with the stratified lymph node metastatic risk in patients with early gastric cancer after endoscopic submucosal dissection. World J Surg Oncol. 2019;17:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Pyo JH, Lee H, Min BH, Lee JH, Choi MG, Lee JH, Sohn TS, Bae JM, Kim KM, Ahn JH, Carriere KC, Kim JJ, Kim S. Long-Term Outcome of Endoscopic Resection vs. Surgery for Early Gastric Cancer: A Non-inferiority-Matched Cohort Study. Am J Gastroenterol. 2016;111:240-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 156] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 17. | Jung DH, Lee YC, Kim JH, Lee SK, Shin SK, Park JC, Chung H, Park JJ, Youn YH, Park H. Additive treatment improves survival in elderly patients after non-curative endoscopic resection for early gastric cancer. Surg Endosc. 2017;31:1376-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Lee SH, Kim MC, Jeon SW, Lee KN, Park JJ, Hong SJ. Risk Factors and Clinical Outcomes of Non-Curative Resection in Patients with Early Gastric Cancer Treated with Endoscopic Submucosal Dissection: A Retrospective Multicenter Study in Korea. Clin Endosc. 2020;53:196-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Hasuike N, Ono H, Boku N, Mizusawa J, Takizawa K, Fukuda H, Oda I, Doyama H, Kaneko K, Hori S, Iishi H, Kurokawa Y, Muto M; Gastrointestinal Endoscopy Group of Japan Clinical Oncology Group (JCOG-GIESG). A non-randomized confirmatory trial of an expanded indication for endoscopic submucosal dissection for intestinal-type gastric cancer (cT1a): the Japan Clinical Oncology Group study (JCOG0607). Gastric Cancer. 2018;21:114-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 20. | Yano T, Ishido K, Tanabe S, Wada T, Azuma M, Kawanishi N, Yamane S, Watanabe A, Katada C, Koizumi W. Long-term outcomes of patients with early gastric cancer found to have lesions for which endoscopic treatment is not indicated on histopathological evaluation after endoscopic submucosal dissection. Surg Endosc. 2018;32:1314-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Conlin A, Kaltenbach T, Kusano C, Matsuda T, Oda I, Gotoda T. Endoscopic resection of gastrointestinal lesions: advancement in the application of endoscopic submucosal dissection. J Gastroenterol Hepatol. 2010;25:1348-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 507] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 23. | Hatta W, Gotoda T, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, Hoteya S, Nakagawa M, Hirano M, Esaki M, Matsuda M, Ohnita K, Yamanouchi K, Yoshida M, Dohi O, Takada J, Tanaka K, Yamada S, Tsuji T, Ito H, Hayashi Y, Nakaya N, Nakamura T, Shimosegawa T. A Scoring System to Stratify Curability after Endoscopic Submucosal Dissection for Early Gastric Cancer: "eCura system". Am J Gastroenterol. 2017;112:874-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 222] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 24. | Ono H, Yao K, Fujishiro M, Oda I, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Ichinose M, Matsui T. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc. 2016;28:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 406] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 25. | Jung DH, Huh CW, Kim JH, Hong JH, Park JC, Lee YC, Youn YH, Park H, Choi SH, Noh SH. Risk-Stratification Model Based on Lymph Node Metastasis After Noncurative Endoscopic Resection for Early Gastric Cancer. Ann Surg Oncol. 2017;24:1643-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Suzuki H, Oda I, Abe S, Sekiguchi M, Nonaka S, Yoshinaga S, Saito Y, Fukagawa T, Katai H. Clinical outcomes of early gastric cancer patients after noncurative endoscopic submucosal dissection in a large consecutive patient series. Gastric Cancer. 2017;20:679-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 27. | Kim ER, Lee H, Min BH, Lee JH, Rhee PL, Kim JJ, Kim KM, Kim S. Effect of rescue surgery after non-curative endoscopic resection of early gastric cancer. Br J Surg. 2015;102:1394-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 28. | Probst A, Schneider A, Schaller T, Anthuber M, Ebigbo A, Messmann H. Endoscopic submucosal dissection for early gastric cancer: are expanded resection criteria safe for Western patients? Endoscopy. 2017;49:855-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 29. | Ryu ES, Chang SJ, An J, Yang JY, Chung JW, Kim YJ, Kim KO, Park DK, Kwon KA, Nam S, Lee WK, Kim JH. Sex-specific differences in risk factors of lymph node metastasis in patients with early gastric cancer. PLoS One. 2019;14:e0224019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Liang XQ, Wang Z, Li HT, Ma G, Yu WW, Zhou HC, Liu HB. Indication for endoscopic treatment based on the risk of lymph node metastasis in patients with undifferentiated early gastric cancer. Asian J Surg. 2020;43:973-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Miyahara K, Hatta W, Nakagawa M, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, Hoteya S, Hirano M, Esaki M, Matsuda M, Ohnita K, Shimoda R, Yoshida M, Dohi O, Takada J, Tanaka K, Yamada S, Tsuji T, Ito H, Aoyagi H, Shimosegawa T. The Role of an Undifferentiated Component in Submucosal Invasion and Submucosal Invasion Depth After Endoscopic Submucosal Dissection for Early Gastric Cancer. Digestion. 2018;98:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Zhao B, Zhang J, Zhang J, Luo R, Wang Z, Xu H, Huang B. Risk Factors Associated with Lymph Node Metastasis for Early Gastric Cancer Patients Who Underwent Non-curative Endoscopic Resection: a Systematic Review and Meta-analysis. J Gastrointest Surg. 2019;23:1318-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Du MZ, Gan WJ, Yu J, Liu W, Zhan SH, Huang S, Huang RP, Guo LC, Huang Q. Risk factors of lymph node metastasis in 734 early gastric carcinoma radical resections in a Chinese population. J Dig Dis. 2018;19:586-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Sunagawa H, Kinoshita T, Kaito A, Shibasaki H, Kaneko K, Ochiai A, Ohtsu A, Nishida T. Additional surgery for non-curative resection after endoscopic submucosal dissection for gastric cancer: a retrospective analysis of 200 cases. Surg Today. 2017;47:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Nakata B, Tendo M, Okuyama M, Nakahara K, Ishizu H, Masuda G, Lee T, Hori T, Ohsawa M, Sato H, Ishikawa T. Additional surgical resection after endoscopic mucosal dissection for early gastric cancer: A medium-sized hospital's experience. Int J Surg. 2016;36:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Hatta W, Gotoda T, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, Hoteya S, Nakagawa M, Hirano M, Esaki M, Matsuda M, Ohnita K, Yamanouchi K, Yoshida M, Dohi O, Takada J, Tanaka K, Yamada S, Tsuji T, Ito H, Hayashi Y, Nakamura T, Nakaya N, Shimosegawa T. Is the eCura system useful for selecting patients who require radical surgery after noncurative endoscopic submucosal dissection for early gastric cancer? Gastric Cancer. 2018;21:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 37. | Hatta W, Gotoda T, Oyama T, Kawata N, Takahashi A, Oka S, Hoteya S, Nakagawa M, Hirano M, Esaki M, Matsuda M, Ohnita K, Shimoda R, Yoshida M, Dohi O, Takada J, Tanaka K, Yamada S, Tsuji T, Ito H, Aoyagi H, Nakamura T, Nakaya N, Shimosegawa T, Masamune A. Is Additional Surgery Always Sufficient for Preventing Recurrence After Endoscopic Submucosal Dissection with Curability C-2 for Early Gastric Cancer? Ann Surg Oncol. 2019;26:3636-3643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Sekiguchi M, Suzuki H, Oda I, Abe S, Nonaka S, Yoshinaga S, Taniguchi H, Sekine S, Kushima R, Saito Y. Risk of recurrent gastric cancer after endoscopic resection with a positive lateral margin. Endoscopy. 2014;46:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Li D, Luan H, Wang S, Zhou Y. Survival benefits of additional surgery after non-curative endoscopic resection in patients with early gastric cancer: a meta-analysis. Surg Endosc. 2019;33:711-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |