Published online Apr 6, 2021. doi: 10.12998/wjcc.v9.i10.2160
Peer-review started: October 27, 2020
First decision: December 27, 2020
Revised: January 10, 2021
Accepted: February 4, 2021
Article in press: February 4, 2021
Published online: April 6, 2021
Processing time: 154 Days and 7.1 Hours
Intra-abdominal infections can be classified into uncomplicated or complicated (peritonitis). Peritonitis is divided into primary, secondary, and tertiary. Tertiary peritonitis is the less common but the most severe among peritonitis stratifications, being defined as a recurrent intra-abdominal infection that occurs 48 h after a well-succeeded control of a secondary peritonitis. This disease has a complex pathogenesis that is closely related to the capacity of the peritoneal cavity to activate immunological processes. Patients who progress to persistent peritonitis are at an increased risk of developing several infectious complications such as sepsis and multiple organ failure syndrome. Moreover, tertiary peritonitis remains an important cause of hospital death mainly among patients with associated risk factors. The microbiological profile of organisms causing tertiary peritonitis is often different from that observed in other types of peritonitis. In addition, there is a high prevalence of multidrug-resistant pathogens causing this condition, and an appropriate and successful clinical management depends on an early diagnosis, which can be made easier with the use of clinical scores presenting a good prediction value during the intensive care unit admission. Complementarily, immediate therapy should be performed to control the infectious focus and to prevent new recurrences. In this sense, the treatment is based on initial antimicrobial therapy and well-performed peritoneal drainage.
Core Tip: Tertiary peritonitis is a major cause of death among surgical patients. However, there is a lack of recent studies compiling the most important data on this issue. In this sense, this review provides a broad overview on that condition, from pathogenesis to treatment, compiling the most updated information on this issue.
- Citation: Marques HS, Araújo GRL, da Silva FAF, de Brito BB, Versiani PVD, Caires JS, Milet TC, de Melo FF. Tertiary peritonitis: A disease that should not be ignored. World J Clin Cases 2021; 9(10): 2160-2169
- URL: https://www.wjgnet.com/2307-8960/full/v9/i10/2160.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i10.2160
Peritonitis is the major cause of severe sepsis in surgical intensive care units (ICUs) worldwide, especially when it comes to its most serious forms[1]. Although remarkable advances in the management of the peritoneal cavity have been achieved as a consequence of greater availability of diagnostic and therapeutic resources, tertiary peritonitis (TP) remains an important cause of death among inpatients, being associated with mortality rates ranging from 30% to 60%[2].
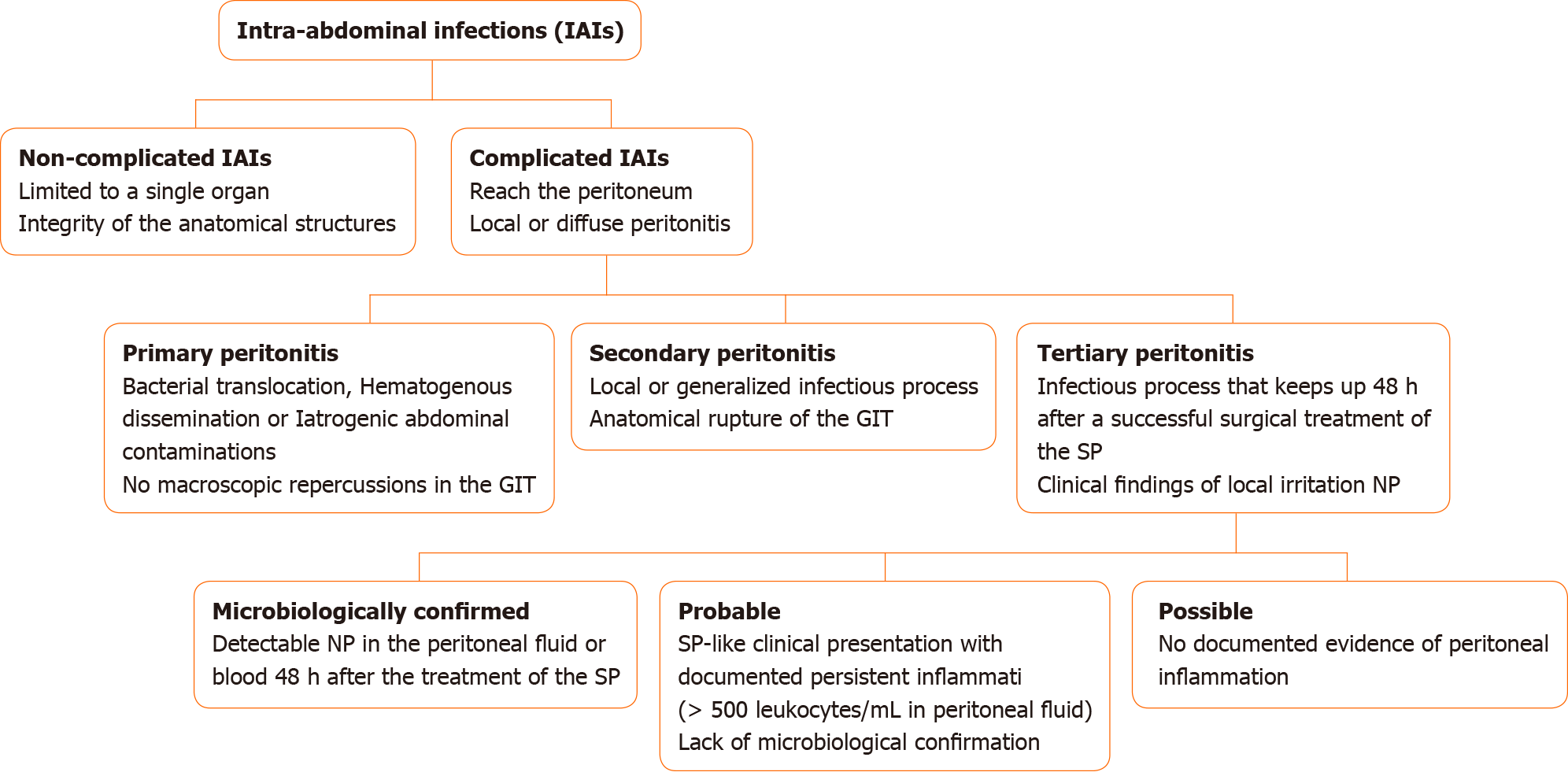
Intra-abdominal infections (IAIs) are commonly classified into non-complicated and complicated IAIs on the basis of the extent of the infection site. Whereas in non-complicated IAIs the infection is limited to a single organ and the integrity of the anatomical structures is maintained, complicated IAIs reach the peritoneum, leading to a local or diffuse peritonitis[3]. The peritonitis, in its turn, can be classified into primary, secondary, or tertiary. Among the causes of primary peritonitis stand out the bacterial translocation, hematogenous dissemination, or iatrogenic abdominal contaminations that are not accompanied by macroscopic repercussions in the gastrointestinal tract (GIT)[4]. The secondary peritonitis (SP) occurs when the infectious process extends in a localized or generalized manner, accompanied by anatomical GIT rupture, resulting from traumatic, surgical, or ischemic processes[5]. Lastly, a peritonitis that keeps up 48 h after successful surgical treatment of an SP is classified as a TP, which initially manifests as local irritation and has nosocomial pathogens as causal agents[4].
Various pathogens are associated with the onset of the TP. The inflammation is favored by the peritoneum capacity to allow the activation, differentiation, and proliferation of immune cells, which leads to complex immune responses against invading microorganisms[6]. Because TP is a life-threatening condition, an accurate and rapid diagnosis is crucial for the appropriate management of the disease[7]. Such diagnosis is made on the basis of clinical manifestations related to the peritoneal and systemic inflammation and can be supported by laboratory findings, imaging, and some score systems including Mannheim Peritonitis Index (MPI), Acute Physiology And Chronic Health Evaluation II (APACHE II), and Simplified Acute Physiology Score (SAPS)[7-11]. The management of affected individuals is made through measures aimed at infection control, which range from antimicrobial therapy to percutaneous drainage or open surgical intervention[12,13].
This review aims to provide an updated overview on TP, from its pathophysiology to its management, as well as to discuss the complications, prognosis, and risk factors associated with this condition.
The peritoneum is essential to provide an immunocompetent cavity system that is able to promote the activation, differentiation, proliferation, and recruitment of components of the immune system[14]. Nonetheless, certain clinical scenarios predispose the colonization of that environment by some microorganisms. In that context, the main pathogens related to the TP are enterococci (15%-35%), coagulase-positive staphylococci (5%-30%), Candida spp (10%-30%), and Escherichia coli (10%-15%), whereas the prevalence of Pseudomonas spp, Enterobacter spp, Bacteroides spp, Klebsiella spp, and Staphylococcus aureus are often lower than 10%[6]. Infections or surgical traumas to the peritoneum trigger an immune response with an initial predominance of monocytes, which produce pro-inflammatory cytokines such as tumor necrosis factor alpha, interleukin (IL)-1β, and interferon-γ aiming to recruit other cells in order to establish a robust immune response. In this process, anti-inflammatory cytokines including IL-4, IL-10, IL-1RA, and IL-13 are also released to control the level of inflammation and protect the host against an exacerbated inflammatory response[15]. These cytokines tend to inhibit the action of monocytes, including their ability to present antigens by reducing the expression of human leukocyte antigen-DR isotype. If there is a decrease of more than 30% in the human leukocyte antigen-DR isotype expression by monocytes, then an “immunological paralysis” can be found, a phenomenon that is believed to be the basis for the development of the TP[16]. In addition, neutrophils seem to affect the microbicidal function of other immune cells, hindering the occurrence of an effective type 1 T helper response and, therefore, playing a role in the above-mentioned immunological phenomenon[17].
The distinct innervations of the parietal and visceral layers of the peritoneum determine the manifestations in patients with peritonitis. Parietal peritonitis manifests as a somatic, acute, constant, local pain and potentially affects abdominal wall muscles. As a result, patients tend to present with abdominal stiffness as a protective reaction. On the other hand, inflammation in the visceral peritoneum often leads to a referred, colicky pain in the medial aspect of the abdomen[8]. Along with the clinical picture of acute abdomen, the TP is commonly accompanied by elevation in body temperature as well as imaging and laboratory findings, being associated with progressive multiple organ failure and prolonged systemic inflammation[18,19].
The first step in the approach of patients with clinical manifestations compatible with peritonitis should be the characterization of the clinical severity through appropriate score systems. Moreover, the hospital service should be prepared for a potential immediate intervention[19]. Relaparotomy is the most commonly used method to diagnose and treat the TP. This procedure may be necessary more than once after the initial surgery and it is considered as a late diagnostic method. Therefore, the identification of clinical features associated with an increased risk of developing TP may lead to an earlier diagnosis[20]. Clinical scoring systems are frequently mentioned in studies as important tools used in the ICU admission, being useful in the evaluation of the severity and clinical outcomes of the TP[21]. The MPI is a scoring tool based on clinical parameters that aims to predict mortality and risk of post-surgical complications in patients with peritonitis[9]. It takes into consideration specific factors of the disease such as the characteristics of the peritoneal fluid (clear, purulent, or fecal) and the degree of the peritonitis[22]. That index used to be applied only in SP, but it also proved to be effective in the prediction of the development of TP[7]. In addition, a study found that scores equal to or less than 32 for that index are associated with a low risk of death, whereas scores greater than 32 are associated with a high risk for this outcome[9]. Other disease-independent scores such as the APACHE II and SAPS II can be used in order to stratify patients based on the risk of death and disease severity. They are obtained from physiological parameters and may help in the decision making in the management of each patient in addition to serving as parameters for the effectiveness of the treatment provided[10,11]. The APACHE II is one of the most well-known and used systems in ICUs, and it is based on 12 physiological variables such as blood pressure, heart rate, temperature, age, and biochemical parameters, predicting mortality and multiple organ failure. A study evaluating this system found that a score equal to or greater than 15 is associated with higher mortality rates than scores below that cutoff[23]. A study including 122 patients demonstrated that individuals who progressed to TP had mean values of APACHE II (12.0 ± 6.3 vs 15.7 ± 4.8, P < 0.001) and MPI (25.2 ± 8.8 vs 28.7 ± 8.0, P = 0.035) scores significantly higher than those who had only SP. Moreover, that study showed higher effectiveness associated with the combined use of these score systems compared to the use of MPI alone (P < 0.001)[21]. The SAPS II is a tool that assists in predicting mortality from 17 variables that are collected in ICU admission, being a prognostic method for TP[24,25]. A study conducted by Weiss et al[26] that included 356 IAI patients demonstrated that the medians of MPI (25.9 vs 31.4, P < 0.001), APACHE II (12.4 vs 20.7, P < 0.001), and SAPS II (31.9 vs 45.6, P < 0.001) were significantly higher in TP patients compared with SP individuals[26].
The serum level of C-reactive protein is another parameter commonly used in that context[27]. Such protein was shown to be increased in the serum of post-surgical patients, but it is not specific for abdominal infections and can be just a consequence of other repercussions from the initial surgery[28]. A study that included 69 patients within the first days after a surgery to treat SP observed significantly higher values of C-reactive protein (265 mg/dL vs 217 mg/dL, P < 0.05) as well as of SAPS II (45.1 vs 28.4; P < 0.005) and MPI (28.6 vs 19.8; P < 0,001) among individuals who progressed to TP compared to those with SP[7].
Clinical suspicion of peritonitis can be elucidated with complementary data from imaging exams. Simple radiographic examination is not very effective in the identification of recurrent peritonitis due to the insignificant presence of abdominal air during the post-surgical period. In contrast, ultrasound examination and computerized tomography are often used and have satisfactory performances in the diagnosis/screening of intra-abdominal acute infections, with the latter having a higher potential for this purpose[2]. Both methods aid in the detection and drainage of abdominal fluid collections[29].
Complementarily, laboratory parameters and microbiological monitoring play pivotal roles in the diagnosis of the TP. Routine microbiological screening is able to identify rapidly infections by nosocomial pathogens and serve as a basis for the search for an infectious focus and for the appropriate antimicrobial choice[30]. Otherwise, laboratory investigation in this setting is made on the basis of the evaluation of leukocytes and procalcitonin, besides the aforementioned C-reactive protein. These parameters can be altered as a consequence of the inflammatory response due to the initial surgery as well; however, considerably high and rising values may indicate a recurrent infection[31]. In the aforementioned study by Weiss et al[1], these parameters were also evaluated, and procalcitonin and temperature elevations showed to be the most sensitive clinical/laboratory parameters to diagnose that condition (sensitivity = 64.5% and 63.4%, respectively). Although these parameters can increase the accuracy of the diagnosis, their usefulness may be reduced in patients who present with sepsis, multiple organ failure, and recurrent inflammations. Of note, even the accuracy of the clinical examination can be reduced due to non-infectious confounding factors such as surgical and reperfusion damages as well as therapies with steroidal anti-inflammatory drugs[1].
There are three practical stratifications for TP according to the different levels of clinical evidence suggesting its diagnosis. A microbiologically confirmed TP is characterized by detectable nosocomial pathogens in the peritoneal fluid or blood 48 h after the treatment of the SP. An SP-like clinical presentation with a documented persistent inflammation (more than 500 leukocytes/µL of peritoneal fluid) and lack of microbiological confirmation after the aforementioned post-surgical period characterizes a probable TP. In contrast, if there is no documented evidence of peritoneal inflammation in that clinical scenario, the condition is defined as a possible TP[4]. Figure 1 summarizes the stratification of patients with IAIs.
Because the delimitation between SP and TP can be difficult in clinical practice, some authors argue that the stratification into three clinical entities for peritonitis may be unnecessary. They suggest the exclusion of the term “tertiary peritonitis” and propose a subclassification of SP in which the presence or absence of anatomic rupture and clinical severity should be considered as distinct phenotypes of a single condition[6,32].
The management of TP involves establishing an effective antimicrobial therapy covering multidrug-resistant pathogens as well as performing procedural approaches in order to control the source of infection. Previous antimicrobial therapies and exposure to the hospital environment are associated with changes in the pattern of microbiological colonization of the patients; and, therefore, IAIs in these circumstances are more likely to involve nosocomial bacteria[12]. Of note, fungi are not uncommon pathogens among patients with bowel involvement, nosocomial infection, previous exposure to antibiotics, immunocompromising conditions, or recurrent infections[13].
Except for primary peritonitis, surgical intervention and/or percutaneous drainage are often required for the treatment of IAIs, and the latter procedure should be the first choice. Open surgical interventions allow surgeons to identify and correct dehiscence or anatomical changes, to debride infected necrotic tissues, and to drain fluids[13]. Sample collections for microbiological analysis including gram stain and cultures should be performed during the procedure. The microbiological analysis is especially important for patients who were previously exposed to antibiotics or who are at a high risk of infection with resistant organisms. Gram staining provides early guidance that aids in the choice of antibiotics and may be the only source of information if cultures do not achieve satisfactory growth[33].
Indication for surgical treatment should be aggressive and aims to eliminate the source of infection, to reduce the peritoneal contaminants, and to avoid a continued peritonitis. The cavity approach should be performed through the former surgical incision, whose length should be extended if necessary. The reduction of peritoneal contamination is done through aspiration of the infectious content, purulent exudates, food debris, or fecal particles, in a radical debridement scheme[34]. Most patients progress with mechanical obstruction and substantial distention of the small intestine, making decompression an important part of the surgical treatment as well as the washing of the abdominal cavity[35]. Of note, the main goal is to solve the intra-peritoneal infectious process in the first re-intervention, thus avoiding new re-interventions, since there is an increase in mortality in patients who undergo multiple re-interventions[36].
Another important measure associated with the definitive resolution of IAI is to avoid planned relaparotomy. A randomized trial including 232 patients compared planned relaparotomy (after 36-48 h of the last approach) vs on-demand approaches (when there are parameters of clinical deterioration). Their results demonstrated that the former group did not experience a statistically significant reduction in the occurrence of unfavorable primary outcomes (morbidity and mortality) associated with severe peritonitis. However, there was a significant reduction in the prevalence of secondary outcomes such as the frequency of relaparotomies and percutaneous drainages as well as the length of hospital and ICU stay[37]. The negative result of programmed relaparotomies seems to be related to a delay in the diagnosis of persistent infection and the onset of multiple organ dysfunction syndrome before the re-approach.
In addition to the mandatory surgical treatment in the management of TP, pharmacological therapy has great importance in mitigating the infectious process. Given the increasing prevalence of infections caused by multidrug-resistant organisms, studies have been evaluating the appropriate selection of antimicrobial drugs and the duration of treatment[38]. Resistance to multiple drugs is not exclusive to nosocomial pathogens; and, otherwise, it can also be seen in community-acquired infections, mainly by extended-spectrum beta-lactamase microorganisms. However, infections by pathogens from a hospital multidrug-resistant microbiota represent a greater challenge for the pharmacological approach in TP[39]. It has to be emphasized that the presence of risk factors related to the patient also leads to the prescription of more aggressive adjuvant therapies.
Given the above, knowing the nosocomial microbiota is crucial to guide the spectrum of the adjuvant therapy. Multidrug-resistant bacteria can be both gram-positive or gram-negative microorganisms[40]. In addition, fungal infections in patients with TP can also be observed, with Candida albicans being the most commonly found pathogen, besides other opportunistic fungi.
Along with the growing number of infections caused by multidrug-resistant pathogens, new drugs and antibiotic schemes have been made available. Among the new drugs and therapeutic combinations stand out some cephalosporins (ceftolozane and ceftazidime) associated with beta-lactamase inhibitors (tazobactam and avibactam) and fluorocyclines (eravacycline). However, their use should be carefully selected in order to prevent the development of bacterial resistance[41]. These new options have been added to the widely used regimens against aerobic and anaerobic gram-negative bacilli such as meropenem, imipenem-cilastatin, doripenem, and piperacillin plus tazobactam, for individual use, and ceftazidime or cefepime associated with metronidazole if an infection by anaerobic microorganism is suspected[42]. In addition, antienterococcal antibiotics are recommended in empirical schemes for patients with nosocomial IAIs, especially those with postoperative infections who were previously treated with cephalosporins. In this scenario, ampicillin or vancomycin can be added for enterococcal coverage[13]. In IAI cases with a documented infection by methicillin-resistant Staphylococcus aureus or an increased risk of colonization by this pathogen due to failure of previous treatment and previous exposure to various antibiotics, an empirical antimicrobial scheme including vancomycin should be instituted[2]. Furthermore, echinocandins were shown to be superior to other drugs in controlling peritonitis by Candida spp in critical patients, whereas the use of azoles is indicated in patients in clinical remission[42].
Furthermore, the duration of antimicrobial therapies directly influences the development of multidrug-resistant organisms. Moreover, a series of studies conducted in countries such as France and the United States (STOP-IT 2015) observed no benefit with the use of prolonged antimicrobial therapy for the control of the infectious focus in patients with peritonitis[43,44]. The most updated guidelines of the Surgical Infection Society indicate the use of antibiotics for no more than 96 h in patients with a controlled infectious focus. On the other hand, patients who have not undergone a procedure aiming for the definitive control of the infectious focus should have their therapy extended for 5-7 d (Table 1)[45].
| Microorganisms | Antimicrobial drugs | Treatment duration (for all regimens) |
| Gram-negative bacilli and multidrug-resistant pathogens | Meropenem or imipenem-cilastatin or piperacillin-tazobactam or doripenem; Anaerobic microorganisms: Ceftazidime or cefepime associated with metronidazole; New schemes: Ceftolozana or ceftazidime + tazobactam or avibactam; Fluorocyclines | Patients with controlled infectious focus: No more than 96 h. Patients who not yet undergone definitive control of the infectious focus: 5-7 d |
| Enterococcus spp | Include ampicillin or vancomycin | |
| MRSA | Include vancomycin | |
| Candida spp | Critical patients: Echinocandins; Patients in clinical remission: Azoles |
Since late diagnosis increases the likelihood of negative outcomes in TP, it is a condition that requires great attention from the medical team. This disease should not be ignored and requires effective and targeted treatment according to the clinical characteristics of each patient in order to avoid clinical complications[2,46].
Some independent factors can increase mortality in TP patients, such as advanced age [odds ratio (OR): 1.06, 95% confidence interval (CI): 1.03-1.1, P < 0.001], cerebrovascular disease (OR: 4.3, 95%CI: 1.40-13.1, P = 0.01), malignant disease (OR: 2.9, 95%CI: 1.3-6.5, P = 0.01), hemodialysis dependence (OR: 3.8, 95%CI: 1.3-11.2, P = 0.02), and liver disease (OR: 4.2, 95%CI: 1.6-15.1, P = 0.03)[2,21]. Individuals with TP have higher rates of multiple organ failure and a higher mortality rate than patients with SP[1,7,21,47]. A recent study by Ballus et al[48] assessed 343 patients with peritonitis who underwent abdominal surgery. Among them, 185 progressed to TP, from which 48% (n = 90, P = 0.02) died. Besides, patients with TP are at a higher risk of developing severe sepsis/septic shock[48].
Among other characteristics associated with a poorer prognosis stand out delayed intervention, inability to obtain satisfactory surgical control, immunosuppression, organ dysfunction, severe peritoneal involvement or diffuse peritonitis, hypo-albuminemia, and low nutritional status[13].
The ICU admission itself predisposes the occurrence of certain infections due to the mechanisms used in the treatment of the patient. In this sense, it is not uncommon to observe cases of pneumonia associated with artificial respiration, contamination through central venous access, or urinary tract infection due to vesical catheterization[26,45]. Therefore, patients with TP are at an increased risk of acquiring additional infections since their period of stay in the ICU tends to be longer than individuals with SP (21.8 ± 14.9 d vs 8.5 ± 7.9 d)[18]. Bacterial resistance is another point that makes recovery difficult and increases the severity of the condition and the length of hospital stay[47].
The aforementioned study by Ballus et al[48] found that some factors may contribute to the development of TP in ill individuals, such as a long stay in ICU (P = 0.01), the necessity of urgent surgery in hospital admission (P = 0.006), the use of parenteral nutrition in ICU patients (P = 0.002), and initial infection in the stomach or duodenal sites (P = 0.011). On the other hand, patients who had localized peritonitis at ICU admission had lower chances of developing TP (P = 0.001).
Although advances have been achieved in our knowledge of TP, much has to be done aiming for a better understanding of this condition. Unfortunately, this review evidences a limited number of current studies on that condition. In this sense, given the high complexity of TP and the absence of a meaningful reduction in its mortality rates throughout the years, it has to be emphasized the importance of conducting original studies that aim for a better comprehension of the condition and for improvements in its management. Of note, an effective diagnosis is essential for an appropriate and rapid therapy, which should include both procedural approaches aiming for the definitive control of the infectious focus and the use of antimicrobial drugs, leading to a lower frequency of complications and unfavorable outcomes.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lutz P S-Editor: Gao CC L-Editor: Filipodia P-Editor: Liu JH
| 1. | Weiss G, Steffanie W, Lippert H. [Peritonitis: main reason of severe sepsis in surgical intensive care]. Zentralbl Chir. 2007;132:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 2. | Evans HL, Raymond DP, Pelletier SJ, Crabtree TD, Pruett TL, Sawyer RG. Tertiary peritonitis (recurrent diffuse or localized disease) is not an independent predictor of mortality in surgical patients with intraabdominal infection. Surg Infect (Larchmt). 2001;2:255-63; discussion 264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Clara L, Rodríguez VM, Saúl P, Domínguez C, Esteban M. [Intra-abdominal infections. Update and recommendations]. Medicina (B Aires). 2018;78:417-426. [PubMed] |
| 4. | Calandra T, Cohen J; International Sepsis Forum Definition of Infection in the ICU Consensus Conference. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med. 2005;33:1538-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 592] [Article Influence: 29.6] [Reference Citation Analysis (1)] |
| 5. | Hartl W, Kuppinger D, Vilsmaier M. [Secondary peritonitis]. Zentralbl Chir. 2011;136:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Martín-López A, Castaño-Ávila S, Maynar-Moliner FJ, Urturi-Matos JA, Manzano-Ramírez A, Martín-López HP. [Tertiary peritonitis: as difficult to define as it is to treat]. Cir Esp. 2012;90:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Chromik AM, Meiser A, Hölling J, Sülberg D, Daigeler A, Meurer K, Vogelsang H, Seelig MH, Uhl W. Identification of patients at risk for development of tertiary peritonitis on a surgical intensive care unit. J Gastrointest Surg. 2009;13:1358-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Ross JT, Matthay MA, Harris HW. Secondary peritonitis: principles of diagnosis and intervention. BMJ. 2018;361:k1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 9. | Budzyński P, Dworak J, Natkaniec M, Pędziwiatr M, Major P, Migaczewski M, Matłok M, Budzyński A. The usefulness of the Mannheim Peritonitis Index score in assessing the condition of patients treated for peritonitis. Pol Przegl Chir. 2015;87:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Al-Khafaji A, Angus DC, Knaus WA. The Acute Physiology and Chronic Health Evaluation II. Article of Knaus et al with expert commentary by Dr Derek Angus. J Crit Care. 2007;22:85-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957-2963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 2193] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 12. | Sitges-Serra A, López MJ, Girvent M, Almirall S, Sancho JJ. Postoperative enterococcal infection after treatment of complicated intra-abdominal sepsis. Br J Surg. 2002;89:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ, O'Neill PJ, Chow AW, Dellinger EP, Eachempati SR, Gorbach S, Hilfiker M, May AK, Nathens AB, Sawyer RG, Bartlett JG. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:133-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 1020] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 14. | Holmes C, Lewis S. Host defense mechanisms in the peritoneal cavity of continuous ambulatory peritoneal dialysis patients. 2. Humoral defenses. Perit Dial Int. 1991;11:112-117. [PubMed] |
| 15. | Bone RC, Grodzin CJ, Balk RA. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest. 1997;112:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 565] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 16. | Buijk SE, Bruining HA. Future directions in the management of tertiary peritonitis. Intensive Care Med. 2002;28:1024-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Leliefeld PH, Wessels CM, Leenen LP, Koenderman L, Pillay J. The role of neutrophils in immune dysfunction during severe inflammation. Crit Care. 2016;20:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 193] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 18. | Nathens AB, Rotstein OD, Marshall JC. Tertiary peritonitis: clinical features of a complex nosocomial infection. World J Surg. 1998;22:158-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 106] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Doklestić SK, Bajec DD, Djukić RV, Bumbaširević V, Detanac AD, Detanac SD, Bracanović M, Karamarković RA. Secondary peritonitis - evaluation of 204 cases and literature review. J Med Life. 2014;7:132-138. [PubMed] |
| 20. | Koperna T, Schulz F. Relaparotomy in peritonitis: prognosis and treatment of patients with persisting intraabdominal infection. World J Surg. 2000;24:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 120] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Panhofer P, Izay B, Riedl M, Ferenc V, Ploder M, Jakesz R, Götzinger P. Age, microbiology and prognostic scores help to differentiate between secondary and tertiary peritonitis. Langenbecks Arch Surg. 2009;394:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Sartelli M. A focus on intra-abdominal infections. World J Emerg Surg. 2010;5:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Bahtouee M, Eghbali SS, Maleki N, Rastgou V, Motamed N. Acute Physiology and Chronic Health Evaluation II score for the assessment of mortality prediction in the intensive care unit: a single-centre study from Iran. Nurs Crit Care. 2019;24:375-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 24. | Allyn J, Ferdynus C, Bohrer M, Dalban C, Valance D, Allou N. Simplified Acute Physiology Score II as Predictor of Mortality in Intensive Care Units: A Decision Curve Analysis. PLoS One. 2016;11:e0164828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (2)] |
| 25. | Agha A, Bein T, Fröhlich D, Höfler S, Krenz D, Jauch KW. ["Simplified Acute Physiology Score" (SAPS II) ina the assessment of severity of illness in surgical intensive care patients]. Chirurg. 2002;73:439-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Weiss G, Meyer F, Lippert H. Infectiological diagnostic problems in tertiary peritonitis. Langenbecks Arch Surg. 2006;391:473-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Malangoni MA. Evaluation and management of tertiary peritonitis. Am Surg. 2000;66:157-161. [PubMed] |
| 28. | Mishra SP, Tiwary SK, Mishra M, Gupta SK. An introduction of Tertiary Peritonitis. J Emerg Trauma Shock. 2014;7:121-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 29. | Wasnik AP, Maturen KE, Kaza RK, Al-Hawary MM, Francis IR. Primary and secondary disease of the peritoneum and mesentery: review of anatomy and imaging features. Abdom Imaging. 2015;40:626-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Roehrborn A, Thomas L, Potreck O, Ebener C, Ohmann C, Goretzki PE, Röher HD. The microbiology of postoperative peritonitis. Clin Infect Dis. 2001;33:1513-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Sartelli M, Abu-Zidan FM, Labricciosa FM, Kluger Y, Coccolini F, Ansaloni L, Leppäniemi A, Kirkpatrick AW, Tolonen M, Tranà C, Regimbeau JM, Hardcastle T, Koshy RM, Abbas A, Aday U, Adesunkanmi ARK, Ajibade A, Akhmeteli L, Akın E, Akkapulu N, Alotaibi A, Altintoprak F, Anyfantakis D, Atanasov B, Augustin G, Azevedo C, Bala M, Balalis D, Baraket O, Baral S, Barkai O, Beltran M, Bini R, Bouliaris K, Caballero AB, Calu V, Catani M, Ceresoli M, Charalampakis V, Jusoh AC, Chiarugi M, Cillara N, Cuesta RC, Cobuccio L, Cocorullo G, Colak E, Conti L, Cui Y, De Simone B, Delibegovic S, Demetrashvili Z, Demetriades D, Dimova A, Dogjani A, Enani M, Farina F, Ferrara F, Foghetti D, Fontana T, Fraga GP, Gachabayov M, Gérard G, Ghnnam W, Maurel TG, Gkiokas G, Gomes CA, Guner A, Gupta S, Hecker A, Hirano ES, Hodonou A, Hutan M, Ilaschuk I, Ioannidis O, Isik A, Ivakhov G, Jain S, Jokubauskas M, Karamarkovic A, Kaushik R, Kenig J, Khokha V, Khokha D, Kim JI, Kong V, Korkolis D, Kruger VF, Kshirsagar A, Simões RL, Lanaia A, Lasithiotakis K, Leão P, Arellano ML, Listle H, Litvin A, Lizarazu Pérez A, Lopez-Tomassetti Fernandez E, Lostoridis E, Luppi D, Machain V GM, Major P, Manatakis D, Reitz MM, Marinis A, Marrelli D, Martínez-Pérez A, Marwah S, McFarlane M, Mesic M, Mesina C, Michalopoulos N, Misiakos E, Moreira FG, Mouaqit O, Muhtaroglu A, Naidoo N, Negoi I, Nikitina Z, Nikolopoulos I, Nita GE, Occhionorelli S, Olaoye I, Ordoñez CA, Ozkan Z, Pal A, Palini GM, Papageorgiou K, Papagoras D, Pata F, Pędziwiatr M, Pereira J, Pereira Junior GA, Perrone G, Pintar T, Pisarska M, Plehutsa O, Podda M, Poillucci G, Quiodettis M, Rahim T, Rios-Cruz D, Rodrigues G, Rozov D, Sakakushev B, Sall I, Sazhin A, Semião M, Sharda T, Shelat V, Sinibaldi G, Skicko D, Skrovina M, Stamatiou D, Stella M, Strzałka M, Sydorchuk R, Teixeira Gonsaga RA, Tochie JN, Tomadze G, Ugoletti L, Ulrych J, Ümarik T, Uzunoglu MY, Vasilescu A, Vaz O, Vereczkei A, Vlad N, Walędziak M, Yahya AI, Yalkin O, Yilmaz TU, Ünal AE, Yuan KC, Zachariah SK, Žilinskas J, Zizzo M, Pattonieri V, Baiocchi GL, Catena F. Physiological parameters for Prognosis in Abdominal Sepsis (PIPAS) Study: a WSES observational study. World J Emerg Surg. 2019;14:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Blot S, De Waele JJ, Vogelaers D. Essentials for selecting antimicrobial therapy for intra-abdominal infections. Drugs. 2012;72:e17-e32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Baron EJ, Miller JM, Weinstein MP, Richter SS, Gilligan PH, Thomson RB Jr, Bourbeau P, Carroll KC, Kehl SC, Dunne WM, Robinson-Dunn B, Schwartzman JD, Chapin KC, Snyder JW, Forbes BA, Patel R, Rosenblatt JE, Pritt BS. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM)(a). Clin Infect Dis. 2013;57:e22-e121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 373] [Cited by in RCA: 330] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 34. | Hudspeth AS. Radical surgical debridement in the treatment of advanced generalized bacterial peritonitis. Arch Surg. 1975;110:1233-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Hau T, Ahrenholz DH, Simmons RL. Secondary bacterial peritonitis: the biologic basis of treatment. Curr Probl Surg. 1979;16:1-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Koperna T, Schulz F. Prognosis and treatment of peritonitis. Do we need new scoring systems? Arch Surg. 1996;131:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 78] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | van Ruler O, Mahler CW, Boer KR, Reuland EA, Gooszen HG, Opmeer BC, de Graaf PW, Lamme B, Gerhards MF, Steller EP, van Till JW, de Borgie CJ, Gouma DJ, Reitsma JB, Boermeester MA; Dutch Peritonitis Study Group. Comparison of on-demand vs planned relaparotomy strategy in patients with severe peritonitis: a randomized trial. JAMA. 2007;298:865-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 208] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 38. | Eisner R, Lippmann N, Josten C, Rodloff AC, Behrendt D. Development of the Bacterial Spectrum and Antimicrobial Resistance in Surgical Site Infections of Trauma Patients. Surg Infect (Larchmt). 2020;21:684-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 39. | Steinbach CL, Töpper C, Adam T, Kees MG. Spectrum adequacy of antibiotic regimens for secondary peritonitis: a retrospective analysis in intermediate and intensive care unit patients. Ann Clin Microbiol Antimicrob. 2015;14:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Karlowsky JA, Hoban DJ, Hackel MA, Lob SH, Sahm DF. Resistance among Gram-negative ESKAPE pathogens isolated from hospitalized patients with intra-abdominal and urinary tract infections in Latin American countries: SMART 2013-2015. Braz J Infect Dis. 2017;21:343-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 41. | Bassetti M, Righi E. New antibiotics and antimicrobial combination therapy for the treatment of gram-negative bacterial infections. Curr Opin Crit Care. 2015;21:402-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 42. | Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. Executive Summary: Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 1046] [Article Influence: 116.2] [Reference Citation Analysis (0)] |
| 43. | Montravers P, Lepape A, Dubreuil L, Gauzit R, Pean Y, Benchimol D, Dupont H. Clinical and microbiological profiles of community-acquired and nosocomial intra-abdominal infections: results of the French prospective, observational EBIIA study. J Antimicrob Chemother. 2009;63:785-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 44. | Sawyer RG, Claridge JA, Nathens AB, Rotstein OD, Duane TM, Evans HL, Cook CH, O'Neill PJ, Mazuski JE, Askari R, Wilson MA, Napolitano LM, Namias N, Miller PR, Dellinger EP, Watson CM, Coimbra R, Dent DL, Lowry SF, Cocanour CS, West MA, Banton KL, Cheadle WG, Lipsett PA, Guidry CA, Popovsky K; STOP-IT Trial Investigators. Trial of short-course antimicrobial therapy for intraabdominal infection. N Engl J Med. 2015;372:1996-2005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 486] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 45. | Mazuski JE, Tessier JM, May AK, Sawyer RG, Nadler EP, Rosengart MR, Chang PK, O'Neill PJ, Mollen KP, Huston JM, Diaz JJ Jr, Prince JM. The Surgical Infection Society Revised Guidelines on the Management of Intra-Abdominal Infection. Surg Infect (Larchmt). 2017;18:1-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 366] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 46. | Hranjec T, Watson CM, Sawyer RG. Peritonitis: Definitions of Primary, Secondary, and Tertiary. In: Vincent JL, Hall JB. Encyclopedia of Intensive Care Medicine; 2012: 1723-1729. [DOI] [Full Text] |
| 47. | Ballus J, Lopez-Delgado JC, Sabater-Riera J, Perez-Fernandez XL, Betbese AJ, Roncal JA. Surgical site infection in critically ill patients with secondary and tertiary peritonitis: epidemiology, microbiology and influence in outcomes. BMC Infect Dis. 2015;15:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Ballus J, Lopez-Delgado JC, Sabater-Riera J, Perez-Fernandez XL, Betbese AJ, Roncal JA. Factors Associated with the Development of Tertiary Peritonitis in Critically Ill Patients. Surg Infect (Larchmt). 2017;18:588-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (2)] |