Published online Jan 6, 2021. doi: 10.12998/wjcc.v9.i1.71
Peer-review started: September 17, 2020
First decision: October 18, 2020
Revised: October 30, 2020
Accepted: November 13, 2020
Article in press: November 13, 2020
Published online: January 6, 2021
Processing time: 105 Days and 20.9 Hours
A proportion of lung cancers show sodium/iodide symporter (NIS) expression. Lung cancers with NIS expression may uptake radioiodine (RAI) and show RAI-avid lesions on RAI scan for differentiated thyroid cancer (DTC) surveillance.
To investigate the possibility of RAI uptake by lung cancer in a cohort with thyroid cancer.
RAI-avid lung cancers were analyzed using a prospectively maintained database of patients with thyroid cancer who were registered at a medical center between December 1, 1976 and May 28, 2018. NIS expression in lung cancer was assessed using immunohistochemical staining.
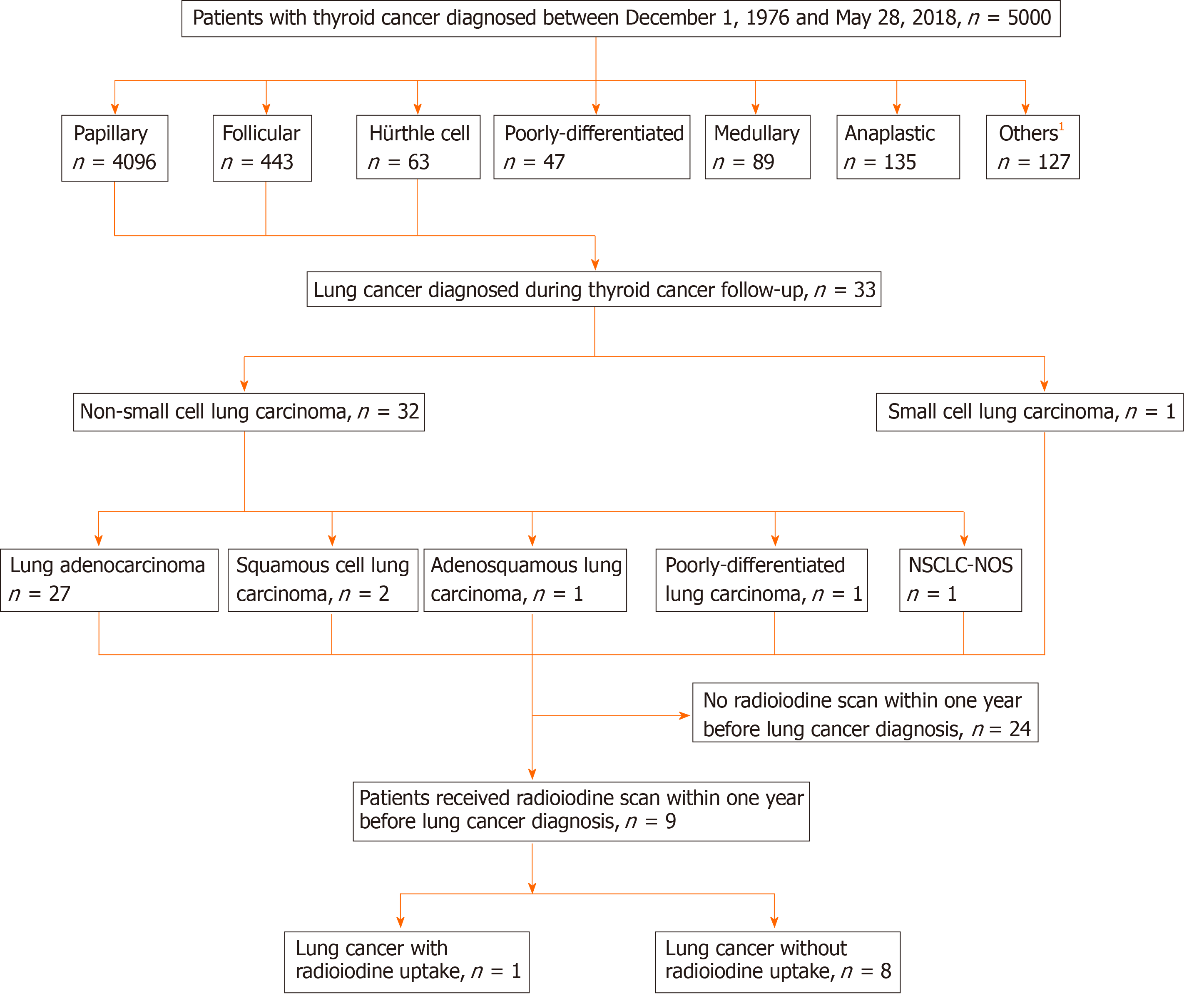
Of the 5000 patients with thyroid cancer from the studied dataset, 4602 had DTC. During follow-up, 33 patients developed primary lung cancer. Of these patients, nine received an iodine-131 (131I) scan within 1 year before the diagnosis of lung cancer. One of these nine lung cancers was RAI-avid. NIS expression was evaluated, and three of the eight available lung cancers revealed NIS expression. The proportions of lung cancer cells with NIS expression were 60%, 15%, and 10%. The RAI-avid lung cancer had the highest level of expression (60%). The RAI-avid lung cancer had a spiculated border upon single-photon emission computed tomography/computed tomography, which led to an accurate diagnosis.
A proportion of lung cancer demonstrates NIS expression and is RAI-avid. Clinicians should be aware of this possibility in the interpretation of RAI scintigraphy.
Core Tip: A radioiodine (RAI) scan is usually performed to detect the existence of differentiated thyroid cancer (DTC). A proportion of lung cancers demonstrate sodium/iodide symporter (NIS) expression. Lung cancers with NIS expression may be able to uptake RAI and show RAI-avid lesions on RAI scan, leading to misinterpretation upon performing this test for DTC surveillance. Single-photon emission computed tomography/computed tomography provides morphologic characterization of the functional tumor, improving diagnostic accuracy over RAI scan.
- Citation: Lu YL, Chen ST, Ho TY, Chan WH, Wong RJ, Hsueh C, Lin SF. Primary lung cancer with radioiodine avidity: A thyroid cancer cohort study. World J Clin Cases 2021; 9(1): 71-80
- URL: https://www.wjgnet.com/2307-8960/full/v9/i1/71.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i1.71
The global incidence of thyroid cancer has increased substantially in the past four decades[1,2]. Thyroid cancer originates from follicular and parafollicular cells. The transformation of the former leads to distinct variants of thyroid malignancies including papillary, follicular, and Hürthle cell cancers (known as differentiated thyroid cancer, DTC), as well as poorly-differentiated and anaplastic thyroid cancers. Medullary thyroid cancer arises from parafollicular cells[3]. DTC accounts for 88% thyroid malignancies[3]. The majority of these patients have been reported to survive for > 10 years after diagnosis following standard treatment with surgery, radioiodine (RAI) therapy, and thyroid hormone replacement/suppression therapy[4]. However, long-term follow-up is crucial as disease recurrence occurs in up to 30% of patients, even decades later[5,6]. The lung is the most common site for distant metastasis in papillary and follicular thyroid cancer, accounting for up to 68.7% of affected patients[6]. Tests that are recommended for the surveillance of DTC recurrence include neck ultrasound, thyroglobulin (Tg)—a tumor marker for DTC—with Tg antibody measurement, and RAI whole-body scanning[7].
RAI whole-body scanning is suggested in the postsurgical follow-up for patients with an intermediate or high risk of disease recurrence[7,8]. An RAI scan has a specificity of 91%-100% and a sensitivity of 27%-55% in detecting metastatic DTC[8]. Despite its high specificity, false positive findings leading to diagnostic errors have been reported in previous studies[9]. The potential mechanisms accounting for false positive RAI uptake are variable, and include ectopic thyroid tissue, sodium/iodide symporter (NIS) expression in non-thyroid tissues, RAI retention in secretions in ducts or cavities, inflammation, RAI contamination, and unknown mechanisms[9].
Lung cancer is one of the most common and serious types of malignancy. It is the leading cause of death from malignancy worldwide, accounting for 18.4% of all cancer mortalities, with only 15% of patients surviving for > 5 years after diagnosis[10]. The four major histological types of lung cancer are adenocarcinoma, squamous cell carcinoma, large cell carcinoma, and small cell lung carcinoma[11]. It is interesting to note that a previous report has shown NIS expression in significant proportions of different types of lung cancer as follows: 76.6% in adenocarcinoma, 36.1% in squamous cell carcinoma, and 20.0% in small cell carcinoma[12]. NIS expression in lung cancer may be functional and confers the ability to accumulate RAI, which is thought to lead to a misinterpretation of RAI scintigraphy when this test is performed for DTC surveillance.
In the present study, we sought to analyze a database of patients with thyroid cancer managed at a medical center to investigate the possibility of RAI-avid lung cancer.
The present study analyzed data from a thyroid cancer registry database at Chang Gung Memorial Hospital, Taiwan. This database was established by JD Lin to facilitate research on thyroid cancer[13,14]. Data on all patients with thyroid cancer managed at this institute were prospectively collected. Clinical characteristics, laboratory data, imaging findings, pathologic reports, treatment, and follow-up results were systemically recorded. A total of 5000 patients were included in this dataset between December 1, 1976 and May 28, 2018. These patients were followed until the time of data cutoff (March 20, 2019).
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded, 5 µm thick tissue sections of lung cancer. Sections were incubated with a rabbit antibody against human NIS (1:50 dilution; Abcam) at room temperature for 30 min, followed by application of a poly-horseradish peroxidase anti-rabbit immunoglobulin G reagent and diaminobenzidine for complex visualization. Staining intensity was graded as negative, weak, moderate, and strong, as previously described[15]. The percentage of lung cancer cells staining positive for NIS was also quantified using visual scoring by a pathologist (CH).
The present study was approved by the Chang Gung Medical Foundation Institutional Review Board (number: 202000365B0) and was conducted in accordance with the ethical principles of the Helsinki Declaration. Informed consent was waived by Chang Gung Medical Foundation Institutional Review Board.
There were 5000 patients with thyroid cancer diagnosed between December 1, 1976 and May 28, 2018 in this database (Figure 1). A total of 4602 patients with DTC were identified, including papillary (n = 4096), follicular (n = 443), and Hürthle cell (n = 63) thyroid cancer. Among them, 33 patients developed lung cancers during follow-up of DTC until March 20, 2019. The histologic types of these cancers were non-small cell lung cancer (NSCLC) (n = 32) and small cell lung carcinoma (n = 1). NSCLC included adenocarcinoma (n = 27), squamous cell carcinoma (n = 2), adenosquamous lung carcinoma (n = 1), poorly-differentiated lung carcinoma (n = 1), and non-small cell lung carcinoma-not otherwise specified (NSCLC-NOS) (n = 1)[16]. The diagnosis of NSCLC-NOS was due to small biopsy sample[16].
Nine of these 33 patients received a RAI scan within 1 year before the histological diagnosis of lung cancer (Figure 1). The characteristics of these nine patients are presented in Table 1. There were three males and six females aged between 44 and 69 years at the time of thyroid cancer diagnosis. All nine patients had papillary thyroid cancer. All of these 9 patients had NSCLC, including eight patients with adenocarcinoma and one patient with NSCLC-NOS[16]. The 8th edition Cancer Staging Manual, American Joint Committee on Cancer was used for tumor staging of thyroid cancer and lung cancer[17]. The interval between the diagnosis of thyroid cancer and that of lung cancer ranged from 0.2 to 18.4 years. The maximal diameters of lung cancer were between 1.7 and 4.3 cm on diagnosis. The indications for RAI use were thyroid remnant ablation (n = 2), elevated Tg level (n = 4), elevated Tg antibody level (n = 1), neck sonography suggesting tumor recurrence (n = 1), and thyroid cancer surveillance (n = 1). All patients received thyroid hormone withdrawal in preparation for the RAI scan. The doses of iodine-131 (131I) administered were between 2 and 200 mCi.
| Patient | Age/gender | Histological type of thyroid cancer | Stage of thyroid cancer | Histological type of lung cancer | Tumor size of lung cancer | Stage of lung cancer | Interval between thyroid cancer and lung cancer diagnosis | Indication and dose of 131I | Interval between 131I administration and lung cancer histological confirmation | 131I uptake in lung cancer | NIS expression in lung cancer (staining intensity/percentage) |
| 1 | 50/F | Papillary | T1aN0bM0 | Adenocarcinoma | 1.7 cm | T1bN0M0 | 1.5 yr | Elevated Tg antibody level/30 mCi | 5.7 mo | Positive | Weak/60% |
| 2 | 50/F | Papillary | T1bN0bM0 | Adenocarcinoma | 2.5 cm | T1bN1M0 | 15.3 yr | Thyroid remnant ablation/30 mCi | 11.3 mo | Negative | Weak/15% |
| 3 | 47/M | Papillary | T2N1aM0 | Adenocarcinoma | 2.4 cm | T2aN0M1a | 0.2 yr | Elevated Tg level/100 mCi | 1.2 mo | Negative | Weak/10% |
| 4 | 44/F | Papillary | TxN0bM0 | Adenocarcinoma | 3.2 cm | T4N3M0 | 18.4 yr | Neck sonography suggesting tumor recurrence/200 mCi | 1.8 mo | Negative | Negative |
| 5 | 66/F | Papillary | TxN0bM0 | Adenocarcinoma | 2.4 cm | T2aN1M0 | 10.2 yr | Thyroid cancer surveillance/2 mCi | 1.7 mo | Negative | Negative |
| 6 | 67/M | Papillary | T1bN1bM0 | Adenocarcinoma | 3.8 cm | T4N2M0 | 3.0 yr | Elevated Tg level/200 mCi | 3.1 mo | Negative | Negative |
| 7 | 55/F | Papillary | T2N1bM0 | Adenocarcinoma | 3.0 cm | T1cN0M0 | 0.3 yr | Thyroid remnant ablation/30 mCi | 0.8 mo | Negative | Negative |
| 8 | 56/F | Papillary | T3aN1aM1 | Adenocarcinoma | 1.8 cm | T2aN2M0 | 7.6 yr | Elevated Tg level/100 mCi | 3.1 mo | Negative | – |
| 9 | 69/M | Papillary | T1aN0bM0 | Non-small cell lung carcinoma-not otherwise specified | 4.3 cm | T2bN2M0 | 1.0 yr | Elevated Tg level/30 mCi | 6.5 mo | Negative | Negative |
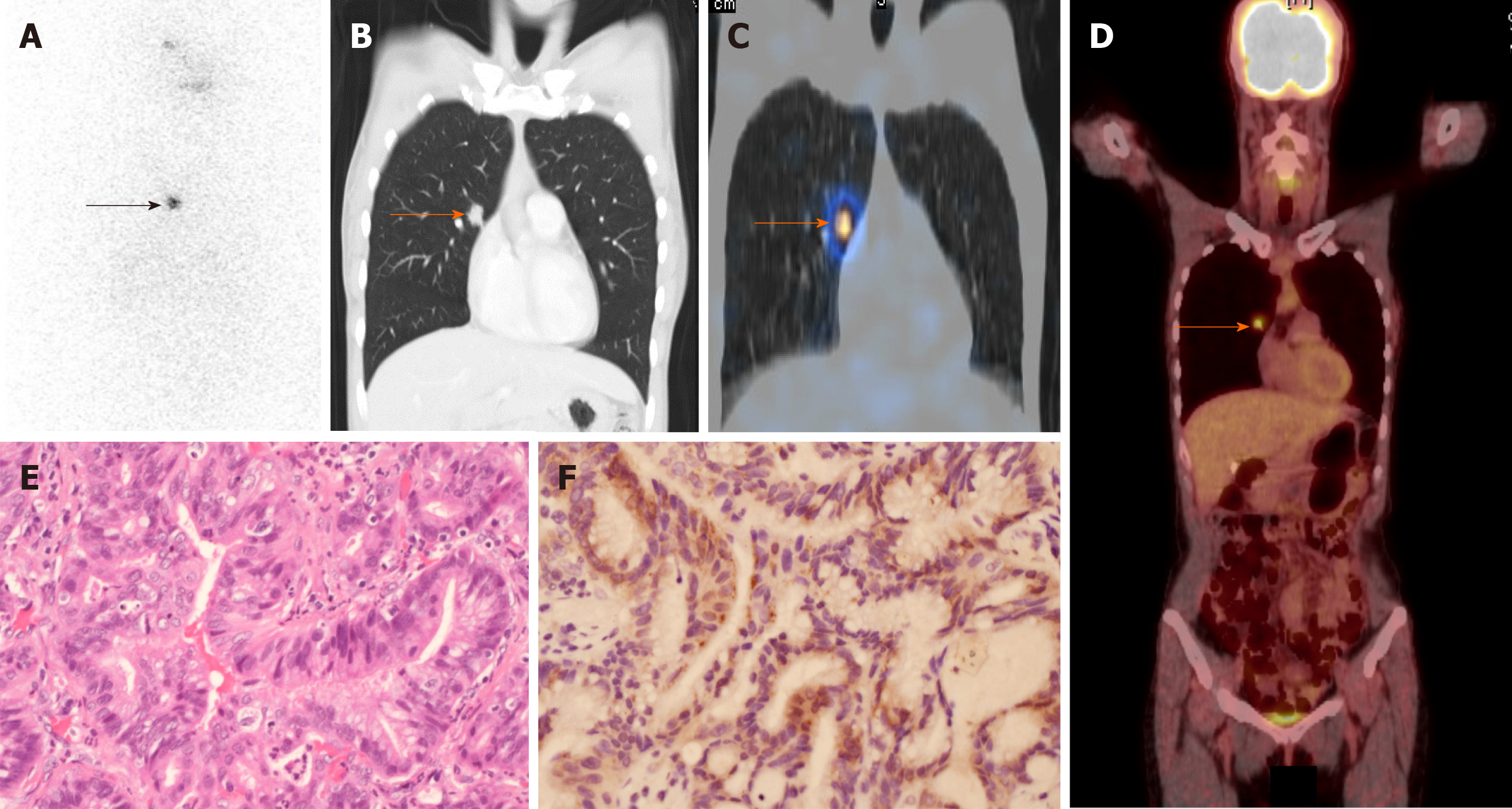
We found that one of the nine lung cancers had 131I avidity on the RAI scan (Figure 1). The details of this patient (patient 1 in Table 1) with RAI-avid lung cancer are described below. A 50-year-old female presented with an incidentally found nodule in the left thyroid region. Thyroid ultrasound revealed an ill-defined 0.9-cm nodule in the left thyroid lobe. Fine-needle aspiration cytology indicated papillary thyroid cancer (Bethesda Diagnostic Category VI)[18]. The patient underwent total thyroidectomy; papillary thyroid cancer was confirmed histologically in the left thyroid gland. In addition, multiple papillary thyroid microcarcinomas with maximal diameters ranging from 0.1 to 0.4 cm were identified in the right thyroid lobe. Thyroid remnant ablation was performed using 30 mCi of 131I at 4 wk following surgery. A RAI whole-body scan performed 7 d after 131I administration revealed no evidence of distant metastasis. Thyroid hormone replacement therapy was initiated soon after thyroid remnant ablation and serum thyroid-stimulating hormone level was maintained at 0.1-0.5 mU/L. One year after thyroidectomy, the patient underwent 131I (30 mCi) treatment at 4 wk after thyroid hormone withdrawal because her initial Tg antibody level was higher than the reference range (261.0 U/mL; reference range, < 60). A subsequent RAI scan for monitoring of potential papillary thyroid cancer recurrence showed RAI uptake in the right chest (Figure 2A). A single-photon emission computed tomography/computed tomography (SPECT/CT) study was performed to identify the morphological features and localize the radioiodine uptake accurately. CT (Figure 2B) and fused SPECT/CT (Figure 2C) revealed RAI accumulation in a spiculated 1.7-cm nodule located in the central area of the right upper lobe of the lung. The spiculated morphology of this single lung tumor gave an impression of primary lung cancer[19]. Consequently, a 18F-fluorodeoxyglucose positron emission tomography/CT (18F-FDG PET/CT) study was conducted to determine the clinical stage of the possible primary lung cancer. The 18F-FDG PET/CT findings revealed that this lung tumor was 18FFDG-avid and likely to be T1bN0M0 disease (Figure 2D). The patient underwent video-assisted right upper lung segmentectomy. The histological characteristics of the resected lung tumor were consistent with lung adenocarcinoma (Figure 2E). Molecular testing using an epidermal growth factor receptor (EGFR) PCR kit and direct sequencing (Qiagen) showed no mutations in exons 18, 19, 20, and 21 of the EGFR gene. Following the lung operation, the patient received periodic chest CT surveillance. During the 5-year follow-up, no evidence of thyroid cancer was found, as evaluated by periodic measurements of serum Tg, Tg antibody, and neck ultrasound.
A previous report demonstrated NIS expression in various types of lung cancer[12]. We sought to evaluate the possibility of NIS expression in lung cancer tumors in our patients (Table 1). Representative sections were stained with hematoxylin and eosin, and slides were reviewed by one pathologist (CH) to confirm the presence of lung cancer before immunohistochemical study for NIS expression. Of the eight lung cancer samples obtained from the pathology archive, three had positive NIS staining (patients 1, 2, and 3). All three specimens demonstrated weakly expressed NIS. The proportions of lung cancer cells expressing NIS were 60% in patient 1, 15% in patient 2, and 10% in patient 3. Of note, the RAI-avid lung cancer had the highest level of NIS expression (60%; Figure 2F).
In the present study, we identified one primary lung cancer that demonstrated higher levels of NIS expression and exhibited the ability to uptake RAI, whereas the other seven lung cancers with lower or undetectable levels of NIS failed to demonstrate RAI avidity on RAI scan. This is consistent with previous studies which reported that the level of NIS expression correlates with the uptake of RAI in thyroid cancer[20].
We observed that one of the nine lung cancers studied had the ability to uptake RAI. The data indicated that RAI scan does not have sufficient sensitivity in the detection of lung cancer. Our results are in line with the fact that the reported cases of lung cancers harboring the ability to uptake RAI is limited. To our knowledge, the first case was reported by Fernandez-Ulloa in 1976 in a 54-year-old man. Since then, five cases have been described (Table 2)[21-26]. The activities of 131I administered were between 5 and 182 mCi in these patients. The histologic types of these lung cancers were diverse and included adenocarcinoma, tubular adenocarcinoma, squamous cell carcinoma, small to medium cell undifferentiated bronchial carcinoma, large cell undifferentiated carcinoma, and mucinous bronchoalveolar carcinoma.
| Case | Author/year | Age/gender | Histological type of thyroid cancer | Dose of 131I | Histological type of lung cancer |
| 1 | Fernandez-Ulloa et al[21]/ 1976 | 54/M | No primary thyroid cancer identified | Not reported | Adenocarcinoma |
| 2 | Acosta et al[22]/1982 | 57/F | No primary thyroid cancer identified | Not reported | Large cell undifferentiated carcinoma |
| 3 | Langsteger et al[23]/1990 | 64/M | Papillary thyroid cancer | 5 mCi | Moderately-differentiated tubular adenocarcinoma |
| 4 | Haubold-Reuter et al[24]/ 1993 | Not reported | Papillary thyroid cancer | 20 mCi | Small to medium cell undifferentiated bronchial carcinoma |
| 5 | Misaki et al[25]/1994 | 71/M | Papillary thyroid cancer | 120 mCi | Squamous cell carcinoma |
| 6 | Malhotra et al[26]/2008 | 52/M | Follicular thyroid cancer | 182 mCi | Mucinous bronchoalveolar carcinoma |
Our patient with RAI-avid lung cancer demonstrates a rare but critical differential diagnosis from pulmonary metastasis of DTC on RAI scan. Whole-body RAI scan is frequently used to determine recurrent and metastatic DTC with high sensitivity[8]. However, RAI uptake can also be seen in normal tissues (salivary gland, breast, thymus, liver, gastric, and colon mucosa), cystic structures (bronchogenic cyst, breast cyst, renal cyst, and hepatic cyst), inflamed tissues (acute respiratory infection, granuloma, cholecystitis, and trauma), benign tumors (meningioma, breast fibroadenoma, hepatic angioma, uterine myoma, and struma ovarii), and malignant non-thyroid tumors (lung, breast, gastric, and ovary cancers)[27,28]. Therefore, RAI scan is subject to interpretation error. Misinterpretation of RAI scan may contribute to improper staging of DTC, unnecessary treatment with RAI, and misdiagnosis of diseases other than DTC.
Our patient revealed RAI uptake in the chest. Various lung diseases, including bronchiectasis, bacterial infection, tuberculosis, aspergilloma, and lung cancers, can lead to false positive RAI uptake in the chest[27,28]. This patient did not have any clinical manifestations of lung infection, which excluded the possibility of infectious lung disease. Therefore, primary lung tumor was noted to be the most likely etiology of false positive RAI uptake in this patient. The spiculated margin found on CT and fused SPECT/CT imaging led to a suspicion of primary lung cancer and resulted in appropriate diagnosis and treatment. SPECT provides information on radioactive tracer distribution by acquiring multiplanar images, whereas CT offers tomographic imaging of the three-dimensional anatomy to give a description of the morphological characteristics and anatomical localization of the area that shows an increased tracer uptake on SPECT[8,29]. SPECT/CT has demonstrated an incremental increase in diagnostic value from 15% to 73.9% with RAI scan after RAI therapy in patients with DTC[30,31]. Furthermore, SPECT/CT increased the detection and localization of RAI foci in lymph node metastases and distant metastases compared with RAI scintigraphy in a study of 147 patients with DTC[32]. Of note, the application of SPECT/CT altered the clinical staging in 6.1% of patients with DTC and changed the therapeutic planning in 2.0% of these patients. Therefore, it is recommended that SPECT/CT should be routinely performed with RAI scintigraphy after RAI therapy to achieve reliable clinical judgment for patients with high risk DTC[32].
Early recognition of lung cancers is pivotal because staging upon diagnosis is important for prognosis. Diameter, morphology, and growth rate are critical factors in the evaluation of the malignant potential of a lung nodule. A pulmonary nodule with a spicular margin is much more likely to be cancerous than that with a smooth, round, and well-defined edge[33]. The spiculation morphology of a lung tumor is highly predictive of cancer with a positive predictive value of 90%[34]. Pulmonary metastases usually show as rounded nodules of variable size in the peripheral of both lungs[35]. In the present study, the RAI-avid lung cancer did not have these features of pulmonary metastases on CT scan.
NIS is encoded by the SLC5A5 gene. The expression of SLC5A5 in lung cancers has been reported in the Human Protein Atlas, although its expression has not been reported to have a prognostic value in lung cancer[36]. NIS expression in lung cancer is of interest particularly because this may offer potential applications in using RAI in the surveillance and treatment of lung cancer. However, two points must be addressed. First, the degree of RAI uptake in lung cancer tissue needs to be clarified. We observed that most lung cancers had low or undetected NIS expression and were not RAI-avid. Second, surgical removal of the thyroid may be mandatory to maximize the efficacy of RAI in the treatment of lung cancer. Otherwise, RAI would most likely result in radioablation of the thyroid gland[37].
Among the nine patients studied who had received an RAI scan within 1 year before the diagnosis of lung cancer, most (8/9) had lung adenocarcinoma. Therefore, our results were primarily generated from this specific type of lung cancer. Further studies are needed to determine the possibility of RAI avidity in other histologic types of lung malignancy.
In the present study, a 1-year period between the RAI scan and histological diagnosis of lung cancer was allowed because of the relatively indolent growth of lung adenocarcinoma, with a mean tumor volume doubling time between 5.9 and 7.4 mo[38,39].
Primary lung cancer that had a high level of NIS expression and RAI avidity was observed to be uncommon. An accurate diagnosis between primary lung cancer and metastatic DTC was challenging based on an RAI scan. The application of SPECT/CT improved diagnostic accuracy over RAI scan alone.
A proportion of lung cancers has sodium/iodide symporter (NIS) expression.
Lung cancers with NIS expression may uptake radioiodine (RAI) and show RAI-avid lesions on RAI scan.
The present study aimed to evaluate the possibility of RAI uptake by lung cancers.
A prospectively maintained database of patients with thyroid cancer at a medical center between December 1, 1976 and May 28, 2018, was analyzed. Immuno-histochemical staining was used to assess NIS expression in lung cancers.
There were 5000 patients with thyroid cancer diagnosed between December 1, 1976 and May 28, 2018, in this database; of these, 4602 patients had differentiated thyroid cancer (DTC). Among those with DTC, 33 patients developed primary lung cancers during follow-up until March 20, 2019. Nine of these patients had an iodine-131 scan within 1 year before the diagnosis of lung cancer. The histological types of lung cancer were adenocarcinoma in eight patients and non-small-cell lung carcinoma-not otherwise specified in one patient. One of these nine lung cancers was RAI-avid. Immunohistochemical staining revealed that three of the eight available lung cancers had NIS expression. The proportions of lung cancer cells with NIS expression in these three lung tumors were 60%, 15%, and 10%, respectively. The RAI-avid lung cancer had the highest level of NIS expression (60%). Of note, the RAI-avid lung cancer had a spiculated border on single-photon emission computed tomography/computed tomography imaging, which led to an accurate diagnosis of primary lung cancer.
A proportion of lung cancers has NIS expression and demonstrates RAI avidity. These findings are significant for clinicians in the interpretation of RAI scintigraphy.
Our data were mainly derived from lung adenocarcinoma. Further studies are mandatory to determine the potential of RAI avidity in the other histologic types of lung cancers.
We acknowledge Prof. Lin JD at Chang Gung Memorial Hospital for kindly providing the dataset.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lin Q S-Editor: Gao CC L-Editor: Webster JR P-Editor: Wang LL
| 1. | Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA. 2017;317:1338-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1536] [Article Influence: 192.0] [Reference Citation Analysis (0)] |
| 2. | Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12:646-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 705] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 3. | Fagin JA, Wells SA Jr. Biologic and Clinical Perspectives on Thyroid Cancer. N Engl J Med. 2016;375:1054-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 641] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 4. | Eustatia-Rutten CF, Corssmit EP, Biermasz NR, Pereira AM, Romijn JA, Smit JW. Survival and death causes in differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2006;91:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 203] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 5. | Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1879] [Cited by in RCA: 1726] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 6. | Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De Vathaire F, Schlumberger M. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892-2899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1047] [Cited by in RCA: 1113] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 7. | Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10769] [Cited by in RCA: 9672] [Article Influence: 1074.7] [Reference Citation Analysis (1)] |
| 8. | Lamartina L, Deandreis D, Durante C, Filetti S. ENDOCRINE TUMOURS: Imaging in the follow-up of differentiated thyroid cancer: current evidence and future perspectives for a risk-adapted approach. Eur J Endocrinol. 2016;175:R185-R202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Triggiani V, Giagulli VA, Iovino M, De Pergola G, Licchelli B, Varraso A, Dicembrino F, Valle G, Guastamacchia E. False positive diagnosis on (131)iodine whole-body scintigraphy of differentiated thyroid cancers. Endocrine. 2016;53:626-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55806] [Article Influence: 7972.3] [Reference Citation Analysis (132)] |
| 11. | Barta JA, Powell CA, Wisnivesky JP. Global Epidemiology of Lung Cancer. Ann Glob Health. 2019;85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 679] [Cited by in RCA: 876] [Article Influence: 146.0] [Reference Citation Analysis (0)] |
| 12. | Kang DY, Lee HW, Choi PJ, Lee KE, Roh MS. Sodium/iodide symporter expression in primary lung cancer and comparison with glucose transporter 1 expression. Pathol Int. 2009;59:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Lin JD, Chao TC, Chou SC, Hsueh C. Papillary thyroid carcinomas with lung metastases. Thyroid. 2004;14:1091-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Lin JD, Lin SF, Chen ST, Hsueh C, Li CL, Chao TC. Long-term follow-up of papillary and follicular thyroid carcinomas with bone metastasis. PLoS One. 2017;12:e0173354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Rizzardi AE, Johnson AT, Vogel RI, Pambuccian SE, Henriksen J, Skubitz AP, Metzger GJ, Schmechel SC. Quantitative comparison of immunohistochemical staining measured by digital image analysis versus pathologist visual scoring. Diagn Pathol. 2012;7:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 317] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 16. | Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, Geisinger K, Hirsch FR, Ishikawa Y, Kerr KM, Noguchi M, Pelosi G, Powell CA, Tsao MS, Wistuba I; WHO Panel. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10:1243-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 3118] [Article Influence: 346.4] [Reference Citation Analysis (0)] |
| 17. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4397] [Article Influence: 549.6] [Reference Citation Analysis (4)] |
| 18. | Cibas ES, Ali SZ; NCI Thyroid FNA State of the Science Conference. The Bethesda System For Reporting Thyroid Cytopathology. Am J Clin Pathol. 2009;132:658-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1033] [Cited by in RCA: 1033] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 19. | Snoeckx A, Reyntiens P, Desbuquoit D, Spinhoven MJ, Van Schil PE, van Meerbeeck JP, Parizel PM. Evaluation of the solitary pulmonary nodule: size matters, but do not ignore the power of morphology. Insights Imaging. 2018;9:73-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 20. | Arturi F, Russo D, Schlumberger M, du Villard JA, Caillou B, Vigneri P, Wicker R, Chiefari E, Suarez HG, Filetti S. Iodide symporter gene expression in human thyroid tumors. J Clin Endocrinol Metab. 1998;83:2493-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Fernandez-Ulloa M, Maxon HR, Mehta S, Sholiton LJ. Iodine 131 uptake by primary lung adenocarcinoma. Misinterpretation of 131I scan. JAMA. 1976;236:857-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Acosta J, Chitkara R, Khan F, Azueta V, Silver L. Radioactive iodine uptake by a large cell undifferentiated bronchogenic carcinoma. Clin Nucl Med. 1982;7:368-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Langsteger W, Lind P, Költringer P, Beham A, Eber O. Misinterpretation of iodine uptake in papillary thyroid carcinoma and primary lung adenocarcinoma. J Cancer Res Clin Oncol. 1990;116:8-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Haubold-Reuter BG, Landolt U, von Schulthess GK. Bronchogenic carcinoma mimicking metastatic thyroid carcinoma. J Nucl Med. 1993;34:809-811. [PubMed] |
| 25. | Misaki T, Takeuchi R, Miyamoto S, Kasagi K, Matsui Y, Konishi J. Radioiodine uptake by squamous-cell carcinoma of the lung. J Nucl Med. 1994;35:474-475. [PubMed] |
| 26. | Malhotra G, Nair N, Menon H, Gujral S, Abhyankar A, Baghel NS, Awasare S, Nabar SJ, Abhyankar S, Kand PG. Bronchoalveolar carcinoma of lung masquerading as iodine avid metastasis in a patient with minimally invasive follicular carcinoma of thyroid. Clin Nucl Med. 2008;33:26-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Oh JR, Ahn BC. False-positive uptake on radioiodine whole-body scintigraphy: physiologic and pathologic variants unrelated to thyroid cancer. Am J Nucl Med Mol Imaging. 2012;2:362-385. [PubMed] |
| 28. | Chudgar AV, Shah JC. Pictorial Review of False-Positive Results on Radioiodine Scintigrams of Patients with Differentiated Thyroid Cancer. Radiographics. 2017;37:298-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Bybel B, Brunken RC, DiFilippo FP, Neumann DR, Wu G, Cerqueira MD. SPECT/CT imaging: clinical utility of an emerging technology. Radiographics. 2008;28:1097-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Grewal RK, Tuttle RM, Fox J, Borkar S, Chou JF, Gonen M, Strauss HW, Larson SM, Schöder H. The effect of posttherapy 131I SPECT/CT on risk classification and management of patients with differentiated thyroid cancer. J Nucl Med. 2010;51:1361-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Chen L, Luo Q, Shen Y, Yu Y, Yuan Z, Lu H, Zhu R. Incremental value of 131I SPECT/CT in the management of patients with differentiated thyroid carcinoma. J Nucl Med. 2008;49:1952-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | Maruoka Y, Abe K, Baba S, Isoda T, Sawamoto H, Tanabe Y, Sasaki M, Honda H. Incremental diagnostic value of SPECT/CT with 131I scintigraphy after radioiodine therapy in patients with well-differentiated thyroid carcinoma. Radiology. 2012;265:902-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Seemann MD, Staebler A, Beinert T, Dienemann H, Obst B, Matzko M, Pistitsch C, Reiser MF. Usefulness of morphological characteristics for the differentiation of benign from malignant solitary pulmonary lesions using HRCT. Eur Radiol. 1999;9:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Winer-Muram HT. The solitary pulmonary nodule. Radiology. 2006;239:34-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 35. | Purandare NC, Rangarajan V. Imaging of lung cancer: Implications on staging and management. Indian J Radiol Imaging. 2015;25:109-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Knut and Alice Wallenberg foundation. The Human Protein Atlas. Expression of SLC5A5 in lung cancer. [cited September 8, 2020]. Available from: https://www.proteinatlas.org/ENSG00000105641-SLC5A5/pathology/Lung+cancer. |
| 37. | Heufelder AE, Morgenthaler N, Schipper ML, Joba W. Sodium iodide symporter-based strategies for diagnosis and treatment of thyroidal and nonthyroidal malignancies. Thyroid. 2001;11:839-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Arai T, Kuroishi T, Saito Y, Kurita Y, Naruke T, Kaneko M. Tumor doubling time and prognosis in lung cancer patients: evaluation from chest films and clinical follow-up study. Japanese Lung Cancer Screening Research Group. Jpn J Clin Oncol. 1994;24:199-204. [PubMed] |
| 39. | Kanashiki M, Tomizawa T, Yamaguchi I, Kurishima K, Hizawa N, Ishikawa H, Kagohashi K, Satoh H. Volume doubling time of lung cancers detected in a chest radiograph mass screening program: Comparison with CT screening. Oncol Lett. 2012;4:513-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |