Published online Jan 6, 2021. doi: 10.12998/wjcc.v9.i1.267
Peer-review started: October 1, 2020
First decision: November 3, 2020
Revised: November 9, 2020
Accepted: November 21, 2020
Article in press: November 21, 2020
Published online: January 6, 2021
Processing time: 92 Days and 4.9 Hours
Immunoglobulin G4-related disease (IgG4-RD) is a multi-system fibroin-flammatory disorder that can involve any organ, including the salivary glands, pancreas, and biliary tree. Treatment of immunoglobulin G4-related sclerosing cholangitis (IgG4-SC) is similar to that for IgG4-RD, but progression is irreversible in some cases. We present a case of IgG4-SC in which an immuno-suppressant induced marked clinical and radiologic improvement.
A 63-year-old male presented with a prominent itching sensation and wholebody jaundice. He showed obstructive-pattern jaundice, an elevated IgG4 level, and infiltration of a large number of IgG4-positive cells in the ampulla of Vater. The imaging findings of intrahepatic duct (IHD) and common bile duct dilation, an elevated serum IgG4 level, and characteristic histological findings led to diagnosis of IgG4-SC that compatible with the 2019 ACR/EULAR classification criteria. We planned to treat the patient with high-dose glucocorticoid (GC), followed by cyclophosphamide pulse therapy. After treatment with high-dose GC and an immunosuppressant, imaging studies showed that IHD dilatation had completely resolved.
Prompt diagnosis and appropriate treatment of IgG4-SC are important. Because there is a risk of relapse of IgG4-SC, the GC dose should be gradually reduced, and a maintenance immunosuppressant should be given.
Core Tip: In this paper, we presented immunoglobulin G4-related sclerosing cholangitis (IgG4-SC) which was diagnosed by ACR/EULAR published new classification criteria and was successfully treated using a glucocorticoid with an immunosuppressant and made approved as imaging follow-up. Prompt diagnosis and appropriate treatment of IgG4-SC are important to induce rapid regression and to prevent irreversible fibrosis and biliary stricture. This case emphasizes the proactive attitude to use immuno-suppressants in treatment with IgG4-SC.
- Citation: Kim JS, Choi WH, Lee KA, Kim HS. Immunosuppressant treatment for IgG4-related sclerosing cholangitis: A case report. World J Clin Cases 2021; 9(1): 267-273
- URL: https://www.wjgnet.com/2307-8960/full/v9/i1/267.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i1.267
Immunoglobulin G4-related disease (IgG4-RD) is an immune-mediated disorder that can induce fibrosis in multiple organs[1]. IgG4-related sclerosing cholangitis (IgG4-SC) is the most common extrapancreatic manifestation of IgG4-RD, and it is the third distinct entity of sclerosing cholangitis[2]. Treatment of IgG4-SC is similar to that for IgG4-RD involving autoimmune pancreatitis (AIP), but progression is irreversible in some cases. We demonstrate a case of IgG4-SC in which an immunosuppressant induced marked clinical and radiologic improvement.
A 63-year-old male was admitted to our hospital with an uncontrolled itching sensation and whole body jaundice.
Six months prior he had experienced intractable itching, which led to suspicion of obstructive cholangitis. Despite endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic retrograde biliary drainage, which ruled out malignancy, the itching became aggravated and obstructive-pattern jaundice developed.
The patient’s medical history was non-specific with the exception of hypertension and diabetes mellitus, which were well controlled by medication. He was ex-smoker of ten years and a social drinker.
No previous illnesses were reported and there was no family history of oncologic and rheumatic diseases.
The physical examination revealed icteric sclerae with multiple itching scratches on the skin.
The patient’s laboratory results were as follows: Hemoglobin 10 g/dL (normal, 13-17 g/dL), hematocrit 30.6% (normal 39%-52%), platelet count 348000/L (normal, 130000-450000/L), aspartate aminotransferase 71 U/L (normal, 0-40 U/L), alanine aminotransferase level 44 U/L (normal, 0-41 U/L), gamma-glutamyl transferase level 233 U/L (normal, 5-61 U/L), alkaline phosphatase level 315 U/L (normal, 35-130 U/L), total bilirubin level 8.6 mg/dL (normal, 0-1.2 mg/dL), BUN level 26.7 mg/dL (normal, 6-20 mg/dL), creatinine level 1.37 mg/dL (normal, 0.5-1.2 mg/dL), erythrocyte sedimentation rate 120 mm/h (normal, 0-20 mm/h), C reactive protein (CRP) level 1.81 mg/dL (normal, 0-0.5 mg/dL), antinuclear antibody titer 1:2560 (nucleolar pattern), IgG level 2416 mg/dL (normal, 700-1600 mg/dL), and serum IgG4 level 465 mg/dL (normal, 0-135 mg/dL).
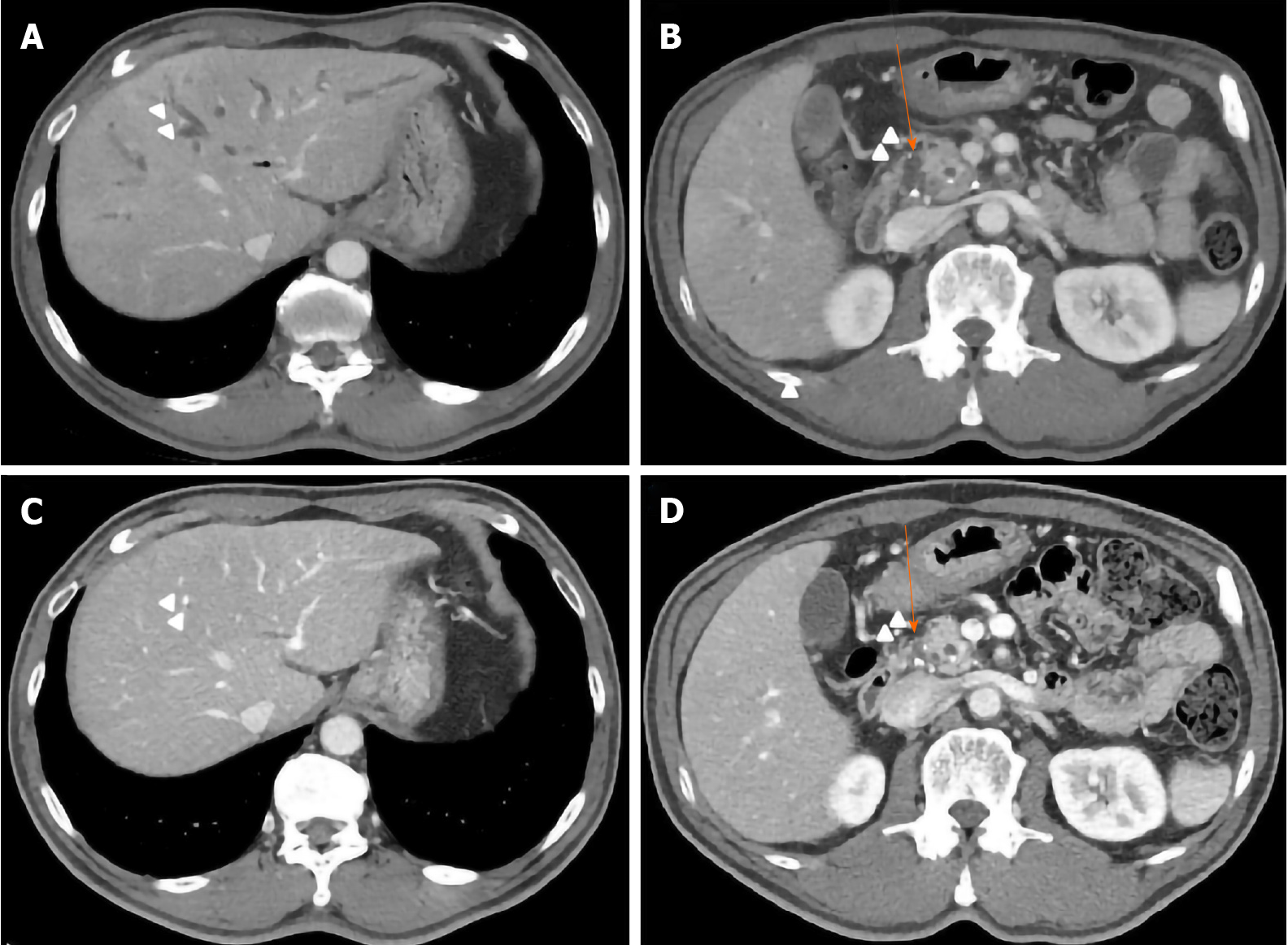
Abdominal computed tomography (CT) showed intrahepatic duct (IHD) dilatation with intraductal soft tissue attenuation and periductal enhancement (Figure 1A). The common bile duct (CBD) was dilated with mild luminal narrowing but without findings of AIP (Figure 1B). There was no definite obstructive lesion or suspected malignancy. CT also showed fibrotic enhancement around the infrarenal aorta.
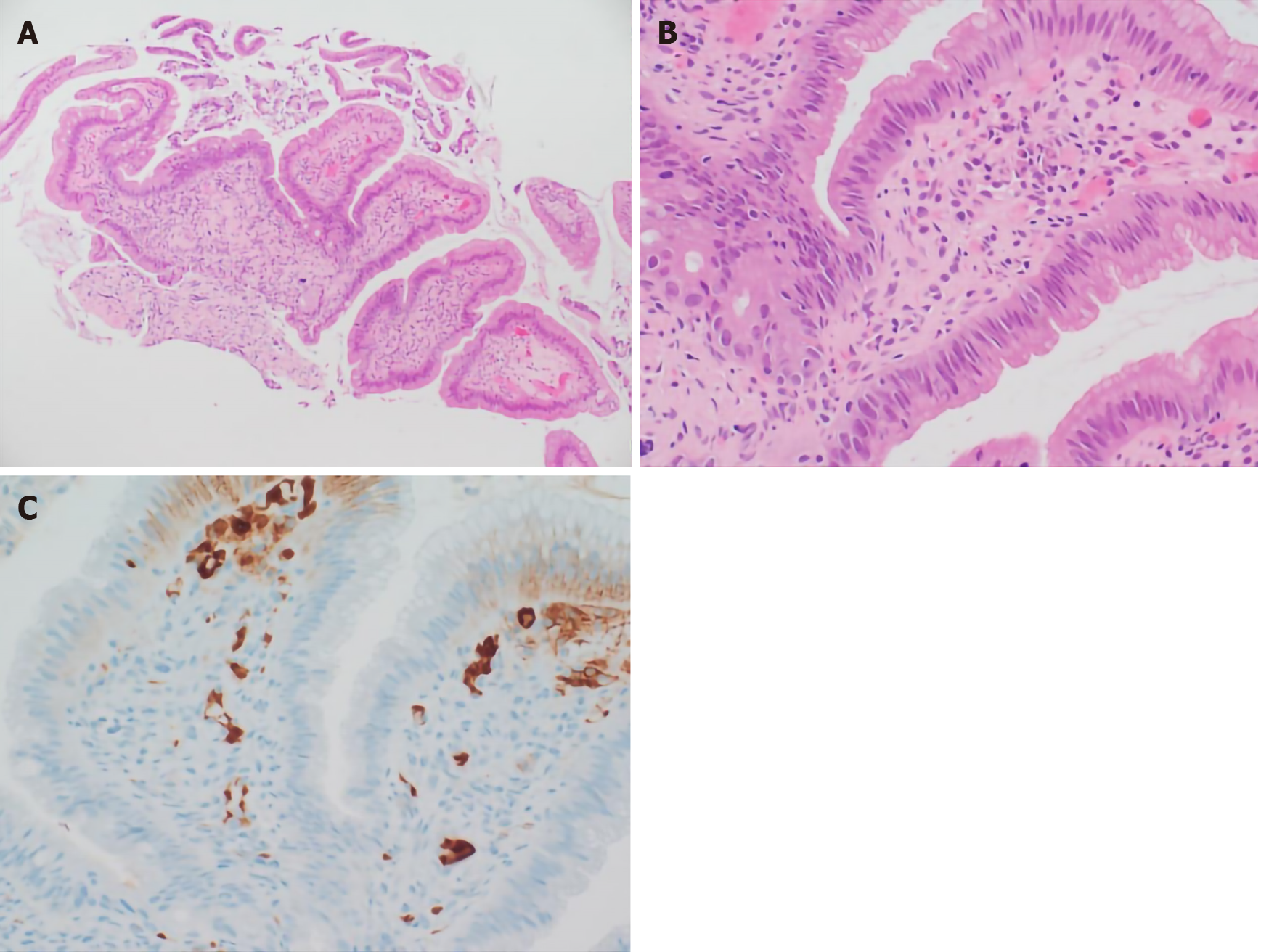
We performed ERCP to obtain an ampulla of Vater (AoV) biopsy for histologic analysis. The AoV biopsy revealed infiltration of inflammatory cells and granulation and many plasma cells in subepithelial stroma (Figure 2A and B). Immunohisto-chemical staining revealed a large number of infiltrating IgG4-positive cells (Figure 2C).
The imaging findings of IHD and CBD dilation, an elevated serum IgG4 level, and characteristic histological findings led to diagnosis of IgG4-SC.
We planned to treat the patient with high-dose systemic glucocorticoid (GC, prednisolone 0.5 mg/kg/d), followed by cyclophosphamide pulse therapy (1000 mg/mo, 6 mo).
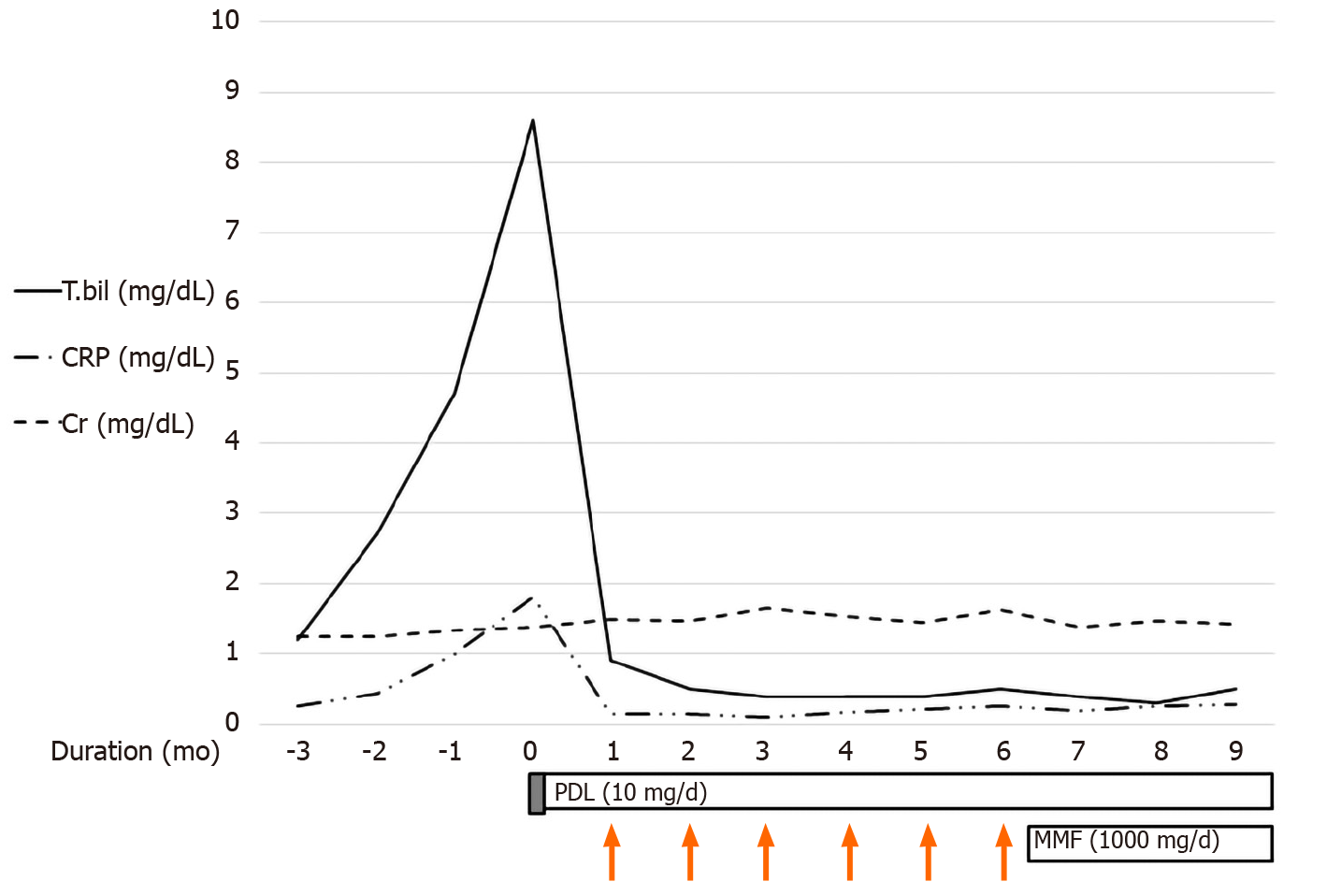
After 3 mo, CT showed that the IHD dilatation with intraductal soft tissue attenuation was almost completely resolved (Figure 1C). CT also showed slight regression of the CBD dilatation with mild luminal narrowing (Figure 1D) and no significant change in the soft tissue attenuation along the infrarenal abdominal aorta. Moreover, the total bilirubin and CRP levels had returned to within the normal ranges. On this basis we tapered the GC to 10 mg/d and added mycophenolate mofeltil (1000 mg/d) for maintenance (Figure 3). There was no recurrence of itching or jaundice at the 1 year follow up. Patient has provided informed consent for publication of the clinical process and images.
IgG4-RD has an immune-mediated fibrotic progression involving multiple organs and has myriad clinical manifestations[2,3]. IgG4-SC is a biliary manifestation of the systemic disorder IgG4-RD. IgG4-SC occurs in one-third of patients with type 1 AIP[4]. The prevalence of isolated IgG4-SC is less than 10% that of IgG4-RD[5]. The cholangio-graphic findings of IgG4-SC involving the hila and/or IHD are similar to those of hilar cholangiocarcinoma or primary sclerosing cholangitis (PSC). Therefore, it is important to differentiate IgG4-SC from the latter two conditions[6]. PSC is a chronic progressive disease with a poor prognosis and does not respond to GC therapy. In our case, IgG4-SC was not accompanied by AIP and could be differentiated from cholangiocarcinoma and PSC.
The Histology, Imaging, Serology, other Organ involvement and Response to therapy (HISORt) criteria of the Mayo Clinic are widely used for diagnosis of IgG4-RD, including IgG4-SC. The HISORt criteria include positive imaging findings, characteristic histological presentations, elevated serum IgG4 level, extrabiliary organ involvement, and a response to GC treatment[5]. The ACR/EULAR published new classification criteria for confident decision into more homogeneous group. The classification process comprises three steps. First, a potential IgG4 RD case must show involvement of at least 1 of 11 possible organs. Second, the case must not meet any of the 32 clinical, serologic, radiologic, and pathologic exclusion criteria. Third, the case must meet eight weighted inclusion criteria[7]. A threshold of 20 points has a specificity of 99.2% and a sensitivity of 85.5% for diagnosis of IgG4 RD; our patient’s score was 48 points. That score comprised the following: Histopathology, 6 points (lymphocytic infiltrate and obliterative phlebitis); immunostaining, 15 points (IgG4:IgG ratio 41%-70% and > 10 IgG4-positive cells/high power field); serum IgG4 concentration, 6 points (two- to five-fold the upper limit of normal); pancreas and biliary tree, 19 points (pancreas and biliary tree involvement); and retroperitoneal involvement, 4 points (diffuse thickening of the abdominal aortic wall). Thus, our patient met both the HISORt and the 2019 ACR/EULAR classification criteria.
The goal of IgG4-SC treatment is to induce rapid regression and to prevent irreversible fibrosis and biliary stricture. The majority of IgG4-RD patients respond to GC therapy. In this case the initial dose was prednisolone 30 to 40 mg/d for 1 mo, followed by tapering over 2 wk, then tapering of 5 mg/d for 3 to 4 mo. The final maintenance prednisolone dose was 5 mg/d. In some IgG4-SC case series, complete resolution of strictures and/or normalization of liver function were reported in up to 67% of the patients[8]. Patients with IgG4-SC are at high risk of relapse; the relapse rate is around 57%. Proximal strictures and a high serum IgG4 level are predictive of relapse. The maintenance option is typically low-dose corticosteroid, with or without immunosuppressive therapy with azathioprine or mycophenolate mofetil[9,10]. The treatment options for IgG4-SC relapse include increasing the dose of, or reintroducing, GC and addition of a second-line immunosuppressant; rituximab is an effective alternative and can lead to both substantial reduction of serum IgG4 concentration and clinical improvement. This agent works by depleting the pool of B cells that replenish the IgG4-secreting plasma cells[11]. In a prospective study of IgG4-RD, the rituximab response rate was 97%, and 77% and 46% of the patients were able to stop GC therapy and had not relapsed after 6 and 12 mo, respectively[12].
We report a case of IgG4-SC that met the 2019 ACR/EULAR IgG4-RD classification criteria and was successfully treated using a GC with an immunosuppressant and made approved as imaging follow-up.
The authors thanks to all colleagues involved in the management of this case.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Salvadori M S-Editor: Huang P L-Editor: A P-Editor: Ma YJ
| 1. | Goodchild G, Pereira SP, Webster G. Immunoglobulin G4-related sclerosing cholangitis. Korean J Intern Med. 2018;33:841-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1856] [Cited by in RCA: 1870] [Article Influence: 143.8] [Reference Citation Analysis (83)] |
| 3. | Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, Okamoto A, Egawa N, Nakajima H. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1011] [Cited by in RCA: 984] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 4. | Topazian M, Witzig TE, Smyrk TC, Pulido JS, Levy MJ, Kamath PS, Chari ST. Rituximab therapy for refractory biliary strictures in immunoglobulin G4-associated cholangitis. Clin Gastroenterol Hepatol. 2008;6:364-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Huggett MT, Culver EL, Kumar M, Hurst JM, Rodriguez-Justo M, Chapman MH, Johnson GJ, Pereira SP, Chapman RW, Webster GJM, Barnes E. Type 1 autoimmune pancreatitis and IgG4-related sclerosing cholangitis is associated with extrapancreatic organ failure, malignancy, and mortality in a prospective UK cohort. Am J Gastroenterol. 2014;109:1675-1683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 182] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 6. | Detlefsen S, Klöppel G. IgG4-related disease: with emphasis on the biopsy diagnosis of autoimmune pancreatitis and sclerosing cholangitis. Virchows Arch. 2018;472:545-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Wallace ZS, Naden RP, Chari S, Choi HK, Della-Torre E, Dicaire JF, Hart PA, Inoue D, Kawano M, Khosroshahi A, Lanzillotta M, Okazaki K, Perugino CA, Sharma A, Saeki T, Schleinitz N, Takahashi N, Umehara H, Zen Y, Stone JH; Members of the ACR/EULAR IgG4-RD Classification Criteria Working Group. The 2019 American College of Rheumatology/European League Against Rheumatism classification criteria for IgG4-related disease. Ann Rheum Dis. 2020;79:77-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 394] [Article Influence: 78.8] [Reference Citation Analysis (1)] |
| 8. | Ghazale A, Chari ST, Zhang L, Smyrk TC, Takahashi N, Levy MJ, Topazian MD, Clain JE, Pearson RK, Petersen BT, Vege SS, Lindor K, Farnell MB. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134:706-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 585] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 9. | Sandanayake NS, Church NI, Chapman MH, Johnson GJ, Dhar DK, Amin Z, Deheragoda MG, Novelli M, Winstanley A, Rodriguez-Justo M, Hatfield AR, Pereira SP, Webster GJ. Presentation and management of post-treatment relapse in autoimmune pancreatitis/immunoglobulin G4-associated cholangitis. Clin Gastroenterol Hepatol. 2009;7:1089-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385:1460-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 794] [Cited by in RCA: 849] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 11. | Khosroshahi A, Bloch DB, Deshpande V, Stone JH. Rituximab therapy leads to rapid decline of serum IgG4 levels and prompt clinical improvement in IgG4-related systemic disease. Arthritis Rheum. 2010;62:1755-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 407] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 12. | Carruthers MN, Topazian MD, Khosroshahi A, Witzig TE, Wallace ZS, Hart PA, Deshpande V, Smyrk TC, Chari S, Stone JH. Rituximab for IgG4-related disease: a prospective, open-label trial. Ann Rheum Dis. 2015;74:1171-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 467] [Article Influence: 46.7] [Reference Citation Analysis (0)] |