Published online Jan 6, 2021. doi: 10.12998/wjcc.v9.i1.252
Peer-review started: September 12, 2020
First decision: September 24, 2020
Revised: October 6, 2020
Accepted: October 27, 2020
Article in press: October 27, 2020
Published online: January 6, 2021
Processing time: 111 Days and 2.9 Hours
Debate exists regarding the use of thermal ablation (TA) to treat papillary thyroid carcinoma (PTC). Some studies have recommended TA as a new, efficient and safe technology for PTC. In this article, we report one case of a residual tumor and central lymph node metastasis (CLNM) after TA for PTC.
A 63-year-old female underwent bilateral ultrasound (US)-guided radiofrequency ablation for PTC. Three months later, she was diagnosed as thyroid cancer with suspected CLNM by US and contrast-enhanced computed tomography. The subsequent fine-needle aspiration (FNA) biopsies were negative. Due to her strong personal preference, she underwent total thyroidectomy and central lymph node dissection. Local tissue adhesion and a difficult dissection were noted during the operation. The pathology of the frozen sections during the operation was still negative. The final pathology results of paraffin-embedded sections revealed residual tumor cells at the edge of the PTC and CLNM.
TA may lead to a residual tumor in patients with PTC. Follow-up using US and FNA biopsy may not be adequate to evaluate the residual tumor. TA should be carefully considered in PTC treatment.
Core Tip: Thermal ablation may lead to a residual tumor in patients with papillary thyroid carcinoma. Follow-up using ultrasound and fine-needle-aspiration biopsy may not be adequate to evaluate the residual tumor. Therefore, thermal ablation of papillary thyroid carcinoma should be carefully considered.
- Citation: Hua Y, Yang JW, He L, Xu H, Huo HZ, Zhu CF. Residual tumor and central lymph node metastasis after thermal ablation of papillary thyroid carcinoma: A case report and review of literature. World J Clin Cases 2021; 9(1): 252-261
- URL: https://www.wjgnet.com/2307-8960/full/v9/i1/252.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i1.252
Recently, thermal ablation (TA) has become an acceptable treatment for benign thyroid nodules, and it has also been used to treat distant metastasis and locoregional recurrence of thyroid cancer. TA was described as an alternative treatment for primary papillary thyroid carcinoma (PTC), particularly in patients who were unwilling or unable to undergo surgery[1-6], and TA was considered an efficacious and safe treatment with rare reports of residual tumor cells, recurrence, and metastasis. In this article, we report one patient with PTC who presented with a residual tumor and central lymph node metastasis (CLNM) after TA.
A 63-year-old female who presented with neck discomfort was admitted to our hospital.
She was diagnosed as bilateral PTC and received bilateral radiofrequency ablation (RFA) at another hospital on March 1, 2019. Prior to RFA, she received ultrasound (US) and fine-needle aspiration (FNA). US showed bilateral multiple thyroid nodules. The nodule in the left lobe was 4.2 mm × 5.2 mm × 4 mm in size, with irregular margins and calcification, and was classified as Thyroid Imaging Reporting and Data System (TI-RADS) 4a. The nodule in the right lobe, which was 11.6 mm × 6.4 mm × 9.3 mm in size, was classified as TI-RADS 3. No lymph node metastasis was detected. FNA biopsy revealed bilateral PTC. She was diagnosed as PTC (T1N0M0), and she subsequently received bilateral RFA.
No special history of past illness was recorded.
The patient denied family history of thyroid disease or malignancy.
No obviously palpable thyroid nodules and suspected lymph nodes could be touched on physical examination.
No abnormal Laboratory examinations, including thyroid function, were detected(.
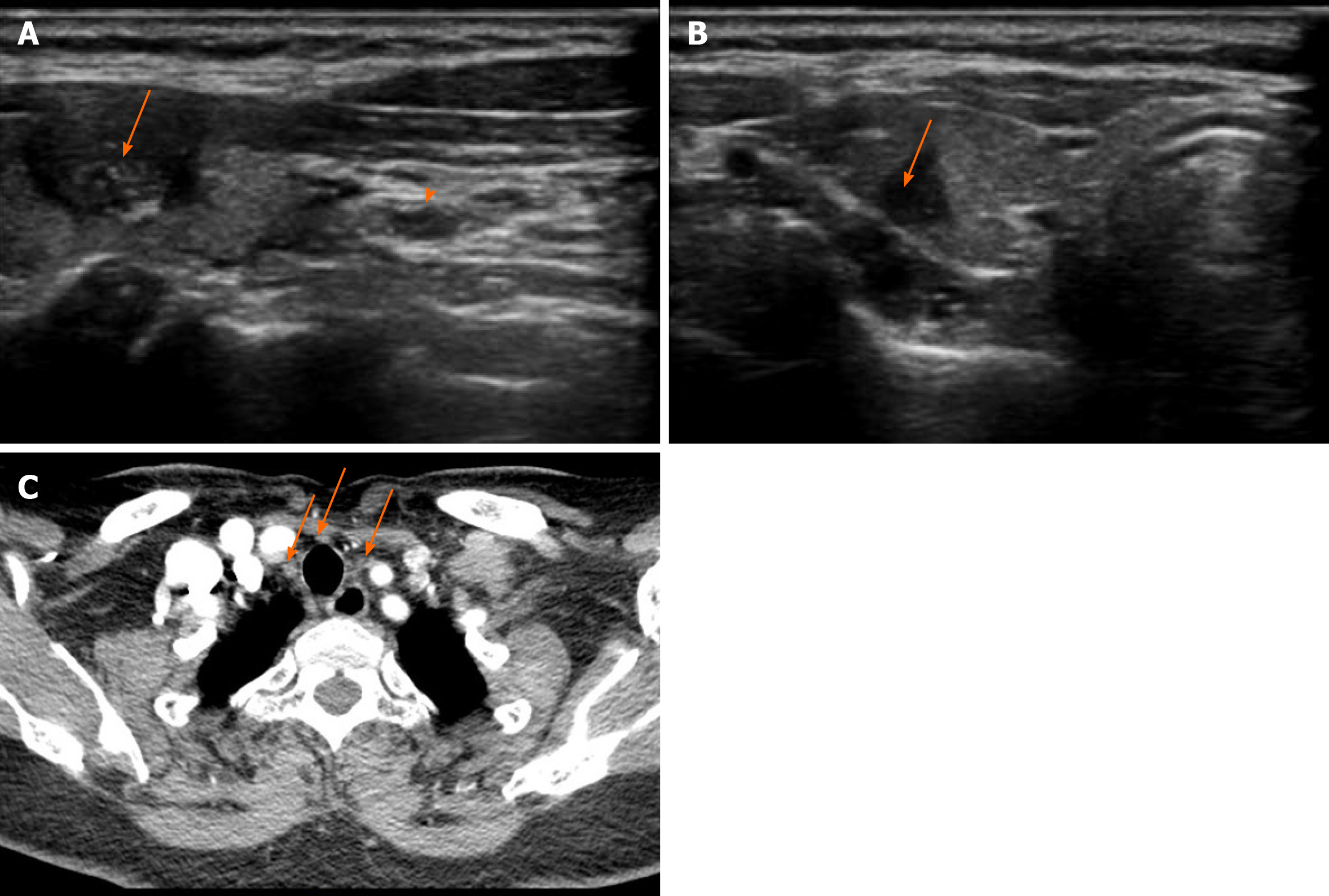
Both US and contrast-enhanced computed tomography performed at our hospital showed bilateral suspected thyroid cancer with CLNM (Figure 1). The nodule in the left lobe (6 mm × 8 mm × 12 mm) with irregular margins and calcification was classified as TI-RADS 4c, and the nodule in the right lobe (5 mm × 5 mm × 7 mm) with unclear margins was classified as TI-RADS 4a by US.
The patient underwent FNA biopsy again, but no PTC cells were detected.
Based on the patient’s strong willingness to undergo a subsequent operation, she underwent total thyroidectomy with central lymph node dissection. In the operation, the thyroid tissue was extensively adhered to the surrounding tissue, and the thyroid tissue, parathyroid tissue, and recurrent laryngeal nerve were relatively difficult to be dissected. The pathology of frozen sections obtained during the operation still revealed no residual PTC cells.
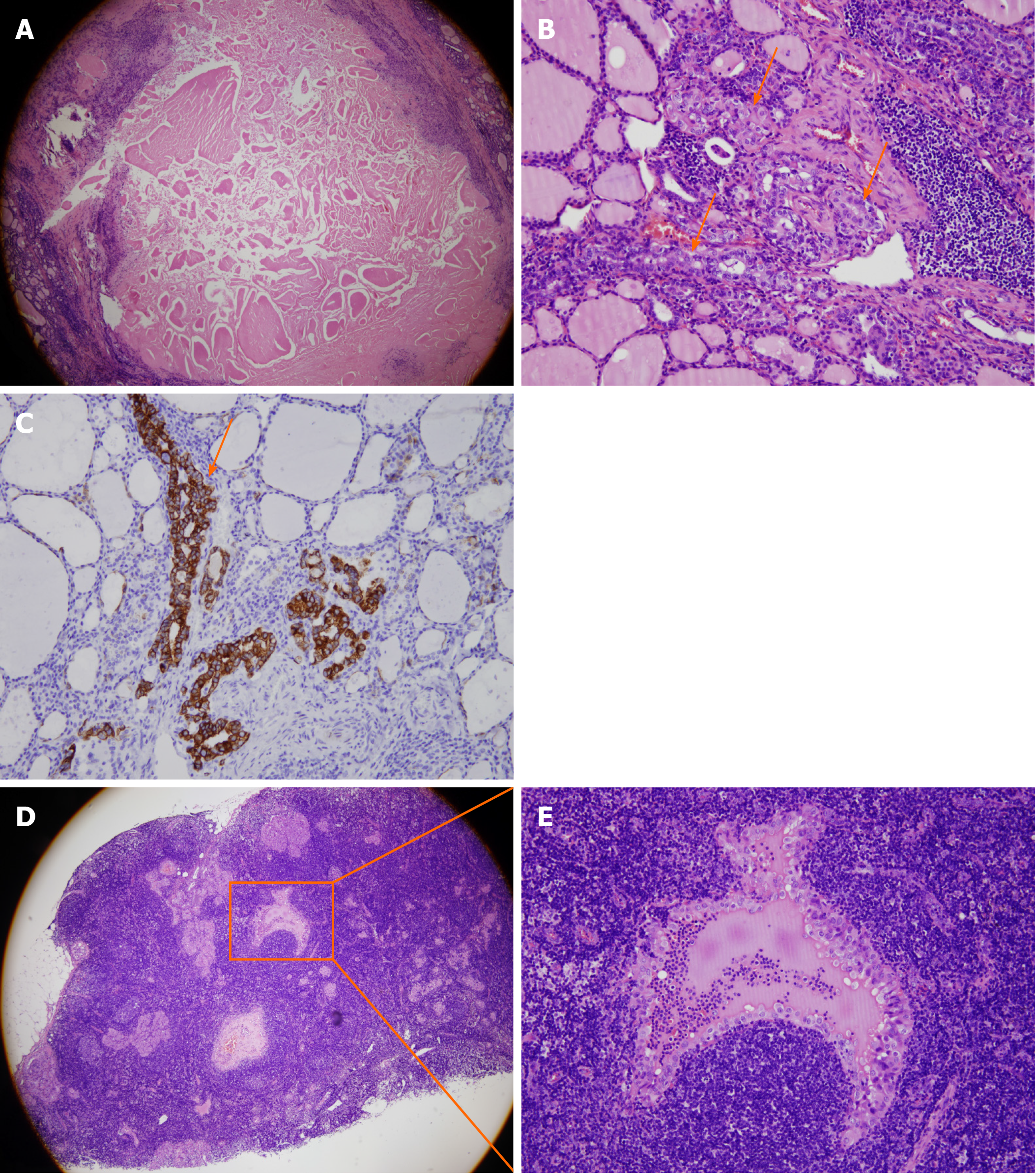
The final pathology of paraffin-embedded sections revealed residual tumor cells along the edge of the tumor and CLNM (4/14+) (Figure 2). The patient was diagnosed as bilateral PTC with CLNM, stage I (T1N1M0) after operation.
The patient was treated with levothyroxine suppression therapy after surgery.
The patient was discharged from the hospital when her condition was stable. No recurrences or metastases were reported at the time of manuscript submission.
The Medline (PubMed) and Cochrane Library were searched using the following search terms: “Papillary thyroid carcinoma”, “radiofrequency ablation”, “thermal ablation”, “laser ablation”, “microwave ablation”, and/or “residual tumor”. This search was performed independently by two reviewers. Regions were not limited. The languages of reference were only English and Chinese, and the references published before April 1, 2020 were collected in this study.
Since the 1990s, TA, including RFA, laser ablation (LA), and microwave ablation (MWA), has been used to treat solid organ tumors, such as liver carcinoma[7], renal cancer[8], lung cancer[9], and prostate cancer[10]. To date, TA has been adopted by National Comprehensive Cancer Network guidelines for some solid tumors. Regarding the use of ablation for thyroid tumors, Pacella et al[11] first reported the use of LA to treat two patients with autonomously functioning thyroid nodules in 2000. Dupuy et al[12] used RFA to treat the regional recurrence of well-differentiated thyroid cancer in 2001. Kim et al[13] first succeeded in using RFA to treat benign thyroid nodules in 2006. Jeong et al[14] showed an 84.1% shrinkage in the average volume of benign thyroid nodules after RFA in 236 patients. Zhang et al[1] reported a prospective study of 98 papillary thyroid microcarcinomas (PTMCs) in 92 patients that explored the efficacy and safety of US-guided RFA for the treatment of low-risk PTMC. No residual tumors, recurrence, or metastasis was detected during the 18-mo follow-up using US, contrast-enhanced US (CEUS), or core-needle biopsy (CNB).
The 2017 Thyroid Radiofrequency Ablation guidelines in Korea[15] recommended the use of RFA for patients with benign thyroid nodules complaining of symptomatic or cosmetic problems, patients with recurrent thyroid cancer at high surgical risk or who refused surgery, and, alternatively, patients with primary thyroid cancer who refused surgery or were unable to undergo an operation. The Italian consensus statement on minimally invasive treatments for benign thyroid nodules[16] suggested that TA may be proposed as a first-line treatment for solid non-functioning thyroid nodules that are benign according to cytology when they cause symptoms. The 2015 American Thyroid Association guidelines[17] proposed TA as a choice for patients with PTC presenting with metastatic disease and suggested that responsiveness to TA is associated with improved survival in patients with distant metastases to various organs; the guidelines also proposed that RFA shows high efficacy in treating individual distant metastases of advanced thyroid cancer with relatively few side effects. A 2018 Chinese expert consensus[18] recommended TA as a treatment for benign thyroid nodules, but the routine use of TA for papillary thyroid microcarcinoma has not been established due to insufficient medical evidence. China also permits TA to be conducted by experienced doctors with more than 2 years of experience performing TA in the thyroid. The preliminary common indications include the following: A thyroid nodule diameter less than 5 mm (or less than 10 mm without invasion of the capsule), PTC confirmed by FNA biopsy before surgery, no cervical lymph node metastasis or distant metastasis, no history of neck irradiation, and anxiety or a desire for minimally invasive treatment.
Numerous studies have been conducted to explore the efficacy and safety of TA for PTC. The PubMed database was searched to identify publications on thermal ablation in treating PTC. The following search terms were used: [(papillary thyroid carcinoma) AND (radiofrequency ablation OR laser ablation OR microwave ablation OR thermal ablation)]. The detailed characteristics of the searched studies are shown in Table 1. Four of them are randomized controlled trials. Eight studies investigated RFA[1,2,19-24], four explored LA[25-28], and six studied MWA[5,6,29-32]. The cohort sizes ranged from six to 421 patients, with the numbers of PTC nodules ranging from six to 440 total nodules. All TA-treated subjects included were ineligible for surgery or refused surgery, except in the study by Luo et al[20] (the inclusion criteria were not mentioned). The main follow-up examinations included US, CEUS, computed tomography, and FNA biopsy or CNB. Yue et al[5] reported three cases of immediate thyroidectomy after MWA, and complete necrosis of the tumor was confirmed. The follow-up periods in these studies ranged from 2 mo to 104 mo. The results from eight studies[1,2,5,6,19,22,23,29] were satisfactory, with no residual cancer cells, recurrences, or metastases. Furthermore, comparisons of the efficacy and safety of RFA, LA, or MWA and surgery for the treatment of PTMC showed that the performance of TA was not inferior to surgery regarding the therapeutic effect, incidence of complications, recurrences, metastases, and disease-free survival[28,29,31,33]. According to recently published reviews and meta-analyses, all types of TA had reasonable safety and efficacy profiles for the treatment of PTMC[34-38]. Choi et al[37] reported that LA was less effective than RFA at reducing the volume of PTMC. Cho et al[38] concluded that TA provides excellent local tumor control in low-risk patients with PTMC, but strict inclusion criteria and technical expertise are required to obtain favorable results. However, the limitations of these studies include an insufficient evidence level, inaccurate comparisons due to different baseline data of the enrolled patients, inconsistent parameters for US-guided ablation, short follow-up durations, and ambiguous clinical risk stratification of PTMC due to insufficient prognostic factors.
| Technique | Ref. | No. of patients/tumors | Follow-up periods/mo, mean ± SD (range) | Follow-up examinations | No. of incomplete ablation | No. of recurrence/mo, post-TA | No. of cervical lymph node metastasis/mo, post-TA |
| RFA | Zhang et al[1], 2016 | 92/98 | 7.8 ± 2.9 (3-18) | US, CEUS, and CNB | 0 | 0 | 0 |
| RFA | Kim et al[19], 2017 | 6/6 | 48.5 ± 12.3 (36-65) | FNA and CNB | 0 | 0 | 0 |
| RFA | Luo et al[20], 2017 | 421/440 | N/A (12-36) | US, CEUS and biopsy | 0 | 4 proven; 5 suspicious (N/A) | 4 (N/A) |
| RFA | Jeong et al[21], 2018 | 6/7 | 19.3 ± 3.5 (15-24) | US and CT | 1 | 0 | 0 |
| RFA | Zhang et al[22], 2019 | 60/60 | N/A (18-60) | US, CEUS, and CNB | 0 | 0 | 0 |
| RFA | Ding et al[23], 2019 | 37/38 | N/A (1-18) | CEUS and CT | 0 | 0 | 0 |
| RFA | Lim et al[2], 2019 | 133/152 | 39 ± 25 (6-104) | US, CT, and biopsy | 0 | 0 | 0 |
| RFA1 | Zhang et al[24], 2020 | 94/94 | 64.2 ± 2.8 (median ± SD) | US, CEUS, CNB, CT, PET, and bone scan | 0 | 1 (N/A) | 0 |
| LA | Zhou et al[25], 2017 | 30/30 | 13.2 (12–24) | US, CEUS, chest X-ray or CT, and FNA | 1 | 0 | 0 |
| LA | Zhang et al[26], 2018 | 64/64 | 25.7 ± 8.2 (12-42) | US, CEUS, and FNA | 2 | 0 | 1 (30) |
| LA | Ji et al[27], 2019 | 37/37 | 16.5 ± 6.9 (12-24) | US, CEUS, and chest radiographs or CT | 8 | 0 | 1 (24) |
| LA1 | Zhou et al[28], 2019 | 36/36 | 49.2 ± 4.5 (30-54) | US, chest X-ray or CT, and FNA | 0 | 1 (42) | 1 (30) |
| MWA | Yue et al[5], 2014 | 21/21 | 11 (3-22) | US, chest X-ray or CT, and biopsy | 0 | 0 | 0 |
| MWA1 | Li et al[29], 2018 | 46/46 | 42 | US, CEUS, and biopsy | 0 | 0 | 0 |
| MWA | Teng et al[6], 2018 | 15/21 | N/A (36-48) | US, CT, and FNA | 0 | 0 | 0 |
| MWA | Teng et al[30], 2019 | 185/206 | 20.7 ± 8.8 (12-36) | US and FNA | 0 | 1 (1) | 0 |
| MWA1 | Li et al[31], 2019 | 168/168 | 25.1 ± 17.3 (2-60) | US and FNA | 0 | 2 (36-44) | 5 (6-44) |
| MWA | Yue et al[32], 2020 | 119/119 | 37.2 ± 20.9 (12-101) | US, CEUS, CNB, and CT | 0 | 0 | 1 (26) |
Few articles have reported problems after TA, such as a residual tumor, needle track seeding, incidental papillary microfoci, lymph node micrometastasis, and possible carcinogenic effects. Incomplete ablation was observed in one RFA study and three LA studies, and second ablations were conducted[21,25-27]. A biopsy confirmed that recurrences and cervical lymph node metastases occurred after the use of all three techniques[20,24,26-28,30-32]. The shortest follow-up durations during which recurrence and cervical lymph node metastasis were detected were 1 mo[30] and 6 mo[31], respectively. Notably, the 1 mo recurrence was another small nodule that had not been detected before MWA. Patients with recurrence or cervical lymph node metastases underwent a second RFA or surgery. Ma et al[39] reported 11 patients with PTC treated with RFA and one patient with PTC treated with MWA who were surgically confirmed to have residual PTC cells (12/12) and lymph node metastases (8/12). In the study by Kim et al[40], one patient underwent robotic thyroid surgery after incomplete RFA for PTC, but the patient had not been diagnosed before RFA. Lee et al[41] observed needle track PTC seeding after a second RFA for thyroid cancer that appeared benign. Ruzzenente et al[42] and Koda et al[43] also reported needle track seeding and rapid tumor progression after RFA for hepatocellular carcinoma. Valcavi et al[4] found two cases of incidental papillary microfoci and one case of lymph node micrometastasis during immediate thyroidectomy surgery after LA for PTMC. Ergul et al[44] reported a rare case of mixed papillary and medullary thyroid carcinoma, which might have occurred due to LA for a benign thyroid nodule 2 years previously.
In our case, tumor shrinkage of bilateral PTC was also detected on follow-up US, but the TI-RADS classifications of the bilateral nodules after TA were still 4. The pathology of the sample obtained through repeated FNA biopsy was negative. However, residual tumor cells were detected at the edge of the PTC. Therefore, we should consider whether US, CEUS, and biopsy are adequate to evaluate the thoroughness of TA. Actually, changes in the tumor size or disappearance on US should not be used to confirm the complete ablation of the tumor. Moreover, the middle portions of the bilateral tumors were completely ablated, while residual tumor cells were mainly located along the edges. In US-guided TA, doctors may generally focus on the middle portion of the nodules. This focus may explain why the pathology results of the FNA biopsy and frozen sections were negative. Metastatic lymph nodes were only detected 3 mo later and may be due to a missed diagnosis before TA or the progression of residual cancer cells after TA. The diagnostic accuracy of US for cervical lymph node metastases in patients with PTC, depending on the experience of the radiologist, was reported to range from 55.8% to 95.2%[45-50]. US showed worse performance in identifying CLNM than lateral lymph node metastases[51]. Therefore, the use of US alone to determine the presence of lymph node metastasis before TA is not sufficient. In addition, because TA exacerbated the adhesion of the thyroid tissue to the surrounding tissue, the dissection of the thyroid tissue, parathyroid tissue, and recurrent laryngeal nerve was difficult. The complication rate might be relatively higher after thyroidectomy. In summary, the efficacy and safety of TA for PTC must be carefully considered. The use of US, CEUS, and CNB or FNA biopsy as follow-up assessments for the efficiency of TA may be insufficient. Additional prospective, multi-center, randomized controlled studies with large populations and longer follow-up periods are necessary in the future.
In conclusion, TA is unable to guarantee complete PTMC ablation or resolve cervical lymph node metastases, the current follow-up methods are not reliable, and subsequent surgery is more difficult after TA. Therefore, TA is not recommended as a routine treatment for PTMC.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang Y S-Editor: Chen XF L-Editor: Filipodia P-Editor: Wang LL
| 1. | Zhang M, Luo Y, Zhang Y, Tang J. Efficacy and Safety of Ultrasound-Guided Radiofrequency Ablation for Treating Low-Risk Papillary Thyroid Microcarcinoma: A Prospective Study. Thyroid. 2016;26:1581-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 153] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 2. | Lim HK, Cho SJ, Baek JH, Lee KD, Son CW, Son JM, Baek SM. US-Guided Radiofrequency Ablation for Low-Risk Papillary Thyroid Microcarcinoma: Efficacy and Safety in a Large Population. Korean J Radiol. 2019;20:1653-1661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 3. | Papini E, Guglielmi R, Gharib H, Misischi I, Graziano F, Chianelli M, Crescenzi A, Bianchini A, Valle D, Bizzarri G. Ultrasound-guided laser ablation of incidental papillary thyroid microcarcinoma: a potential therapeutic approach in patients at surgical risk. Thyroid. 2011;21:917-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Valcavi R, Piana S, Bortolan GS, Lai R, Barbieri V, Negro R. Ultrasound-guided percutaneous laser ablation of papillary thyroid microcarcinoma: a feasibility study on three cases with pathological and immunohistochemical evaluation. Thyroid. 2013;23:1578-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Yue W, Wang S, Yu S, Wang B. Ultrasound-guided percutaneous microwave ablation of solitary T1N0M0 papillary thyroid microcarcinoma: initial experience. Int J Hyperthermia. 2014;30:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Teng D, Sui G, Liu C, Wang Y, Xia Y, Wang H. Long-term efficacy of ultrasound-guided low power microwave ablation for the treatment of primary papillary thyroid microcarcinoma: a 3-year follow-up study. J Cancer Res Clin Oncol. 2018;144:771-779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 7. | Rossi S, Di Stasi M, Buscarini E, Cavanna L, Quaretti P, Squassante E, Garbagnati F, Buscarini L. Percutaneous radiofrequency interstitial thermal ablation in the treatment of small hepatocellular carcinoma. Cancer J Sci Am. 1995;1:73-81. [PubMed] |
| 8. | Zlotta AR, Wildschutz T, Raviv G, Peny MO, van Gansbeke D, Noel JC, Schulman CC. Radiofrequency interstitial tumor ablation (RITA) is a possible new modality for treatment of renal cancer: ex vivo and in vivo experience. J Endourol. 1997;11:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 270] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Dupuy DE, Zagoria RJ, Akerley W, Mayo-Smith WW, Kavanagh PV, Safran H. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol. 2000;174:57-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 428] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 10. | Chin JL, Billia M, Relle J, Roethke MC, Popeneciu IV, Kuru TH, Hatiboglu G, Mueller-Wolf MB, Motsch J, Romagnoli C, Kassam Z, Harle CC, Hafron J, Nandalur KR, Chronik BA, Burtnyk M, Schlemmer HP, Pahernik S. Magnetic Resonance Imaging-Guided Transurethral Ultrasound Ablation of Prostate Tissue in Patients with Localized Prostate Cancer: A Prospective Phase 1 Clinical Trial. Eur Urol. 2016;70:447-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Pacella CM, Bizzarri G, Guglielmi R, Anelli V, Bianchini A, Crescenzi A, Pacella S, Papini E. Thyroid tissue: US-guided percutaneous interstitial laser ablation-a feasibility study. Radiology. 2000;217:673-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 133] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Dupuy DE, Monchik JM, Decrea C, Pisharodi L. Radiofrequency ablation of regional recurrence from well-differentiated thyroid malignancy. Surgery. 2001;130:971-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 160] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Kim YS, Rhim H, Tae K, Park DW, Kim ST. Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid. 2006;16:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 197] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 14. | Jeong WK, Baek JH, Rhim H, Kim YS, Kwak MS, Jeong HJ, Lee D. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008;18:1244-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 348] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 15. | Kim JH, Baek JH, Lim HK, Ahn HS, Baek SM, Choi YJ, Choi YJ, Chung SR, Ha EJ, Hahn SY, Jung SL, Kim DS, Kim SJ, Kim YK, Lee CY, Lee JH, Lee KH, Lee YH, Park JS, Park H, Shin JH, Suh CH, Sung JY, Sim JS, Youn I, Choi M, Na DG; Guideline Committee for the Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology. 2017 Thyroid Radiofrequency Ablation Guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018;19:632-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 444] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 16. | Papini E, Pacella CM, Solbiati LA, Achille G, Barbaro D, Bernardi S, Cantisani V, Cesareo R, Chiti A, Cozzaglio L, Crescenzi A, De Cobelli F, Deandrea M, Fugazzola L, Gambelunghe G, Garberoglio R, Giugliano G, Luzi L, Negro R, Persani L, Raggiunti B, Sardanelli F, Seregni E, Sollini M, Spiezia S, Stacul F, Van Doorne D, Sconfienza LM, Mauri G. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperthermia. 2019;36:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 17. | Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10769] [Cited by in RCA: 9672] [Article Influence: 1074.7] [Reference Citation Analysis (1)] |
| 18. | Thyroid Tumor Ablation Experts Group of Chinese Medical Doctor Association, Chinese Association of Thyroid Oncology, Interventional Ultrasound Committee of Chinese College of Interventionalists, Tumor Ablation Committee of Chinese College of Interventionalists, The Society of Interventional Therapy of China Anti-cancer Association, The Society of Minimally Invasive Therapy in Cancer of China Anti-cancer Association. Expert Consensus on Thermal Ablation for Thyroid Benign Nodes, Microcarcinoma and Metastatic Cervical Lymph Nodes (2018 Edition). Zhongguo Zhongliu. 2018;27:768-773. [DOI] [Full Text] |
| 19. | Kim JH, Baek JH, Sung JY, Min HS, Kim KW, Hah JH, Park DJ, Kim KH, Cho BY, Na DG. Radiofrequency ablation of low-risk small papillary thyroidcarcinoma: preliminary results for patients ineligible for surgery. Int J Hyperthermia. 2017;33:212-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 20. | Luo YK, Zhang MB. Ultrasound-Guided Radiofrequency Ablation of Low-Risk Papillary Thyroid Microcarcinoma: a Prospective Study. Ultrasound in Med & Biol. 2017;43:S240-S241. [DOI] [Full Text] |
| 21. | Jeong SY, Baek JH, Choi YJ, Chung SR, Sung TY, Kim WG, Kim TY, Lee JH. Radiofrequency ablation of primary thyroid carcinoma: efficacy according to the types of thyroid carcinoma. Int J Hyperthermia. 2018;34:611-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Zhang Y, Zhang MB, Luo YK, Li J, Zhang Y, Tang J. Effect of chronic lymphocytic thyroiditis on the efficacy and safety of ultrasound-guided radiofrequency ablation for papillary thyroid microcarcinoma. Cancer Med. 2019;8:5450-5458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Ding M, Tang X, Cui D, Chi J, Shi Y, Wang T, Zhai B, Li P. Clinical outcomes of ultrasound-guided radiofrequency ablation for the treatment of primary papillary thyroid microcarcinoma. Clin Radiol. 2019;74:712-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Zhang M, Tufano RP, Russell JO, Zhang Y, Zhang Y, Qiao Z, Luo Y. Ultrasound-Guided Radiofrequency Ablation Versus Surgery for Low-Risk Papillary Thyroid Microcarcinoma: Results of Over 5 Years' Follow-Up. Thyroid. 2020;30:408-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 25. | Zhou W, Jiang S, Zhan W, Zhou J, Xu S, Zhang L. Ultrasound-guided percutaneous laser ablation of unifocal T1N0M0 papillary thyroid microcarcinoma: Preliminary results. Eur Radiol. 2017;27:2934-2940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 26. | Zhang L, Zhou W, Zhan W, Peng Y, Jiang S, Xu S. Percutaneous Laser Ablation of Unifocal Papillary Thyroid Microcarcinoma: Utility of Conventional Ultrasound and Contrast-Enhanced Ultrasound in Assessing Local Therapeutic Response. World J Surg. 2018;42:2476-2484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 27. | Ji L, Wu Q, Gu J, Deng X, Zhou W, Fan X, Zhou F. Ultrasound-guided percutaneous laser ablation for papillary thyroid microcarcinoma: a retrospective analysis of 37 patients. Cancer Imaging. 2019;19:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Zhou W, Ni X, Xu S, Zhang L, Chen Y, Zhan W. Ultrasound-guided laser ablation versus surgery for solitary papillary thyroid microcarcinoma: a retrospective study. Int J Hyperthermia. 2019;36:897-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 29. | Li J, Liu Y, Liu J, Qian L. Ultrasound-guided percutaneous microwave ablation versus surgery for papillary thyroid microcarcinoma. Int J Hyperthermia. 2018;34:653-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 30. | Teng DK, Li HQ, Sui GQ, Lin YQ, Luo Q, Fu P, Du JR, Jin CX, Wang H. Preliminary report of microwave ablation for the primary papillary thyroid microcarcinoma: a large-cohort of 185 patients feasibility study. Endocrine. 2019;64:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 31. | Li J, Liu Y, Liu J, Yang P, Hu X, Qian L. A comparative study of short-term efficacy and safety for thyroid micropapillary carcinoma patients after microwave ablation or surgery. Int J Hyperthermia. 2019;36:640-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 32. | Yue WW, Qi L, Wang DD, Yu SJ, Wang XJ, Xu HX, Wang SR. US-guided Microwave Ablation of Low-Risk Papillary Thyroid Microcarcinoma: Longer-Term Results of a Prospective Study. J Clin Endocrinol Metab. 2020;105:dgaa128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 33. | Xu B, Zhou NM, Cao WT, Gu SY. Comparative study on operative trauma between microwave ablation and surgical treatment for papillary thyroid microcarcinoma. World J Clin Cases. 2018;6:936-943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Radzina M, Cantisani V, Rauda M, Nielsen MB, Ewertsen C, D'Ambrosio F, Prieditis P, Sorrenti S. Update on the role of ultrasound guided radiofrequency ablation for thyroid nodule treatment. Int J Surg. 2017;41 Suppl 1:S82-S93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | Tong M, Li S, Li Y, Li Y, Feng Y, Che Y. Efficacy and safety of radiofrequency, microwave and laser ablation for treating papillary thyroid microcarcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019;36:1278-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 36. | Chen J, Cao J, Qiu F, Huang P. The Efficacy and The Safety of Ultrasound-guided Ablation Therapy for Treating Papillary Thyroid Microcarcinoma. J Cancer. 2019;10:5272-5282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Choi Y, Jung SL. Efficacy and Safety of Thermal Ablation Techniques for the Treatment of Primary Papillary Thyroid Microcarcinoma: A Systematic Review and Meta-Analysis. Thyroid. 2020;30:720-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 38. | Cho SJ, Baek JH, Chung SR, Choi YJ, Lee JH. Thermal Ablation for Small Papillary Thyroid Cancer: A Systematic Review. Thyroid. 2019;29:1774-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 39. | Ma B, Wei W, Xu W, Wang Y, Guan H, Fan J, Zhao Z, Wen D, Yang S, Wang Y, Chang B, Ji Q. Surgical Confirmation of Incomplete Treatment for Primary Papillary Thyroid Carcinoma by Percutaneous Thermal Ablation: A Retrospective Case Review and Literature Review. Thyroid. 2018;28:1134-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 40. | Kim HY, Ryu WS, Woo SU, Son GS, Lee ES, Lee JB, Bae JW. Primary papillary thyroid carcinoma previously treated incompletely with radiofrequency ablation. J Cancer Res Ther. 2010;6:310-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Lee CU, Kim SJ, Sung JY, Park SH, Chong S, Baek JH. Needle track tumor seeding after radiofrequency ablation of a thyroid tumor. Jpn J Radiol. 2014;32:661-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Ruzzenente A, Manzoni GD, Molfetta M, Pachera S, Genco B, Donataccio M, Guglielmi A. Rapid progression of hepatocellular carcinoma after Radiofrequency Ablation. World J Gastroenterol. 2004;10:1137-1140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 117] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 43. | Koda M, Murawaki Y, Hirooka Y, Kitamoto M, Ono M, Sakaeda H, Joko K, Sato S, Tamaki K, Yamasaki T, Shibata H, Shimoe T, Matsuda T, Toshikuni N, Fujioka S, Ohmoto K, Nakamura S, Kariyama K, Aikata H, Kobayashi Y, Tsutsui A. Complications of radiofrequency ablation for hepatocellular carcinoma in a multicenter study: An analysis of 16,346 treated nodules in 13,283 patients. Hepatol Res. 2012;42:1058-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 44. | Ergul E, Gozetlik EO. Does percutaneous thyroid laser ablation induce mixed papillary and medullary thyroid carcinoma? Acta Chir Belg. 2010;110:228-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 45. | Shimamoto K, Satake H, Sawaki A, Ishigaki T, Funahashi H, Imai T. Preoperative staging of thyroid papillary carcinoma with ultrasonography. Eur J Radiol. 1998;29:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 124] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 46. | Stulak JM, Grant CS, Farley DR, Thompson GB, van Heerden JA, Hay ID, Reading CC, Charboneau JW. Value of preoperative ultrasonography in the surgical management of initial and reoperative papillary thyroid cancer. Arch Surg. 2006;141:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 266] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 47. | Kim E, Park JS, Son KR, Kim JH, Jeon SJ, Na DG. Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography. Thyroid. 2008;18:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 295] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 48. | Choi YJ, Yun JS, Kook SH, Jung EC, Park YL. Clinical and imaging assessment of cervical lymph node metastasis in papillary thyroid carcinomas. World J Surg. 2010;34:1494-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 49. | Lee DW, Ji YB, Sung ES, Park JS, Lee YJ, Park DW, Tae K. Roles of ultrasonography and computed tomography in the surgical management of cervical lymph node metastases in papillary thyroid carcinoma. Eur J Surg Oncol. 2013;39:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 50. | Wei Q, Wu D, Luo H, Wang X, Zhang R, Liu Y. Features of lymph node metastasis of papillary thyroid carcinoma in ultrasonography and CT and the significance of their combination in the diagnosis and prognosis of lymph node metastasis. J BUON. 2018;23:1041-1048. [PubMed] |
| 51. | Zhao H, Li H. Meta-analysis of ultrasound for cervical lymph nodes in papillary thyroid cancer: Diagnosis of central and lateral compartment nodal metastases. Eur J Radiol. 2019;112:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 152] [Article Influence: 25.3] [Reference Citation Analysis (0)] |