Published online Jan 6, 2021. doi: 10.12998/wjcc.v9.i1.232
Peer-review started: August 26, 2020
First decision: November 3, 2020
Revised: November 5, 2020
Accepted: November 13, 2020
Article in press: November 13, 2020
Published online: January 6, 2021
Processing time: 127 Days and 22 Hours
Aortic dissection (AD) is a life-threatening condition with a high mortality rate without immediate medical attention. Early diagnosis and appropriate treatment are critical in treating patients with AD. In the emergency department, patients with AD commonly present with classic symptoms of unanticipated severe chest or back pain. However, it is worth noting that atypical symptoms of AD are easily misdiagnosed.
A 51-year-old woman was first diagnosed with scapulohumeral periarthritis due to left shoulder pain. After careful examination of her previous medical history and contrast-enhanced computed tomography angiography, the patient was diagnosed with a new type A AD after chronic type B dissection in the ascending aorta. The patient was successfully treated with surgical replacement of the dissected aortic arch and remains in good health.
New retrograde type A AD after chronic type B dissection is relatively rare. It is worth noting that a physician who has a patient with suspected AD should be vigilant. Both patient medical history and imaging tests are crucial for a more precise diagnosis.
Core Tip: We report a case of new retrograde type A aortic dissection, which developed after type B aortic dissection. This case is special for only presenting mild left shoulder pain. Hence, detailed medical history and imaging tests are crucial for patients with atypical symptoms.
- Citation: Yin XB, Wang XK, Xu S, He CY. Type A aortic dissection developed after type B dissection with the presentation of shoulder pain: A case report. World J Clin Cases 2021; 9(1): 232-235
- URL: https://www.wjgnet.com/2307-8960/full/v9/i1/232.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i1.232
Aortic dissection (AD) is a severe condition that usually occurs in the emergency department (ED). Once diagnosed, urgent medical management involves reducing blood pressure and the heart rate[1]. Chest or back pain is the most common symptom of AD. Pain is usually described as a tearing or stabbing pain that is projected in the anterior chest or interscapular area. Vasovagal events such as sweating, vomiting, and fainting may also occur. Typical pain tends to be observed by the emergency physician, but painless AD or AD with atypical symptoms might mislead the diagnosis[2]. Here, we report an atypical case of mild left shoulder pain that was finally diagnosed as a new type A AD after a 10-year history of type B AD.
A 51-year-old woman presented with mild left shoulder pain.
Shoulder pain started 7 d previously when the patient was walking. No sweating, fatigue, or nausea was reported by the patient. She visited the local hospital and underwent physical examination and a left shoulder X-ray, but with no significant findings. The treating physician suspected possible scapulohumeral periarthritis and discharged the patient with oral analgesics. After taking NSAIDs for three days, her shoulder pain did not improve, and the patient came to our ED for further investigation.
She was diagnosed with AD (Stanford type B) for more than 10 years. However, she did not receive any surgical intervention and only had antihypertensive medications.
The patient had no remarkable personal and family history.
At the time of admission, physical examination revealed an elevated blood pressure of 139/93 mmHg, heart rate of 78 bpm, and oxygen saturation of 98% in room air. No movement limitation of the left shoulder was observed.
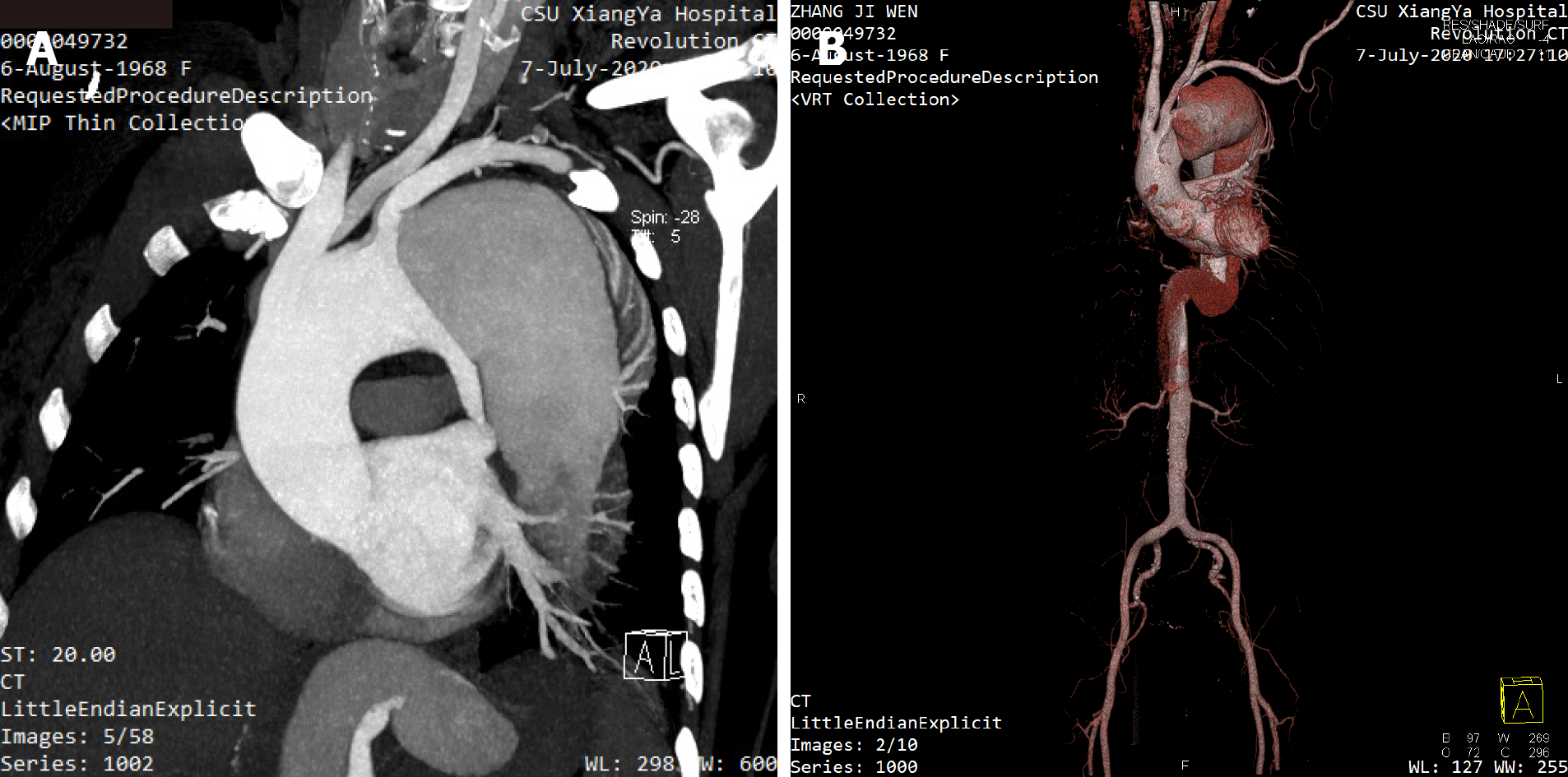
Emergent contrast-enhanced computed tomography angiography confirmed the diagnosis of a new type A AD with a sizable false lumen (Figure 1).
New type A AD after type B AD.
The patient was immediately transferred to the intensive care unit (ICU) for more precise blood pressure control as well as preoperative preparation. The patient was managed surgically with a modified elephant trunk stent-graft one day after hospitalization. She was then transferred back to the ICU to monitor vital signs and postoperative care.
The patient was discharged in a good general condition after 15 d of hospitalization.
This case report describes a rare clinical event due to an untreated type B AD for 10 years ago, which gradually developed into a new type A AD with mild left shoulder pain.
Only a few studies have mentioned shoulder pain as the chief complaint in AD. Ueno et al[3] reported a case of Stanford B-type AD in which the initial complaints were toothache and left shoulder pain[3]. They considered the shoulder pain as a radiation pain, which was related to the communication between the aorta and somatic or pharyngeal nerves via the autonomic nervous system. The other two AD cases reported that shoulder pain was related to complications from splenic rupture or splenic hamartoma[4,5]. In our case, the etiology of shoulder pain is still unclear. This might be caused by compression of the false lumen to the thorax.
Although there are no literature reports on shoulder pain after new type A AD, the possibility of its occurrence should be considered in the case of a history of type B AD. The phenomenon of new proximal or retrograde dissection progression into the ascending aorta is commonly associated with thoracic endovascular aortic repair (TEVAR) in the descending thoracic aorta. Compared to the estimated occurrence rate of new type A AD (1.3% to 4.0%), it is even more frequent (up to 7%) after TEVAR for type B AD[6]. Studies have shown that it is probably caused by stent-graft-induced iatrogenic aortic injury[7].
In this case, the patient was misdiagnosed as having scapulohumeral periarthritis by a local hospital. We speculated that the patient who had mild left shoulder pain misled the judgment of the physician.
Shoulder pain may mislead the diagnosis of the patient as the most common symptom of AD is a sudden onset of tearing chest or abdominal pain associated with hypertension. An emergency physician can easily recognize the typical symptoms and make the correct diagnosis of AD. However, a wide range of atypical presentations also exists that may prevent the clinical decision, especially in the emergency setting, where approximately one-third of AD patients with chest pain are initially diagnosed as having acute coronary syndrome[2]. In addition, painless AD with atypical presentations, such as fatigue or neurological symptoms, make the diagnosis even more complicated[8]. Avoiding a delay in diagnosis or misdiagnosis will subsequently decrease mortality and morbidity, especially in patients who present with atypical manifestations.
The ED physician should be more careful in treating patients with an AD medical history. Furthermore, the necessary imaging tests and detailed medical history are crucial for a more precise diagnosis.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Grawish M S-Editor: Fan JR L-Editor: Webster JR P-Editor: Li JH
| 1. | Riou B, Plaisance P, Lecomte F, Soulat L, Orcel P, Mazoit JX. Comparison of two doses of ketoprofen to treat pain: a double-blind, randomized, noninferiority trial. Fundam Clin Pharmacol. 2014;28:20-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Marroush TS, Boshara AR, Parvataneni KC, Takla R, Mesiha NA. Painless Aortic Dissection. Am J Med Sci. 2017;354:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Ueno M, Omori K, Yanagawa Y. A case of Stanford B type aortic dissection in a patient whose initial complaints were a toothache and left shoulder pain. J Emerg Trauma Shock. 2015;8:69-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Seyama Y, Tanaka N, Suzuki Y, Nagai M, Furuya T, Nomura Y, Ishii J, Nobori M. Spontaneous rupture of splenic hamartoma in a patient with hepatitis C virus-related cirrhosis and portal hypertension: a case report and review of the literature. World J Gastroenterol. 2006;12:2133-2135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Guo X, Pang X, Liu Z. Non-Traumatic Splenic Rupture After Open Surgery for Type A Aortic Dissection: A Case Report and Literature Review. Heart Surg Forum. 2020;23:E315-E317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Wu YH, Yeh IJ, Liu KT. Spontaneous pneumocephalus and subdural hemorrhage after sneezing. Am J Emerg Med 2018; 36: 1928.e1-1928. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Charlton-Ouw KM, Sandhu HK, Leake SS, Miller CC 3rd, Afifi RO, Azizzadeh A, Estrera AL, Safi HJ. New type A dissection after acute type B aortic dissection. J Vasc Surg. 2018;67:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Mussa FF, Horton JD, Moridzadeh R, Nicholson J, Trimarchi S, Eagle KA. Acute Aortic Dissection and Intramural Hematoma: A Systematic Review. JAMA. 2016;316:754-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 336] [Article Influence: 37.3] [Reference Citation Analysis (0)] |