Published online Jan 6, 2021. doi: 10.12998/wjcc.v9.i1.204
Peer-review started: June 24, 2020
First decision: October 18, 2020
Revised: October 22, 2020
Accepted: November 12, 2020
Article in press: November 12, 2020
Published online: January 6, 2021
Processing time: 190 Days and 21.9 Hours
Myeloid neoplasm (MN) with eosinophilia and rearrangement of platelet-derived growth factor receptor beta (PDGFRB) shows a good therapeutic response to imatinib in adults. MN is rarely found in children, and the efficacy of imatinib on pediatric patients remain unclear.
We report 2 pediatric cases diagnosed with MN with eosinophilia and PDGFRB rearrangement who were treated with imatinib. Case 1 was a 1-year-old girl admitted to the hospital because of “abdominal distension with hyperleukocytosis for 3 mo”. She had leukocytosis, anemia, and eosinophilia (the absolute eosinophil count (AEC) was 8960/μL), and her fluorescence in situ hybridization (FISH) test revealed that PDGFRB rearrangement was detected in 70% of 500 interphase cells. Case 2 was a 2-year-old girl admitted to the hospital because of “recurrent fever and rashes for 1 mo”. Her blood cell count showed an AEC of 3540/μL. The FISH test revealed that PDGFRB rearrangement was detected in 71% of 500 interphase cells. Both patients were diagnosed as MN with eosinophilia and PDGFRB rearrangement. Imatinib was added into their treatment regimen. As expected, complete hematologic remission was achieved after 1 mo of treatment, and symptoms disappeared.
Although MN with eosinophilia and PDGFRB rearrangement usually occurs in adults, it can be found in children. The therapeutic benefits of imatinib in these 2 pediatric patients were consistent with its reported effects in adult patients.
Core Tip: In the present report, we describe 2 pediatric patients diagnosed as myeloid neoplasm (MN) with eosinophilia and platelet-derived growth factor receptor beta (PDGFRB) rearrangement and reviewed the relative literature to analyze the clinical and therapeutic features of this rare clinical entity. Although MN with PDGFRB rearrangement rarely occurs in children, awareness should be increased for the possibility of this disease. Detection of the mutant gene by fluorescence in situ hybridization is necessary once the disease is suspected. Imatinib had a considerable effect on children, though the dose of imatinib is still unclear. Moreover, additional attention should be paid regarding the prognosis, life expectancy, side effects, and quality of life of these pediatric patients.
- Citation: Wang SC, Yang WY. Myeloid neoplasm with eosinophilia and rearrangement of platelet-derived growth factor receptor beta gene in children: Two case reports. World J Clin Cases 2021; 9(1): 204-210
- URL: https://www.wjgnet.com/2307-8960/full/v9/i1/204.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i1.204
Myeloid neoplasms (MN) with eosinophilia and rearrangement of platelet-derived growth factor receptor beta (PDGFRB) usually occurs in adults, while it is rarely found in children[1]. Identification of the genes involved in the pathogenesis of MN is crucial for the guidance of treatment. Imatinib is recommended for adult patients with PDGFRB rearrangement due to its sustained hematologic remission and low toxicity[2]. Literature review showed that only six children diagnosed as MN with PDGFRB rearrangement had been treated with imatinib. In the present report, we describe 2 pediatric patients diagnosed as MN with eosinophilia and PDGFRB rearrangement and reviewed the relative literature to analyze the clinical and therapeutic features of this rare clinical entity.
Chief complaint: A 1-year-old girl was admitted to the hospital because of “abdominal distension with hyperleukocytosis for 3 mo”.
History of present illness: This girl was found to have a bulged abdominal lump 3 mo ago, and the routine blood tests showed that she had leukocytosis [white blood cell (WBC) count 56000/μL] and anemia (9.5 g/dL hemoglobin). She was admitted to the Institute of Hematology and Blood Diseases Hospital for further examination.
History of past illness: The patient had no history of other significant medical conditions.
Personal and family history: The patient had no significant personal and family history.
Physical examination: Physical examination revealed a pale appearance and splenomegaly.
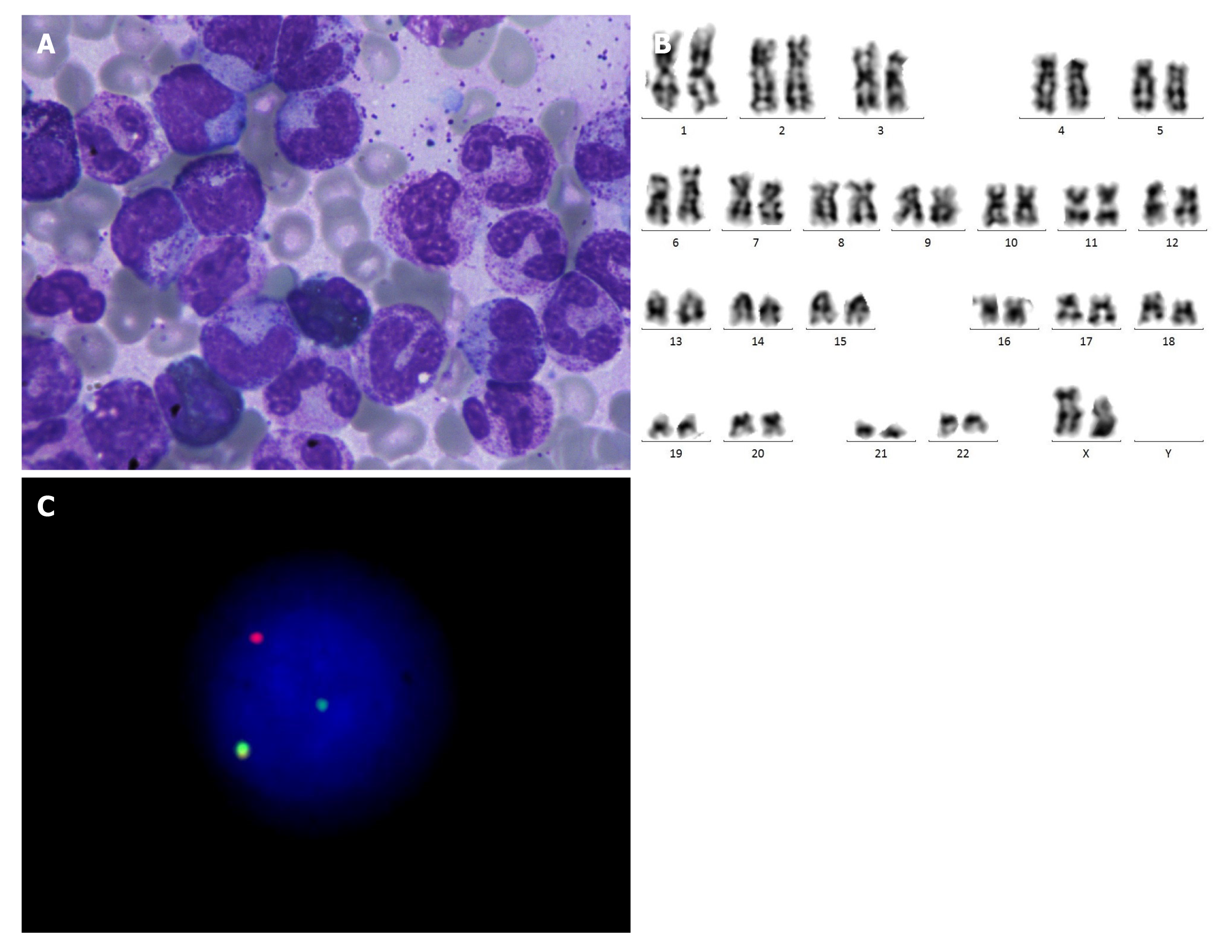
Laboratory examinations: Her blood cell count showed that the WBC was 112000/μL, hemoglobin was 10.2 g/dL, and platelet count was 333000/μL. Differential blood count was 69% neutrophils, 20% lymphocytes, 3% monocytes, and 8% eosinophils, with an absolute monocyte count (AMC) of 8960/μL. Bone marrow (BM) biopsy showed extreme myelomonocytic hyperplasia, neutrophils were prominently increased (mainly in metamyelocyte and stab granulocyte), eosinophils were relatively increased (12%), erythrocytes were decreased, and some small megakaryocytes were observed (Figure 1A). Karyotype analysis of the BM was 46, XX in 20/20 metaphases (Figure 1B). Fluorescence in situ hybridization (FISH) test revealed that PDGFRB rearrangement was detected in 70% of 500 interphase cells (Figure 1C). Moreover, whole-genome sequencing revealed no mutations, and quantitative polymerase chain reaction was negative.
Imaging examinations: Ultrasound of the abdomen supported medium splenomegaly, measuring 9.1 cm × 3.4 cm × 4.4 cm with no abnormal function of the liver.
Diagnostic assessment: BM aspiration showed that the proportion of medullary blasts was less than 20%, and the negative results of the BCR-ABL fusion gene excluded BCl-ABL+ chronic myelogenous leukemia. Regarding the other fusion gene, the FISH test showed no rearrangement of platelet-derived growth factor receptor alpha (PDGFRA), cytokine receptor-like factor 2 (CRLF2), or mixed-lineage leukemia (MLL) gene, and the P53/CEP17 mutational analyses were negative. Her clinical performance and laboratory examination met the diagnostic criteria of myeloid neoplasm with eosinophilia.
Chief complaint: A 2-year-old girl was admitted to the hospital because of “recurrent fever and rashes for 1 mo”.
History of present illness: The girl was found with a subcutaneous nodule on her extremity 1 mo ago, and her parents were not concerned until onset of recurrent fever. She went to our hospital for further examination.
History of past illness: The patient had no history of other significant medical conditions.
Personal and family history: The patient had no significant personal and family history.
Physical examination: Physical examination showed a pale appearance and splenomegaly.
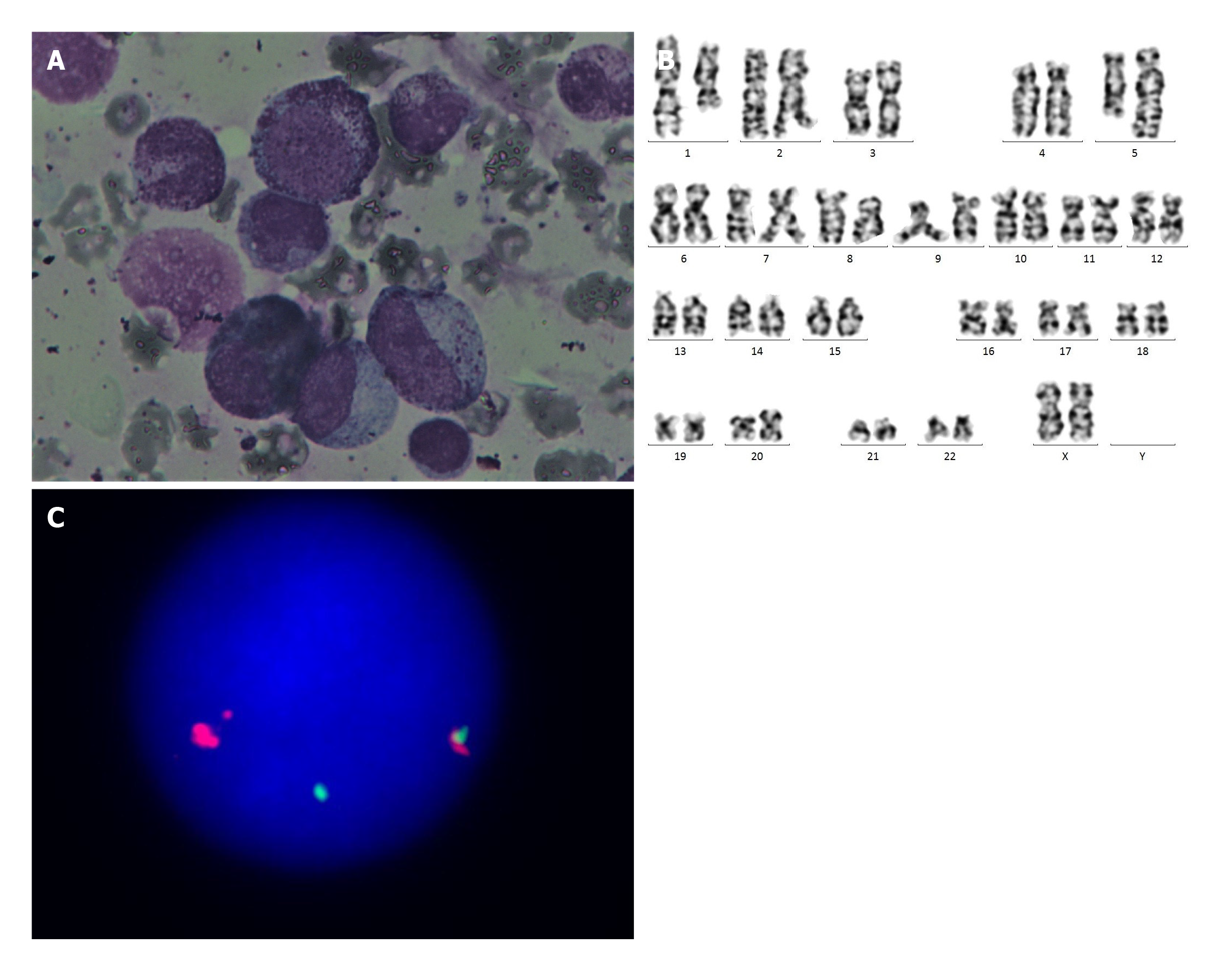
Laboratory examinations: Her routine blood test revealed that the WBC count was 34000/μL, hemoglobin was 7.1 g/dL, and platelet count was 36000/μL × 109/L. Differential blood count showed that the proportions of neutrophils and lymphocytes were 55.4% and 22.2%, respectively, the proportion of monocytes was 11.4% with an AMC of 3900/μL, and the proportion of eosinophils was 10.4% with an absolute eosinophil count of 3540/μL. BM biopsy displayed myelomonocytic hyperplasia and granulocytosis with left shift, eosinophils were relatively increased (13.5%), and the megakaryocytes were decreased (Figure 2A). Cytogenetic analysis showed that the karyotype was 46, XX, t(1;5)(q21;q33)[19]/46,XX[1] in 20/20 metaphases (Figure 2B). There was no evidence of the BCR-ABL fusion gene, and the FISH test showed no rearrangement of PDGFRA, CRLF2, or MLL. P53/CEP17 mutational analyses were negative. The FISH test revealed that PDGFRB rearrangement was detected in 71% of 500 interphase cells (Figure 2C).
Imaging examinations: Ultrasound of the abdomen showed the enlarged liver with 3 cm was below the left costal margin, and the enlarged spleen was below the pelvic rim.
Diagnostic assessment: BM aspiration showed that the proportion of medullary blasts was less than 20%, and the negative results of the BCR-ABL fusion gene excluded BCl-ABL+ chronic myelogenous leukemia.
Based on clinical performance and laboratory examination, both of their diagnoses were revised as MN with eosinophilia and PDGFRB rearrangement according to revised 2016 World Health Organization classification of MN and eosinophilic disorders[1].
Taking into consideration several previous case reports and the physical condition of these pediatric patients, we suggested 100 mg imatinib (200 mg/m2) per day for their treatment. Both of them responded exquisitely to imatinib.
The patient had a good response to imatinib therapy and achieved complete hematologic remission (CHR) after 1 mo. She was on maintenance therapy and remained in good condition during the 1 year of close follow-up.
Complete resolution of leukocytosis and normal spleen size were achieved after 1 mo. The patient was closely followed up every 2 mo. Until now, the patient still receives 100 mg of imatinib per day, and hematologic remission and normal size of spleen have remained for more than 2 years.
In 2008, the World Health Organization has endorsed a semi-molecular classification scheme of disease subtypes “myeloid neoplasms with eosinophilia and rearrangement of PDGFRA, PDGFRB, or fibroblast growth factor receptor 1 (FGFR1)”. One previous study[3] revealed that the incidence of myeloproliferative neoplasms (MPN) with PDGFRB rearrangement is 1.8% of MPN (10/556), with a median age of 61 years. The identification of MN patients with rearrangements of PDGFRA/B has significant implications for treatment and prognosis. It has been reported that high rates (> 90%) of CHR and complete molecular remissions (CMR) have been achieved in adult MN patients on imatinib therapy. These durable responses can be translated into excellent progression-free and overall survival, while hematopoietic stem cell transplantation (HSCT) remains the only approach to cure the disease[4]. Since this disorder is rarely described in children, there is no guideline for pediatric MN patients with PDGFRB rearrangement, and the existing diagnosis and treatment are based on the guidelines for adult patients.
We searched PubMed and CNKI databases for the terms “child, PDGFRB rearrangement and imatinib”. There are only six reported cases[5-9] of pediatric MN patients with PDGFRB rearrangement (Table 1). The patients’ age ranged from 0 to 8 years. All the children presented with leukocytosis, eosinophilia, and splenomegaly, and their molecular biological examination show the PDGFRB rearrangement. All patients had an abnormal karyotype involved in translocation of 5q31-33, which is a hotspot for diverse chromosomal aberrations in rearrangements of PDGFRB and formation of PDGFRB fusion genes (except one whose karyotype was unknown). Other common symptoms include anemia (6/8), thrombocytopenia (3/8), fever, and rashes. All of them received imatinib therapy; the initial doses range from 340 mg/m2 to 200 mg/m2, and the dose was reduced to 100-185 mg/m2 during maintenance therapy. Children achieved the hematologic response after the use of imatinib, and the complete cytogenetic response and CMR was usually achieved at 1 mo after the therapy, which is consistent with its effect on adults.
| Diagnosis | Age | Sex | Karyotype | Dose of imatinib | Duration to complete remission | Clinical character | Ref. |
| MDS/MPD syndrome with eosinophilia | 11 mo | Female | t(1;5)(q23;q33) | - | 5 mo | Anemia, leukocytosis eosinophilia, and thrombocytopenia hepatosplenomegaly. | Wilkinson et al[5] |
| JMML | Newborn | Male | t(1;5) q21;q33) | 370 mg/m2 | 1 mo | Leukocytosis with eosinophilia, thrombocytopenia and anemia hepatomegaly, | Abraham et al[6] |
| CEL | 8 yr | Male | t(1;5)(q21; 33) | 200mg/m2 daily | 1 mo | Anemia and leukocytosis eosinophilia, hepatosplenomegaly. | Li et al[7] |
| Myeloid neoplasms associated with the PDGFRB rearrangement | 1mo | Male | t(1;5) (q21; 33) | 340 mg/m2/d---170 mg/m2/d | 1 mo | Anemia and leukocytosis eosinophils, hepatosplenomegaly. | Abraham et al[8] |
| Myeloid neoplasms associated with the PDGFRB rearrangement | 4 yr | Male | Not clear | 340 mg/m2/d---145 mg/m2/d | 1 mo | Leukocytosis; eosinophils hepatosplenomegaly. | Abraham et al[8] |
| Myeloid neoplasms associated with the PDGFRB rearrangement | 19 mo | Male | t(5;14)(q33;q32) | 200 mg/m2/d | 1 wk | Leukocytosis; eosinophilia, hepatosplenomegaly. | Zhang et al[9] |
| Myeloid neoplasms associated with the PDGFRB rearrangement | 1 yr | Female | Normal | 200 mg/m2 | 1 mo | Leukocytosis; anemia, eosinophilia, hepatosplenomegaly. | |
| Myeloid neoplasms associated with the PDGFRB rearrangement | 2 yr | Female | t(1;5)(q21;q33) | 200 mg/m2 | 1 mo | Leukocytosis; anemia, thrombocytopenia, eosinophilia, hepatosplenomegaly, fever, rashes. |
The common side effects of imatinib are nausea, vomiting, tiredness, edema, myelotoxicity, and so on. The treatment was suspended in one patient[7] for 1 mo because of the gastrointestinal reaction and was restarted due to the molecular relapse, and the patient achieves hematologic release after 2 mo. These findings suggest that temporary suspension of the drug may cause relapse, while patients can still benefit from such therapy when the treatment is resumed. Other patients are tolerant to the drug. One of the patients[9] underwent HSCT because of the inconvenience of taking daily pills. Besides, there was no resistance to imatinib reported in children.
Consistent with these patients, both of our patients presented with leukocytosis, eosinophilia, and splenomegaly, their BM aspiration showed myelomonocytic hyperplasia, and the proportion of medullary blasts was less than 20%, which reminded us of the possibility of myeloproliferative diseases. Considering that the eosinophilia with no obvious cause is a possible hematological neoplasm with clonal eosinophilia, we tested their peripheral blood analysis for PDGFRA, PDGFRB, FGFR1, etc. by FISH[10]. PDGFRB is usually associated with reciprocal translocations of 5q31–33 region thus presenting an abnormal karyotype. However, the karyotype in case 1 was normal, while the PDGFRB rearrangement detected by FISH was positive. This condition has been reported in adult cases, which may be caused by cryptic rearrangements, suggesting the necessity of the detection by molecular technology.
After clarifying the diagnosis and reviewing imatinib treatment in adults, as well as the patients before, we suggested 100 mg imatinib (200 mg/m2) per day for their treatment. Both patients we reported achieved hematologic release within 1 mo after initiation of the imatinib treatment, attained clinical remissions, and remained in sustained remission. Collectively, imatinib had a considerable effect on children. Patients could rapidly achieve CHR and CMR after the use of imatinib.
Although MN with eosinophilia and rearrangement of PDGFRB rarely occurs in children, we should increase our awareness for the possibility of this disease. PDGFRA, PDGFRB, or FGFR1 by FISH are necessary once the disease is suspected. Moreover, we should pay more attention to the prognosis, life expectancy, side effects, and quality of life of these pediatric patients. The dose of imatinib for treatment is still unclear. For more information about pediatric MN with eosinophilia and rearrangement of PDGFRB, a multicenter clinical study with long-term follow up is required.
I would like to express my gratitude to all those who helped me during the writing of this report. Thanks for the patients, thanks for the guidance of doctors and nurses, thanks for my parents always supporting me.
Manuscript source: Unsolicited manuscript
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rolla G S-Editor: Liu M L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Shomali W, Gotlib J. World Health Organization-defined eosinophilic disorders: 2019 update on diagnosis, risk stratification, and management. Am J Hematol. 2019;94:1149-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 2. | David M, Cross NC, Burgstaller S, Chase A, Curtis C, Dang R, Gardembas M, Goldman JM, Grand F, Hughes G, Huguet F, Lavender L, McArthur GA, Mahon FX, Massimini G, Melo J, Rousselot P, Russell-Jones RJ, Seymour JF, Smith G, Stark A, Waghorn K, Nikolova Z, Apperley JF. Durable responses to imatinib in patients with PDGFRB fusion gene-positive and BCR-ABL-negative chronic myeloproliferative disorders. Blood. 2007;109:61-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Arefi M, García JL, Peñarrubia MJ, Queizán JA, Hermosín L, López-Corral L, Megido M, Giraldo P, de las Heras N, Vanegas RJ, Gutiérrez NC, Hernández-Rivas JM. Incidence and clinical characteristics of myeloproliferative neoplasms displaying a PDGFRB rearrangement. Eur J Haematol. 2012;89:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Baer C, Muehlbacher V, Kern W, Haferlach C, Haferlach T. Molecular genetic characterization of myeloid/Lymphoid neoplasms associated with eosinophilia and rearrangement of PDGFRA, PDGFRB, FGFR1 or PCM1-JAK2. Haematologica. 2018;103:e348-e350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Wilkinson K, Velloso ER, Lopes LF, Lee C, Aster JC, Shipp MA, Aguiar RC. Cloning of the t(1;5)(q23;q33) in a myeloproliferative disorder associated with eosinophilia: involvement of PDGFRB and response to imatinib. Blood. 2003;102:4187-4190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Abraham SM, Salama ME, Jacobsen JR, Hancock J, Fluchel M. Myeloid Neoplasm with PDGFRB Translocation, t(1;5)(q21;q33): A Congenital Presentation of An Imatinib Responsive Congenital JMML with Eosinophilia. Blood. 2010;116:4092. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Li Z, Yang R, Zhao J, Yuan R, Lu Q, Li Q, Tse W. Molecular diagnosis and targeted therapy of a pediatric chronic eosinophilic leukemia patient carrying TPM3-PDGFRB fusion. Pediatr Blood Cancer. 2011;56:463-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Abraham S, Salama M, Hancock J, Jacobsen J, Fluchel M. Congenital and childhood myeloproliferative disorders with eosinophilia responsive to imatinib. Pediatr Blood Cancer. 2012;59:928-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 9. | Zhang XY, Liu TF, Li CW, Li QH, Zhu XF. [Pediatric myeloid neoplasms associated with eosinophilia and platelet-derived growth factor receptor beta gene rearrangement: a case report and literature review]. Zhonghua Er Ke Za Zhi. 2018;56:34-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Butt NM, Lambert J, Ali S, Beer PA, Cross NC, Duncombe A, Ewing J, Harrison CN, Knapper S, McLornan D, Mead AJ, Radia D, Bain BJ; British Committee for Standards in Haematology. Guideline for the investigation and management of eosinophilia. Br J Haematol. 2017;176:553-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |