Published online Jan 6, 2021. doi: 10.12998/wjcc.v9.i1.197
Peer-review started: May 27, 2020
First decision: November 25, 2020
Revised: November 25, 2020
Accepted: December 6, 2020
Article in press: December 6, 2020
Published online: January 6, 2021
Processing time: 218 Days and 14.8 Hours
Pulmonary thromboembolism (PTE) is a serious postoperative complication that can occur after a fracture. Generally, PTE is caused by the falling off of lower extremity deep vein thrombosis (LEDVT) after lower limb fracture surgery. LEDVT and PTE after upper extremity fracture surgery are very rare. PTE is one of the most common clinical causes of sudden death. Venous thromboembolism includes PTE and DVT. We experienced one case of LEDVT and PTE after distal ulna and radius fracture surgery. The purpose of our report is to raise awareness for orthopedic surgeons that PTE can occur after distal ulna and radius fracture surgery, and patients with high risk factors should be considered for prevention and treatment of thrombosis in a timely manner.
We report a 51-year-old Chinese male who had severe fractures of the left distal ulna, radius and little finger after a motorcycle accident. The patient underwent external fixation, open reduction and internal fixation. On the third post-operative day, computed tomographic pulmonary angiography showed PTE. Doppler ultrasonography showed thrombus formation in the bilateral posterior tibial veins. After a period of anticoagulation therapy, on the 25th d after the PTE, computed tomographic pulmonary angiography showed that thrombus in both sides of the pulmonary artery disappeared. Furthermore, about 4 mo after the PTE, thrombosis in the deep veins of the lower limbs disappeared. About 1 year after the surgery, X-rays showed good fracture healing, and the function of the wrist joint recovered well.
Though rare, PTE can occur after distal ulna and radius fracture surgery and patients with high risk factors should be considered for prevention and treatment of thrombosis in a timely manner.
Core Tip: We report a 51-year-old Chinese male who had severe fractures in the left distal ulna, radius and little finger after a motorcycle accident. The patient underwent external fixation, open reduction and internal fixation. On the third postoperative day after the second surgery, he was diagnosed with pulmonary thromboembolism and lower extremity deep vein thrombosis. After 25 d of anticoagulation therapy, computed tomographic pulmonary angiography showed that the thrombus in the pulmonary artery disappeared. This case raises awareness for orthopedics that pulmonary thromboembolism can occur after upper extremity surgery, and that patients with high risk factors should be considered for prevention and treatment of thrombosis in a timely manner.
- Citation: Lv B, Xue F, Shen YC, Hu FB, Pan MM. Pulmonary thromboembolism after distal ulna and radius fractures surgery: A case report and a literature review. World J Clin Cases 2021; 9(1): 197-203
- URL: https://www.wjgnet.com/2307-8960/full/v9/i1/197.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i1.197
There are three main predisposing factors that can lead to deep vein thrombosis (DVT): damage to the lining of the vein, slow movement of blood in the circulation and a high blood coagulation state[1]. If pulmonary thromboembolism (PTE) is not diagnosed and treated promptly, it is associated with a mortality rate as high as 30% and accounts for 5%-10% of inpatient hospital deaths in the United States[2]. DVT is more common in the lower extremity after lower extremity fracture surgery. The incidence of venous thromboembolism (VTE) after upper extremity fracture is about 1.3%; the incidence of VTE after mid- and/or distal ulna and/or radius fracture is about 0.95%[3]. We present the case of a 51-year-old man who was diagnosed with a lower extremity DVT and PTE after distal ulna and radius fracture surgery. Our patient was informed that data concerning the case would be submitted for publication.
A 51-year-old male was admitted to our hospital on August 28, 2013 after a motorcycle accident. The patient complained of pain, swelling and bleeding in the left upper limb for 2 h.
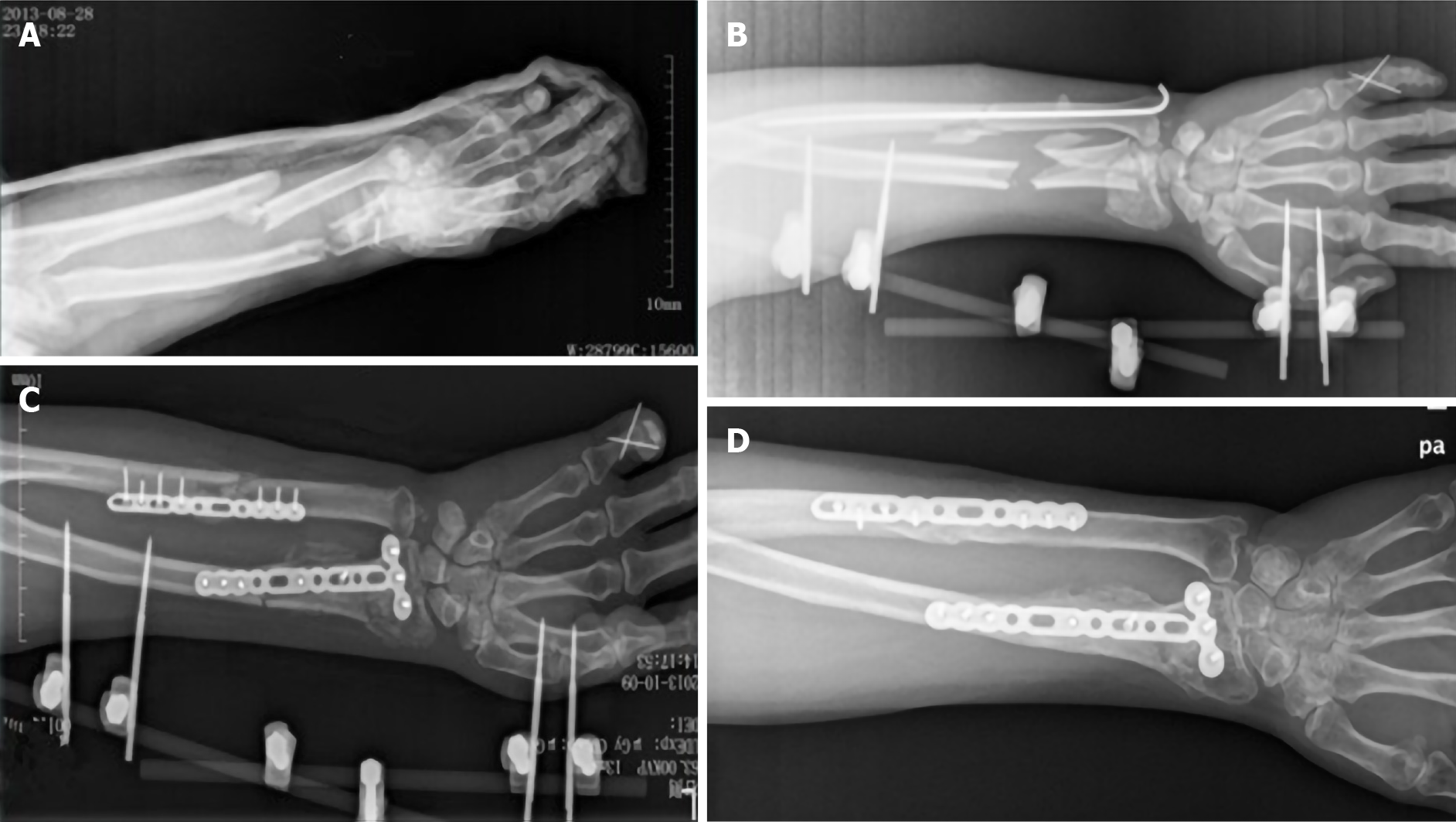
The patient had a motorcycle accident on August 28, 2013. The left little finger landed first, then the left wrist flexed and landed and the dorsal skin of the left forearm was punctured by ulna and radius fractures causing pain, swelling and bleeding of the left upper limb. The skin of the left hand felt normal after the trauma, and finger activity was normal. He was sent to our hospital for emergency treatment for debridement, and his left arm was fixed with long arm plaster. The X-ray radiography showed fractures of the left distal ulna, radius and little finger as well as wrist joint dislocation (Figure 1A). The injury occurred only in the left upper limb, and no other organs or tissues were injured.
The patient was hospitalized for further surgical treatment. External fixation and Kirschner wire were used to stabilize the left distal ulna, radius and little finger (Figure 1B). The patient walked every day after the operation. The swelling of the left forearm was reduced 12 d after the first surgery. Open reduction and internal fixation were performed under general anesthesia to stabilize the left distal ulna and radius. Because some of the bone in the distal radius was lost due to compression, we took about 10 g of bone from the left ilium to fill (Figure 1C). The operation continued for 4 h. Broad-spectrum antibiotic was used to prevent infection, and drugs were used to promote blood flow and microcirculation. After the anesthesia recovery period, the patient told us that he suffered severe pain and could hear the noise from the electric drill during the operation, but he was unable to communicate this during the operation. This led to psychological trauma. The patient became very sensitive to pain and remained in bed until the third postoperative day.
On the third postoperative morning, when he got out of bed and was going to the toilet, he was unable to stand, owing to double lower limb weakness and pain. The double lower limbs were not tumid, and the skin color was normal. When he received a shot for the intravenous infusion, he was very nervous and suddenly felt chest pain and asthma and had breathing difficulty. Additionally, his double lung breaths sounded thick and had a large number of dry and wet rales. The partial pressure of arterious blood oxygen was 7.2 kPa (normal range 11-13 kPa), arterial oxygen saturation was 88.8% (normal range 91.9%-99.0%), and central venous pressure was 11 cmH2O (normal range 5-10 cmH2O). After oxygen therapy, peripheral capillary hemoglobin oxygen saturation was 92%, blood pressure was 158/110 mmHg, heart rate was 110 bpm, and respiratory rate was 30 bpm. The brain natriuretic peptide troponin I was negative. The serum D-dimer level was 17.48 μg/mL. The bedside electrocardiograph showed sinus tachycardia. Wells and revised Geneva scores were 9 and 11, respectively. The patient was diagnosed with high clinical probability of PTE based on these Wells and revised Geneva scoring systems[4,5]. We first considered PTE.
The patient had no previous surgery or medical problems or family history of blood clotting disorders. Previously, the patient had no respiratory or circulatory problems.
The patient was a 51-year-old male, weighed 90 kg and was 175 cm tall. His body mass index (BMI) was 29.4 kg/m2. He started drinking heavily around age 17 (about 500 mL of wine a day). He did not smoke. None of his family has suffered from VTE.
Left forearm dorsal skin was pricked by ulna and radius fracture ends, swelling deformity, bleeding, left wrist activity limitations. The skin of the left hand felt normal after the trauma, and finger activity was normal.
On the morning of the first day after the motorcycle accident, the serum D-dimer level was 0.80 μg/mL (the reference range < 1.00 μg/mL). When pulmonary embolism occurred, the serum D-dimer level was 17.48 μg/mL. The routine blood and biochemical indicators were not significantly abnormal.
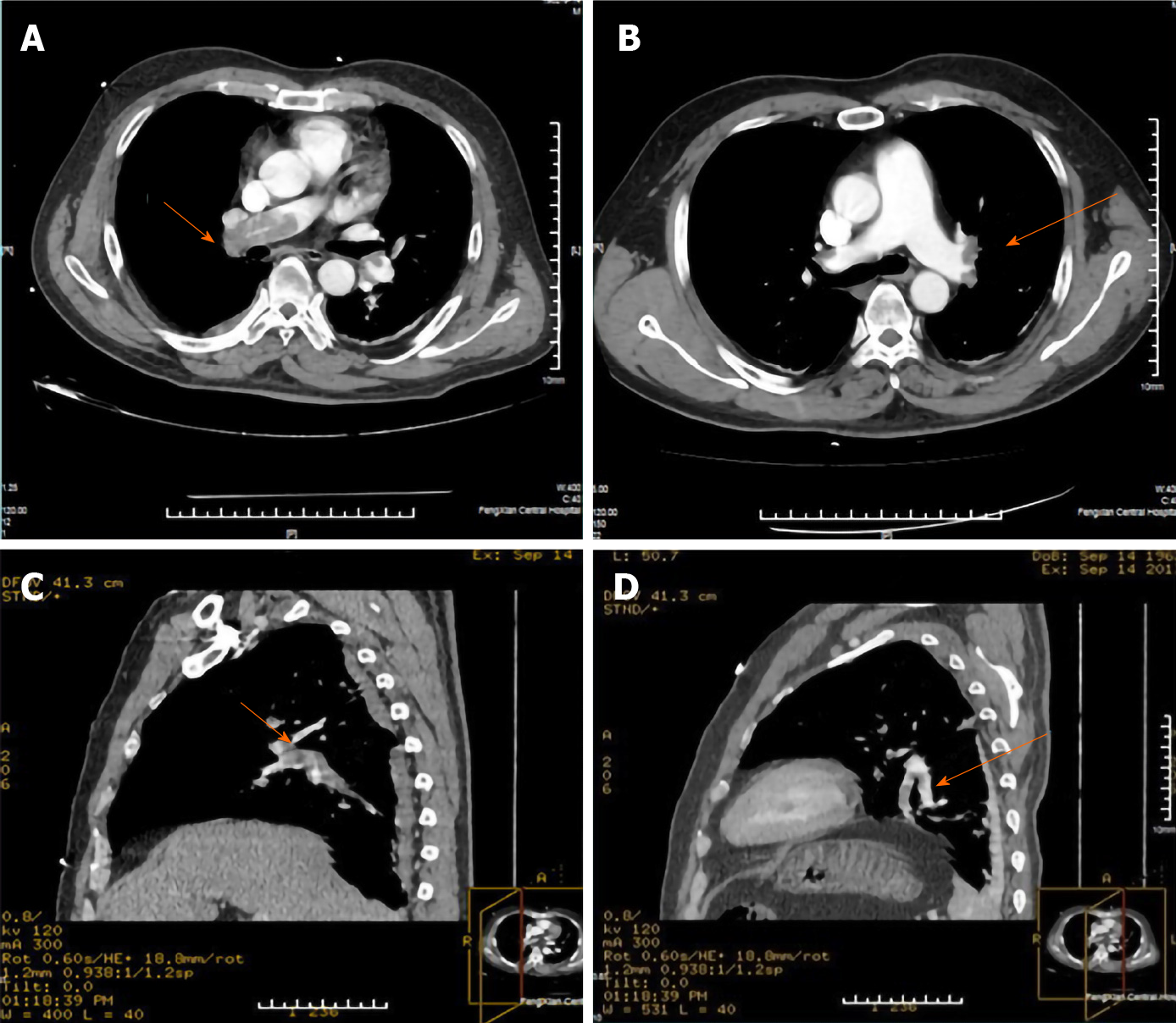
Computed tomographic pulmonary angiography (CTPA) showed intravascular wirelike, sheet filling defects in both sides of the pulmonary artery trunks and its branches; the large shadow in the right pulmonary trunk was about 56 mm × 16 mm (Figure 2). Doppler ultrasonography showed no significant anomalies in the upper extremity deep vein within the bilateral posterior tibial veins of the lower limb thrombus formation. Echocardiography did not show patent foramen ovale and a right to left shunt.
Based on the clinical findings, along with the serum D-dimer, CTPA and upper and lower extremity Doppler ultrasonography, the patient was diagnosed with PTE, LEDVT after distal ulna and radius fracture surgery.
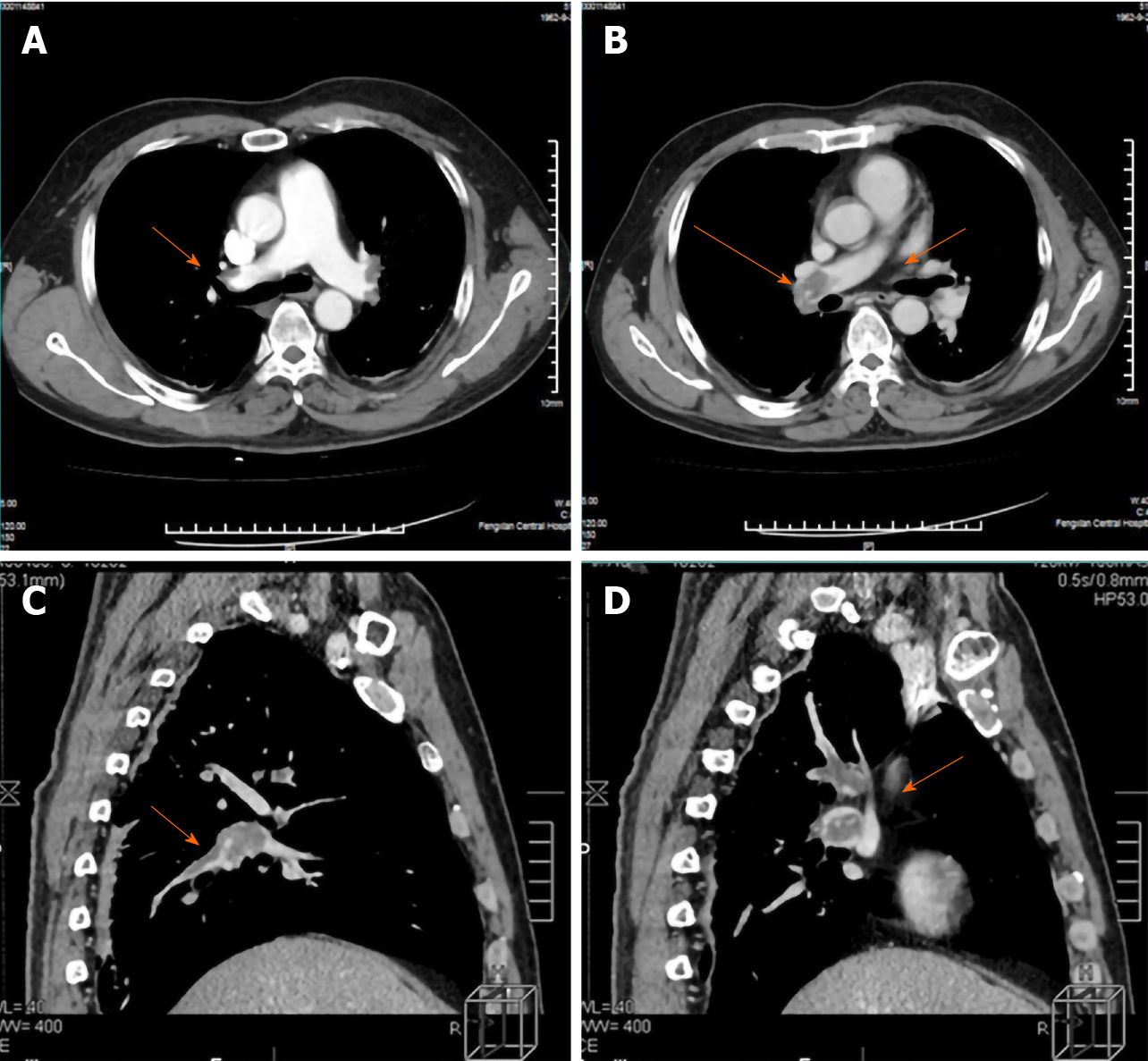
The patient was immediately given oxygen therapy and transferred to the intensive care unit. The patient’s body position remained in one place to prevent deep vein thrombosis of the lower limbs breaking off and increasing pulmonary embolism. Low molecular weight heparin sodium and warfarin sodium were used for anticoagulation. The drugs were used to promote local blood flow, and antibiotics were used to prevent lung infection. On the same day, subcutaneous injections of low molecular weight heparin sodium (17500 IU) was administered. In the first 9 days after PTE, low molecular weight heparin sodium (10000 IU) was administered subcutaneously every day in the morning and at night. On the ninth day after PTE, re-examination of the CTPA showed that the thrombus in both sides of the pulmonary artery was not significantly reduced (Figure 3). Reexamination of the deep venous vascular ultrasound showed thrombosis in both the superficial femoral and deep femoral veins and intravascular thrombosis of the bilateral soleus. The anticoagulation effect was not ideal, as there was a need to adjust anticoagulation. The 10th to 31st days after PTE, low molecular weight heparin sodium (15000 IU) was administered subcutaneously every day at morning, noon and night. From the 32nd day after PTE, we stopped using low molecular heparin sodium.
On the sixth day after PTE, the patient said his right lower limb was painful, and an additional 2.5 mg of warfarin sodium was used every day. On the 14th and 15th day after PTE, the dose of warfarin sodium was increased to 3.125 mg. On the 16th to 18th day after PTE, the dose of warfarin sodium was increased to 3.75 mg. On the 19th to 22nd day after PTE, the dose of warfarin sodium was increased to 5 mg. On the 23rd to 35th day after PTE, the dose of warfarin sodium was increased to 6.25 mg. On the 31st d after PTE, the international normalized ratio (INR) was 1.47 (normal range 0.69-1.33, INR should be controlled in the 2-3 range, according to the guidelines). On the 40th day after PTE, the INR was 2.74, and the warfarin sodium was reduced to 5 mg. On the 43rd day after PTE, the INR was 2.96, and the warfarin sodium was reduced to 4.375 mg. We continued to administer 4.375 mg of warfarin sodium every night for 1 yr.
After 25 d of anticoagulant treatment, CTPA showed the thrombus in both sides of the pulmonary artery had disappeared. About 4 mo after PTE, thrombosis in the deep veins of the lower limbs had disappeared. On the 44th d after the PTE, the patient was discharged and continued oxygen therapy for about 3-4 mo. About 7 mo after PTE, the patient’s respiratory function returned to near the previous level. About 2 mo after the second surgery, the external fixation was dismantled. About 1 year after the second surgery, radiographs showed good fracture healing (Figure 1D), and the left wrist function recovered.
PTE after upper extremity fracture is rare. There are no common guidelines for PTE after upper extremity fractures. Common clinical manifestations of DVT include swelling, redness, tenderness, and the presence of collateral superficial veins. Common clinical manifestations of PTE comprise a sudden onset of dyspnea, chest pain, syncope, hemoptysis, tachycardia and tachypnoea. Because the above clinical manifestations are not specific, further auxiliary examinations are necessary in time. D-dimer has high sensitivity, low specificity and a high negative predictive value in predicting VTE[6]. If the plasma D-dimer is negative, VTE can be largely excluded. Doppler ultrasonography has high sensitivity and specificity in the diagnosis of DVT, which is the preferred method for the diagnosis of DVT at present[7]. CTPA has a high sensitivity for PTE, which is currently the preferred test for diagnosing PTE[8,9]. The treatment of PTE mainly includes anticoagulation and thrombolysis.
For patients with hemodynamic instability, thrombolytic therapy is preferred to restore pulmonary reperfusion in time. In contrast, for patients with hemodynamic stability, anticoagulant therapy is generally preferred[10,11]. The patient reported suffered from severe pain during the operation. This led to psychological trauma. The patient became very sensitive to pain and remained in bed until the third postoperative day. Upper limb fractures and surgical trauma led to a high blood coagulation state. The patient was bedridden for three consecutive days after surgery, and the activity of both lower limbs was significantly reduced, resulting in slow movement of the blood in the circulation of both lower limbs.
The patient was a 51-year-old male who weighed 90 kg and was 175 cm tall. His BMI was 29.4 kg/m2. Asians and the west people belong to different races. The BMI obesity standard of WHO is not very suitable for Chinese people, so the reference standard of BMI obesity in China is established as BMI ≥ 28 kg/m2. The patient was obese. Combined with the above factors, LEDVT and PTE occurred. The case we reported did not have shock symptoms, so we immediately transferred him to the intensive care unit to monitor his vital signs. At the same time, we immediately gave anticoagulant treatment and adjusted the dosage of anticoagulant drugs according to the treatment results. The patient ultimately recovered. For patients with decreased activity of daily living and depression after operation, clinicians should encourage patients to get out of bed early after fracture surgery and give anticoagulation therapy in time if necessary.
PTE can occur after distal ulna and radius fracture surgery, and patients with high risk factors should be considered for the prevention and treatment of thrombosis.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nava-Zavala AH S-Editor: Zhang L L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Wolberg AS, Aleman MM, Leiderman K, Machlus KR. Procoagulant activity in hemostasis and thrombosis: Virchow's triad revisited. Anesth Analg. 2012;114:275-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 222] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 2. | Schleyer AM, Schreuder AB, Jarman KM, Logerfo JP, Goss JR. Adherence to guideline-directed venous thromboembolism prophylaxis among medical and surgical inpatients at 33 academic medical centers in the United States. Am J Med Qual. 2011;26:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Nayar SK, Kuwabara AM, Flores JM, Osgood GM, LaPorte DM, Shafiq B. Venous Thromboembolism in Upper Extremity Fractures. J Hand Surg Asian Pac Vol. 2018;23:320-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Wells PS, Ginsberg JS, Anderson DR, Kearon C, Gent M, Turpie AG, Bormanis J, Weitz J, Chamberlain M, Bowie D, Barnes D, Hirsh J. Use of a clinical model for safe management of patients with suspected pulmonary embolism. Ann Intern Med. 1998;129:997-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 594] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 5. | Le Gal G, Righini M, Roy PM, Sanchez O, Aujesky D, Bounameaux H, Perrier A. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 652] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 6. | Philbrick JT, Heim S. The d-dimer test for deep venous thrombosis: gold standards and bias in negative predictive value. Clin Chem. 2003;49:570-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Johnson SA, Stevens SM, Woller SC, Lake E, Donadini M, Cheng J, Labarère J, Douketis JD. Risk of deep vein thrombosis following a single negative whole-leg compression ultrasound: a systematic review and meta-analysis. JAMA. 2010;303:438-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 8. | van Beek EJ, Brouwerst EM, Song B, Stein PD, Oudkerk M. Clinical validity of a normal pulmonary angiogram in patients with suspected pulmonary embolism--a critical review. Clin Radiol. 2001;56:838-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 115] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Moores LK, Jackson WL Jr, Shorr AF, Jackson JL. Meta-analysis: outcomes in patients with suspected pulmonary embolism managed with computed tomographic pulmonary angiography. Ann Intern Med. 2004;141:866-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Konstantinides SV. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3145-3146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, Stevens SM, Vintch JRE, Wells P, Woller SC, Moores L. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3434] [Article Influence: 381.6] [Reference Citation Analysis (2)] |