Published online Jan 6, 2021. doi: 10.12998/wjcc.v9.i1.190
Peer-review started: May 23, 2020
First decision: September 14, 2020
Revised: October 17, 2020
Accepted: November 9, 2020
Article in press: November 9, 2020
Published online: January 6, 2021
Processing time: 222 Days and 21.8 Hours
Pituitary metastasis is an uncommon manifestation of systemic malignant tumors. Moreover, hyperprolactinemia and overall hypopituitarism caused by metastatic spread leading to the initial symptoms are rare.
A 53-year-old male patient was admitted to our hospital with complaints of bilateral blurred vision, dizziness, polyuria, nocturia, severe fatigue and somnolence, decreased libido, and intermittent nausea and vomiting for more than 6 mo. During the last 7 d, the dizziness had worsened. Laboratory investigations revealed overall hypofunction of the pituitary gland, but the patient had an elevated serum prolactin level (703.35 mg/mL). Preoperative magnetic resonance imaging revealed a tumor in the sellar region, accompanied by intratumoral hemorrhage and calcification. Thus, transnasal subtotal resection of the lesion in the sellar region was performed. The histopathological and immunohistochemical examinations of the resected lesion revealed metastasis of lung adenocarcinoma to the pituitary gland. Oral hydrocortisone (30 mg/d) and levothyroxine (25 mg/d) were given both pre- and postoperatively. Post-operatively, the clinical symptoms were significantly improved. However, 4 mo following the surgery, the patient succumbed due to multiple organ failure.
Hyperprolactinemia is one of the markers of poor prognosis in patients with carcinoma that metastasizes to the pituitary gland. Exogenous hormone supplementation plays a positive role in relieving the symptoms of patients and improving quality of life.
Core Tip: Pituitary metastasis is an uncommon manifestation of systemic malignant tumors. Moreover, hyperprolactinemia and overall hypopituitarism caused by metastatic spread leading to the initial symptoms are very rare. We report a rare case of hyperprolactinemia caused by pituitary metastasis of lung adenocarcinoma and reviewed the relevant literature. We found that hyperprolactinemia is one of the markers of poor prognosis in patients with carcinoma metastatic to the pituitary gland, and exogenous hormone supplementation plays a positive role in relieving the symptoms of patients and improving their quality of life.
- Citation: Liu CY, Wang YB, Zhu HQ, You JL, Liu Z, Zhang XF. Hyperprolactinemia due to pituitary metastasis: A case report. World J Clin Cases 2021; 9(1): 190-196
- URL: https://www.wjgnet.com/2307-8960/full/v9/i1/190.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i1.190
Pituitary metastasis is a rare occurrence. Its prevalence, among all resected pituitary tumors is about 1%[1]. In this group of patients, various case series have reported a median survival of less than a year[2,3]. Review of the literature reveals that hyperpro-lactinemia caused by pituitary metastasis is still rarely observed. Moreover, pituitary metastasis resulting in the first symptom of systemic spread is not common[4]. Even though tumor resulting in stroke often occurs in pituitary macroadenomas, it is rarely reported in pituitary metastasis. In this case report, we present a rare case of hyper-prolactinemia caused by pituitary metastasis resulting in overall hypopituitarism as the first symptom.
A 53-year-old male patient was admitted to our department with complaints of bilateral blurred vision, dizziness, polyuria, nocturia, severe fatigue, somnolence, decreased libido, and intermittent nausea and vomiting for more than 6 mo.
The patient’s symptoms started a little more than 6 mo ago with bilateral blurred vision and dizziness, which had worsened over the last 7 d. There were no obvious symptoms of dyspnea, cough, expectoration, or hemoptysis.
There was no past history of liver or kidney disease or malignant tumor, and no recent history of oral intake of drugs.
The patient had a history of smoking for more than 30 years and had lost 10 kg of body weight within the last 3 mo (baseline body weight: 61 kg).
Physical examination revealed lethargy, delicate and pale skin, decreased visual acuity in the right eye (only perception of light), and bilateral temporal hemianopsia. On auscultation, the breath sounds in both lungs were slightly coarse, and there were no clinical symptoms of enlarged superficial lymph nodes or other discomfort.
Examination of pituitary function revealed low serum levels of gonadotropin and sex hormones (Table 1). Free serum cortisol levels (8:00 am) were found to be as low as 67.16 nmol/L. The serum prolactin (PRL) levels were elevated (703.35 mIU/mL), as was the 24-h urine output (4.6 L/d).
| Hormone | Measurement level, preoperative | Measurement level, postoperative | Normal level |
| TSH | 4.990 μIU/mL | 8.100 μIU/mL | 0.27–4.2n μIU/mL |
| T4 | 3.89 pmol/L | 4.67 pmol/L | 12.0–22.0 pmol/L |
| T3 | 4.66 pmol/L | 1.81 pmol/L | 3.1–6.8 pmol/L |
| FSH | 0.470 mIU/mL | 0.530 mIU/mL | 1.27–19.26 mIU/mL |
| LH | < 0.2 mIU/mL | 0.030 mIU/mL | 1.24 –8.62 mIU/mL |
| GH | 0.207 ng/mL | 0.389 ng/mL | 0.003–0.971 ng/mL |
| PRL | 703.35 mIU/mL | 578.86 mIU/mL | 55.97–278.36 mIU/mL |
| 24-h urine output | 4.600 L/24 h | 3.900 L/24 h | 1.5-2.5 L/24 h |
| Free cortisol (8:00 am) | 67.16 nmol/L | 141.60 nmol/L | 240–619 nmol/L |
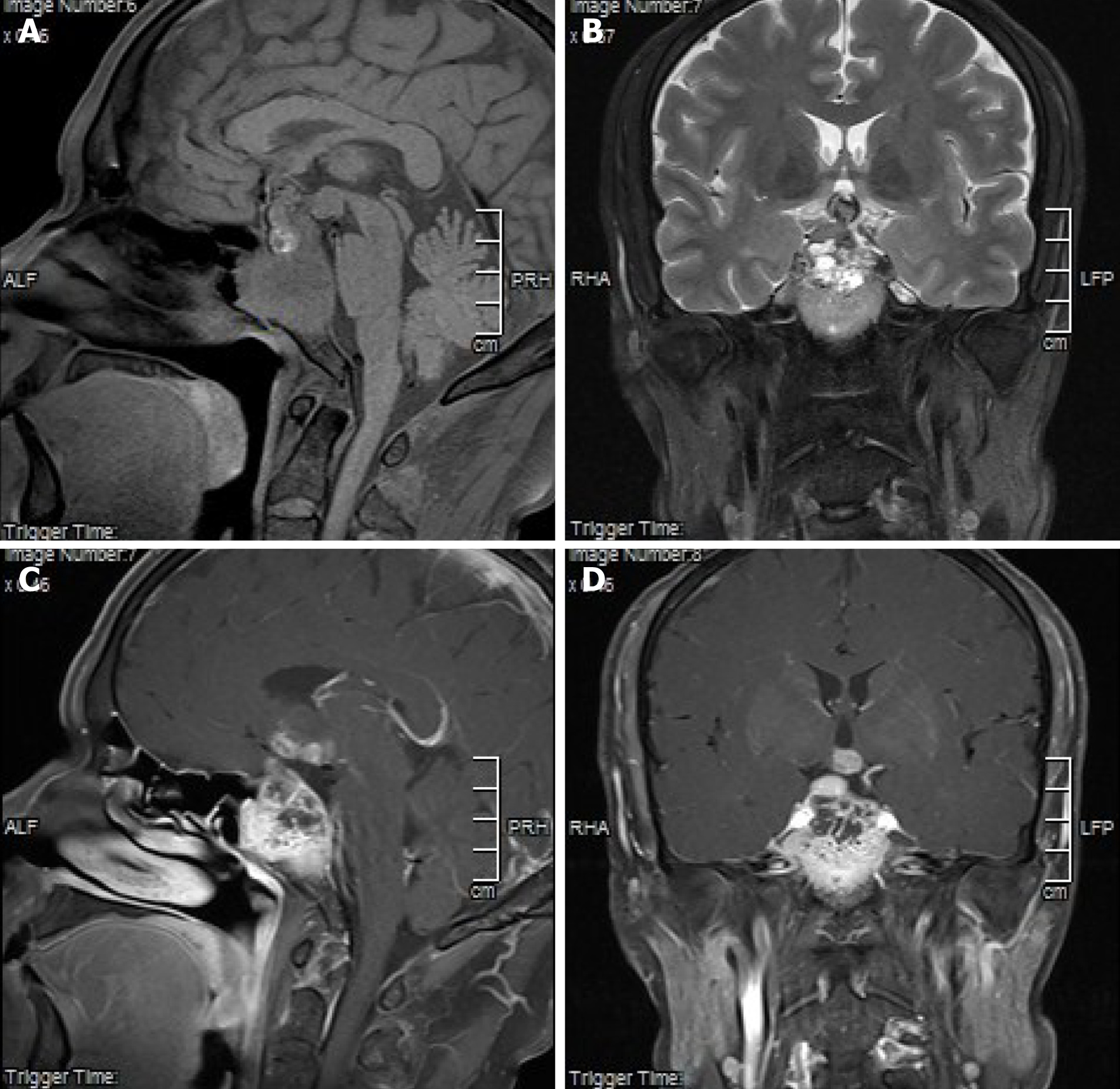
Magnetic resonance imaging (MRI) of the pituitary revealed unclear borders of the sella turcica; a mass (5.37 cm × 3.20 cm × 3.04 cm) protruding from the sella turcica and sphenoid sinus; iso- and spot-like high and low intensity signals on T1 weighted images (T1WIs; Figure 1A); and high, slightly high, and low intensity signals on T2Wis (Figure 1B). The enhanced scan was not uniformly enhanced, and the pituitary gland and its stalk were not clearly visible. The optic nerve and papillary body were surrounded by the mass. The mass protruded into the third ventricle and invaded the bilateral cavernous sinus and internal carotid artery. Thus, only a small part of the clivus was visible (Figure 1C and D). Moreover, a multi-row computed tomography (CT) of the pituitary gland revealed bony destruction around the sella turcica. Plain chest CT revealed a massive high-density shadow (3.5-3.7 cm) in the upper lobe of the right lung, the boundaries of which were unclear. There was a marginal burr, and the possibility of peripheral lung cancer was considered.
Therefore, based on the findings described above, the preliminary diagnosis was invasive pituitary adenoma, the nature of which was difficult to determine.
Preoperatively, oral administration of hydrocortisone (30 mg/d) and levothyroxine (25 mg/d) resulted in significant improvement in the symptoms of fatigue and somnolence. One week later, transnasal subtotal resection of the lesion in the sellar region was performed and tumor tissue was sent for histopathological and immunohistochemical examinations. During the surgery, a solid tumor with abundant blood vessels was found to occupy the sphenoid sinus. Moreover, the tumor was found to invade the surrounding structures and adhered closely to the surrounding tissues, suggesting its malignant nature. Postoperatively, the symptoms of dizziness and visual acuity were significantly improved. The levels of pituitary hormones were re-examined and serum PRL was found to be decreased (578.86 mIU/mL) (Table 1). Moreover, the oral supplementation of both hydrocortisone and levothyroxine was continued.
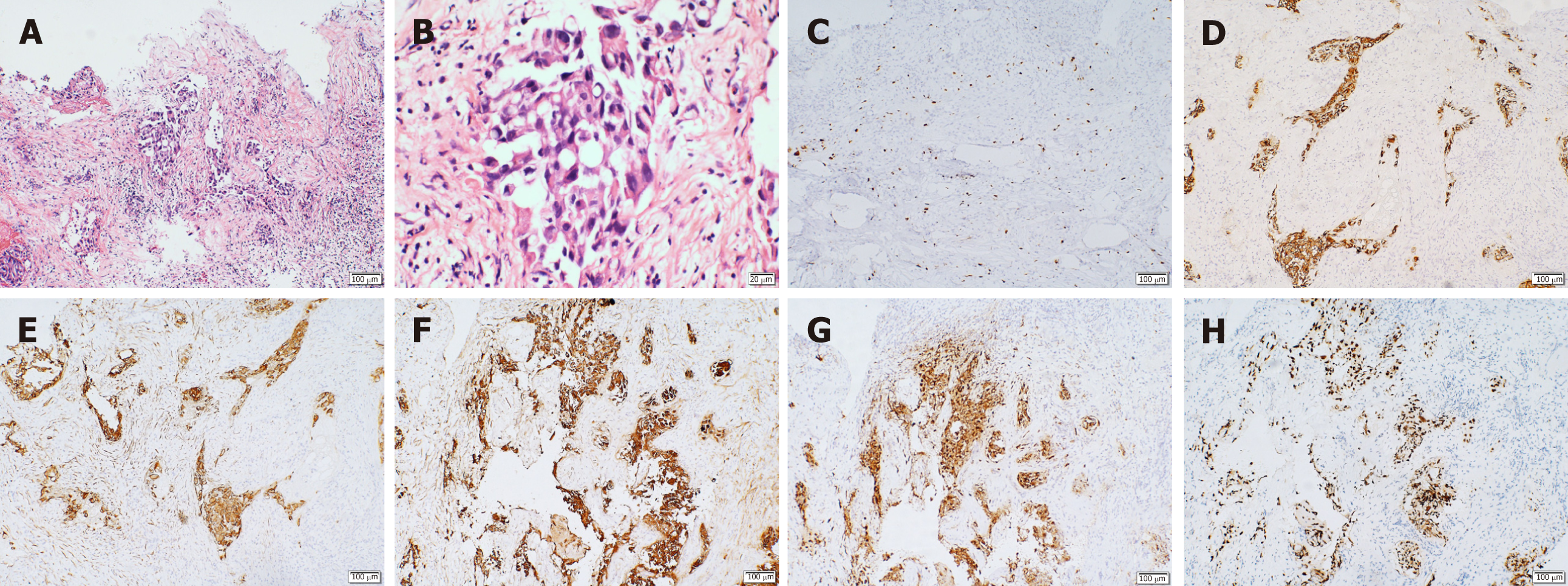
The tumor tissue was fixed in 10% formalin. The MaxVision method was used for immunohistochemistry. The first antibody was anti-Ki-67, anti-Cytochrome c (anti-Cyt), anti-cytokeratin 7 (CK7), anti-CK-pan, anti-thyroid transcription factor 1 (TTF-1), anti-napsin A, anti-calmodulin 5.2 (CAM5.2), anti-CK5/6, anti-p63, anti-Sal-like protein 4 (anti-SALL4), anti-chromogranin (anti-CgA), anti-Sy, and anti-Smuri 100. The second antibody was treated with the ready-to-use rapid immunohistochemical MaxVision HRP Kit. The chromogenic agent was hypersensitive diaminobenzidine. The findings of the histopathological and immunohistochemical examinations were consistent with adenocarcinoma or adenosquamous carcinoma. The immuno-histochemical results showed that the tissue was positive for Ki-67 (20%), CK-pan, TTF-1, CK7, CAM5.2, and napsin A and negative for CK5/6, p63, SALL4, CgA, Sy, and Smuri 100, suggesting the metastasis of non-small cell lung cancer (NSCLC) (T2N0M1b) (Figure 2). Due to financial issues, the patient and his family members decided to go back to the local hospital for further treatment. However, 4 mo following the operation, the patient succumbed due to multiple organ failure.
Tumor metastasis to the pituitary gland is rarely observed in clinics, and there is no significant gender difference in its incidence. The most common cancers metastasizing to the pituitary gland are that of breast and lung[2,5,6]. A recent review of the case series demonstrated that diabetes insipidus and pituitary dysfunction are the most common clinical symptoms, and the posterior pituitary is more likely to be the site of metastasis than the anterior pituitary because of the difference in the surrounding anatomy and blood distribution[3]. This is consistent with the results of previous studies[6]. With the enhancement in screening and diagnostic techniques and the extension of human life span, pituitary metastatic tumors are found frequently, although rarely, but the cases of hyperprolactinemia caused by metastatic spread are still rare. Review of the related literature to pituitary metastasis resulted in only 4 cases with serum PRL level > 200 ng/mL caused by metastatic disease (PRL > 200 ng/mL is used as a diagnostic criteria for prolactinoma)[7].
A PubMed search for cases and series related to pituitary metastasis published as of 2020 was conducted with "neurosurgical disease" and "pituitary metastasis" used as keywords. In addition, references cited in the selected articles were manually searched and reviewed to identify other potentially eligible studies. The patient data were extracted, and the clinical patient data were described and counted. As shown in Table 2[1,8-10], the average age of cases at the onset of symptoms was 64 years, and the incidence rate in males was 80%, which is higher than that of females. Amongst the primary tumors, there were two cases of lung cancer and one case each of colorectal, thyroid, and liver cancer. All patients had varying degrees of visual field impairment, and 80% of the cases (liver, lung, and colorectal cancer) had oculomotor nerve palsy and required surgical resection of the tumor. In 80% of the patients, the transnasal resection of lesions, in the sellar region, was the preferred approach. Postoperatively, the clinical symptoms of the patients were relieved, especially due to optic nerve decompression. While, in one patient, the transcranial approach for resection of the tumor was used and the outcome was poor. The median postoperative survival was 3.9 mo, with the longest and the shortest survival period being 9 mo and 2 wk, respectively. Thus, the overall prognosis remains poor.
| Patients | Ref. | Gender | Age in yr | Clinical symptoms | PRL level | Mode of operation | Postoperative symptoms | Primary tumor | Postoperative survival time |
| 1 | Yao et al[8] | M | 67 | Headache, left eyelid prolapse | 1022 ng/mL | Endoscopic transsphenoidal surgery | Headache relief | Lung adenocarcinoma | 3 mo |
| 2 | Thewjitcharoen et al[9] | M | 65 | Visual impairment, left eyelid prolapse | 254 ng/mL | Transsphenoidal subtotal resection | Vision returned to normal | Colorectal cancer | 9 mo |
| 3 | Stojanović et al[10] | F | 67 | Visual impairment | 1270 mU/L | Transcranial surgery | No improvement | Thyroid carcinoma | 2 wk |
| 4 | Komninos et al[1] | M | 68 | Headache, visual impairment | 438.6 ng/mL | Transsphenoidal decompression | Headache relief | Liver cancer | 3 mo |
| 5 | Present case | M | 53 | Visual impairment, dizziness | 703 mIU/mL | Transnasal subtotal sphenotomy | Improvement of vision and dizziness | Lung adenocarcinoma | 4 mo |
It is worth mentioning a case report by Yao et al[8], where metastatic tumor cells secreting PRL were confirmed by both immunohistochemistry and Western blotting, and it was speculated that the abnormal activation of pan-catenin regulated the transcription of PRL. In this case, a large mass occupying the sellar region and presenting with hyperprolactinemia strongly suggested a PRL pituitary adenoma. However, postoperatively, it proved to be NSCLC metastasis, and almost no PRL could be detected in the serum[8]. In our case, because the tumor was mainly located below the hypothalamic area and in the sphenoid sinus, the possibility of increased PRL secretion caused by hypothalamic inhibition was almost nonexistent. Therefore, in the present case, there was a reason to suspect the presence of a malignant tumor secreting PRL, although the chances were extremely rare. As the family refused to perform gene sequencing, there is a lack of evidence supporting our hypothesis.
It is generally believed that the diagnosis of prolactinoma can be confirmed by the radiological identification of sellar masses and elevated serum PRL levels (> 200 ng/mL)[7]. However, this criteria needs to be reconsidered. The preoperative diagnosis of pituitary metastatic tumors mainly depends on radiological examination. A study demonstrating MRI findings that may be beneficial in diagnosing pituitary metastasis included thickening of the pituitary stalk, disappearance of the posterior pituitary bright spots, and iso-intensity on T1WI and T2WI[11]. As the malignant tumor grows in an invasive manner, the bony structures around the sellar and parasellar regions are often destroyed. Castle-Kirszbaum et al[3], in a case series, hypothesized that the development of diabetes insipidus or ophthalmoplegia caused by any pituitary lesion indicate its metastatic nature. The findings in the present case support this hypothesis. However, the confirmed diagnosis of pituitary metastasis requires pathological evidence.
It is still unclear whether pituitary metastases can be treated by surgery. Gilard et al[2], in their study, demonstrated that the surgical resection of the pituitary gland has no effect on survival, but it can relieve the symptoms of patients, especially due to optic nerve decompression, which is reflected by visual improvement in our patient. Based on the tumor growth pattern, different surgical approaches can be chosen. The tumor usually tends to grow at the bottom of the sellar and subsellar diaphragm; thus, the endoscopic transsphenoidal approach is the first choice. When the tumor is located in the upper pituitary or the sellar diaphragm, the skull base approach of the optic-carotid or interoptic space may be the first choice, which is beneficial in attaining hemostasis of the operative area and protecting the surrounding structures. It also prevents the leakage of cerebrospinal fluid and maximizes the resection of the tumor. However, comparison of the surgical methods and postoperative outcomes in five patients suggest that the transnasal resection of tumors in the sellar region should be the first choice. At present, there are no uniform standards advocating postoperative radiotherapy and chemotherapy. However, an exceptional case was reported by Atallah-Yunes et al[12], in which radiotherapy resulted in a significant reduction in tumor size. Finally, a study by Caponnetto et al[13] confirmed that hyperprolactinemia may be one of the factors of poor prognosis of tumors metastatic to the pituitary gland.
Hyperprolactinemia and overall hypopituitarism caused by pituitary metastasis leading to the initial symptoms are rare. Review of the relevant literature reveals that hyperprolactinemia is one of the indicators of poor prognosis. Preoperative and postoperative exogenous supplementation of hormones to correct pituitary hypofunction plays a positive role in relieving clinical symptoms and improving quality of life. Moreover, surgical resection results in improvement of visual symptoms.
The authors would like to thank the patient for agreeing to provide his medical history.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chien CR, Exbrayat JM, Hata M S-Editor: Huang P L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Komninos J, Vlassopoulou V, Protopapa D, Korfias S, Kontogeorgos G, Sakas DE, Thalassinos NC. Tumors metastatic to the pituitary gland: case report and literature review. J Clin Endocrinol Metab. 2004;89:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 266] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 2. | Gilard V, Alexandru C, Proust F, Derrey S, Hannequin P, Langlois O. Pituitary metastasis: is there still a place for neurosurgical treatment? J Neurooncol. 2016;126:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Castle-Kirszbaum M, Goldschlager T, Ho B, Wang YY, King J. Twelve cases of pituitary metastasis: a case series and review of the literature. Pituitary. 2018;21:463-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Teears RJ, Silverman EM. Clinicopathologic review of 88 cases of carcinoma metastatic to the putuitary gland. Cancer. 1975;36:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Zoli M, Mazzatenta D, Faustini-Fustini M, Pasquini E, Frank G. Pituitary metastases: role of surgery. World Neurosurg. 2013;79:327-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Fassett DR, Couldwell WT. Metastases to the pituitary gland. Neurosurg Focus. 2004;16:E8. [PubMed] |
| 7. | Casanueva FF, Molitch ME, Schlechte JA, Abs R, Bonert V, Bronstein MD, Brue T, Cappabianca P, Colao A, Fahlbusch R, Fideleff H, Hadani M, Kelly P, Kleinberg D, Laws E, Marek J, Scanlon M, Sobrinho LG, Wass JA, Giustina A. Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin Endocrinol (Oxf). 2006;65:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 473] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 8. | Yao H, Rui W, Zhang Y, Liu Y, Lin S, Tang H, Zhao W, Wu Z. Prolactin-Secreting Lung Adenocarcinoma Metastatic to the Pituitary Mimicking a Prolactinoma: A Case Report. Neurosurgery. 2019;85:E773-E778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Thewjitcharoen Y, Shuangshoti S, Lerdlum S, Siwanuwatn R, Sunthornyothin S. Colorectal cancer manifesting with metastasis to prolactinoma: report of a case involving symptoms mimicking pituitary apoplexy. Intern Med. 2014;53:1965-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Stojanović M, Pekić S, Doknić M, Miljić D, Cirić S, Diklić A, Tatić S, Joksimović M, Manojlović-Gačić E, Skender-Gazibara M, Popović V. What's in the Image? Eur Thyroid J. 2013;1:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | van Seters AP, Bots GT, van Dulken H, Luyendijk W, Vielvoye GJ. Metastasis of an occult gastric carcinoma suggesting growth of a prolactinoma during bromocriptine therapy: a case report with a review of the literature. Neurosurgery. 1985;16:813-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Atallah-Yunes SA, Clark J, Samanani S, Soe M. Small Cell Lung Cancer with Pituitary Metastasis Presenting as Secondary Adrenal Insufficiency: A Case Report and Literature Review. Am J Case Rep. 2019;20:207-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Caponnetto S, Iannantuono GM, Barchiesi G, Magri V, Gelibter A, Cortesi E. Prolactin as a Potential Early Predictive Factor in Metastatic Non-Small Cell Lung Cancer Patients Treated with Nivolumab. Oncology. 2017;93:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |