Published online Jan 6, 2021. doi: 10.12998/wjcc.v9.i1.183
Peer-review started: May 27, 2020
First decision: September 13, 2020
Revised: September 30, 2020
Accepted: November 12, 2020
Article in press: November 12, 2020
Published online: January 6, 2021
Processing time: 218 Days and 22.4 Hours
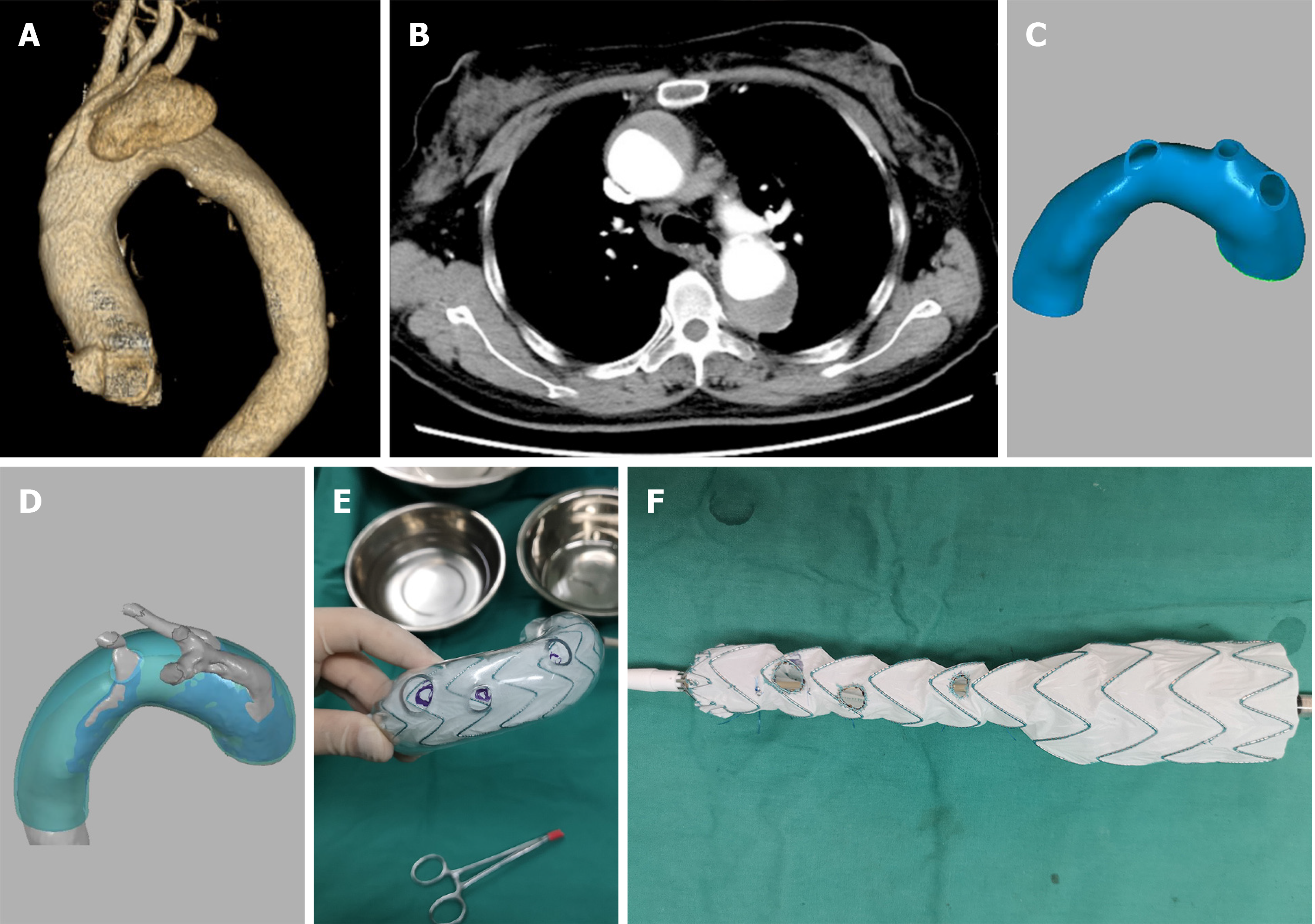
A 63-year-old female was diagnosed with acute Stanford type A aortic dissection. The patient had pain in the chest and back for 1 wk. The computed tomography angiography (CTA) showed Stanford type A aortic dissection (Myla type III aortic arch). The intimal tear was located at the top of the aortic arch and retrograded to the ascending aorta.
Preoperatively, a three-dimensional (3D)-printed model of the aortic arch was made according to CTA data. Then, under the guidance of the 3D-printed aortic model, a pre-fenestrated stent-graft was customized, and the diameter of the stent-graft was reduced intraoperatively by surgeons. 3D printing, triple pre-fenestration, and reduced diameter techniques were used during the surgery. The CTA examinations were performed at the 3rd mo and 1st year after the surgery; the results showed that the aortic dissection was repaired without endoleak, and all three branches of the aortic arch remained unobstructed.
Applying the triple pre-fenestration technique for aortic arch lesions was feasible and minimally invasive in our case. The technique provides a new avenue for thoracic endovascular aortic repair of Stanford type A aortic dissection.
Core Tip: In this work, we report a treatment of Stanford type A aortic dissection by triple pre-fenestration, reducing diameter, and three-dimensional-printing technique. We performed a well-designed total endovascular repair in a patient in which the dissection involved three branches of aortic arch and the ascending aorta. Moreover, we discussed the advantage of three-dimensional-printing technique to guide the application of modified triple pre-fenestrated stent-graft to treat Stanford type A aortic dissection. Temporarily reducing stent diameter was also vital for success of the operation.
- Citation: Zhang M, Tong YH, Liu C, Li XQ, Liu CJ, Liu Z. Treatment of Stanford type A aortic dissection with triple pre-fenestration, reduced diameter, and three-dimensional-printing techniques: A case report. World J Clin Cases 2021; 9(1): 183-189
- URL: https://www.wjgnet.com/2307-8960/full/v9/i1/183.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i1.183
Stanford type A aortic dissection (TAAD) is an extremely dangerous disease in which the dissection involves the aortic arch or ascending aorta. Traditional thoracotomy surgery is traumatic and has many postoperative complications[1]. Performing endovascular repair in the aortic arch also has high rates of mortality and complications, so the ascending aorta is perhaps the last frontier in thoracic endovascular aortic repair (TEVAR)[2]. Currently, the in situ fenestration technique is widely used in TAAD, but it requires the establishment of temporary bypass, which can increase the risk of cerebral infarction[3]. Customized pre-fenestrated stent-grafts can adapt to the anatomical variation in the aortic arch. We reported a case of TAAD that was successfully treated by triple pre-fenestration, reduced diameter, and three-dimensional (3D)-printing techniques.
Sudden chest pain that lasted for 1 wk.
A 63-year-old female patient was admitted to the Department of Vascular Surgery of our hospital due to sudden chest pain in October 2018.
The patient had a history of coronary heart disease and underwent coronary stent implantation in May 2017. She had hypertension for more than 10 years and was treated regularly with nifedipine.
The patient’s parents were healthy, and her personal history was normal.
Upon admission, the patient's body temperature was 36.5 °C, pulse was 72 times/min, and blood pressure was 130/74 mm/Hg. Her breathing was steady, averaging 14 breaths per min. The patient had no chest deformities, and the breath sounds in both lungs were normal. There was no abnormal uplift in her precardiac region and no obvious expansion of her cardiac dullness boundary. Her heart rate was regular at 72 beats/min, and there was no pathological murmur. There were no positive signs in the abdomen and no swelling in the lower limbs.
There were no significant abnormalities in preoperative routine blood tests, routine stool tests, or biochemical examinations. The occult blood test of urine analysis showed weak positive results. The value of D-dimer was 1.87 mg/L in plasma.
The computed tomography angiography (CTA) scan showed Stanford type A aortic dissection (Myla type III aortic arch). The intimal tear was located at the top of the aortic arch and retrograded to the ascending aorta (Figure 1). A series of operations and imaging examinations were performed to address the intractable situation (Table 1).
| Date | October 25, 2018 | November 3, 2018 | February 6, 2019 | November 9, 2019 |
| Operation | Preoperative CTA | Stent implantation of thoracic aorta, innominate artery, left carotid artery, and left subclavian artery | 1st postoperative CTA | 2nd postoperative CTA |
Stanford type A aortic dissection, coronary heart disease (postoperative coronary stent implantation), and hypertension.
Preoperatively, a 3D-printed model of the aortic arch was made according to CTA data. The original computed tomography scan data were input into Mimics software (Materialise’s interactive medical image control system; Materialise Co., Leuven, Belgium) for 3D reconstruction. The 3D reconstruction data were input into design software (Geomagic Studio 2014; Geomagic Co., Morrisville, NC, United States) for further pre-processing. The computer-aided design for the mathematical model of arteries could be obtained by using a reverse engineering technique to reconstruct the nonparametric surface of arteries. To simulate the situation after stent implantation, we dilated the narrow true lumen of the aortic dissection. Geomagic Design Direct 2014 (Geomagic Co.) was used to locate the positions of the three branches of the aortic arch. Then, we used it to design the 3D printing guide plate and print the model. Finally, the 3D-printed model was disinfected with ethylene oxide (Figure 1).
After successful anesthesia, the guidewire and gold-standard catheter were inserted through the right femoral artery, entering the ascending aorta. Angiography was performed to evaluate the dissection conditions. This was consistent with the computed tomography scan results and indicated that the dissection was located at the aortic arch and involved the ascending aorta.
A covered aortic stent-graft (40 mm × 200 mm; Captivia, Medtronic Co., Fridley, MN, United States) was released in a 3D printing model to determine the pre-fenestrated positions. We used an electric pen (CIRX Co., China) to make the pre-fenestration (10 mm for the innominate artery, 7 mm for the left carotid artery, and 5 mm for the left subclavian artery). A dehaired platinum spring coil (Cook Co., Bloomington, IN, United States) was sewn up to the edge of the fenestration and the large curved side of the aortic arch with 5-0 nonabsorbent sutures (JNJ Co., New Brunswick, NJ, United States) to serve as a marker during the operation. Additionally, 4-0 nonabsorbent sutures (JNJ Co.) and V18 guidewires (Boston Scientific Co., Marlborough, MA, United States) were used to reduce the diameter of the stent to 24 mm (Figure 1).
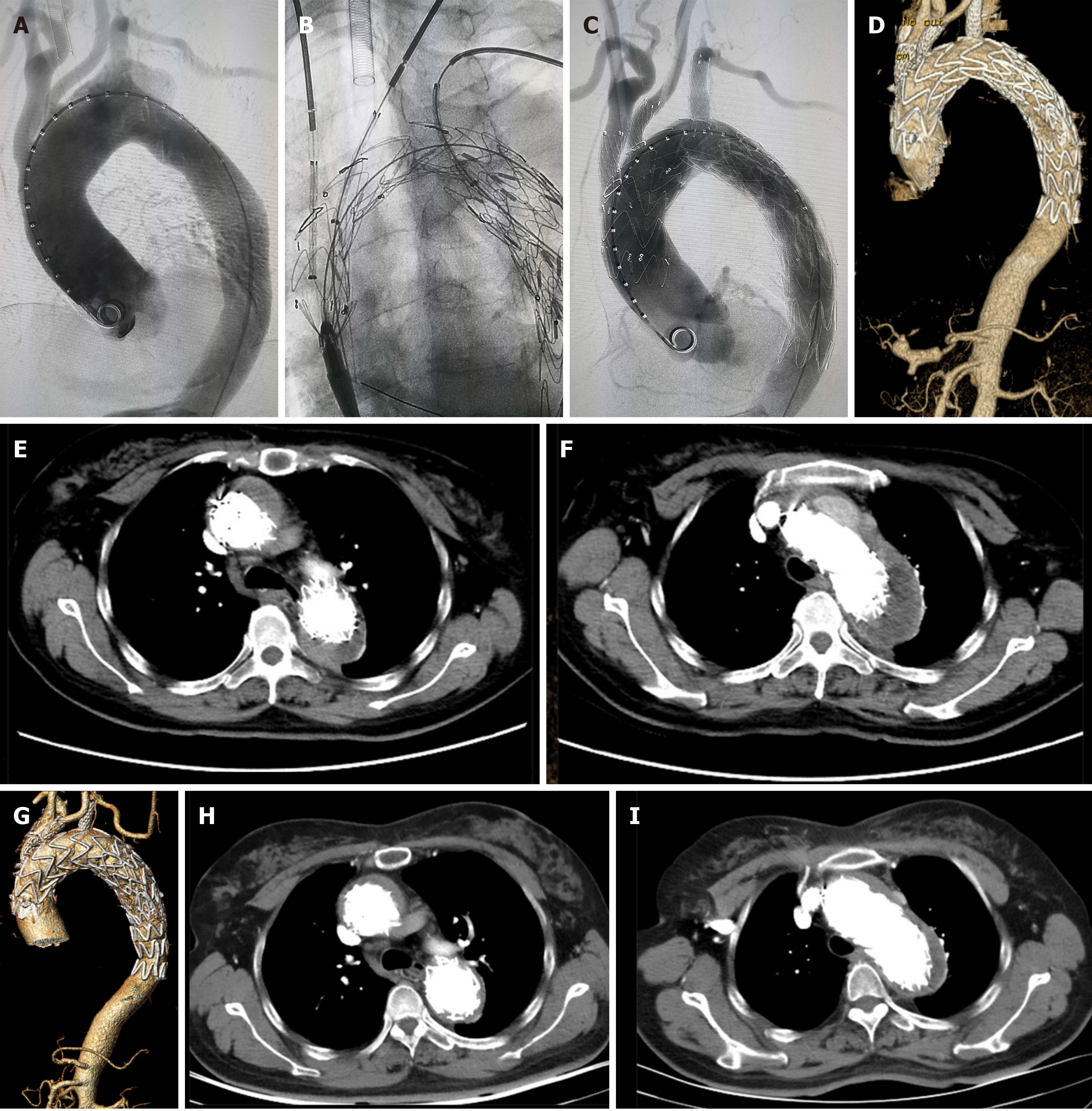
Subsequently, we returned the modified stent-graft to the conveying sheath. Bilateral cervical and left elbow incisions were made, and the vascular sheaths were inserted into the bilateral carotid arteries and the left brachial artery. A soft guidewire with a catheter was passed from the right femoral artery to the descending aorta and then was exchanged with a stiff guidewire. A covered stent-graft (32 mm × 80 mm; Ankura, Life Tech Co., China) was released at the descending aorta as a distal restrictive stent. The modified stent-graft was released in the aortic arch. The directions of the marks and fenestrations were adjusted to the large curved side of the aortic arch. The stent-graft was released, and the diameter of the stent-graft was reduced. The guidewires were super-selectively sent through the fenestrations into the modified stent-graft. A covered stent-graft (13 mm × 40 mm; Fluency, Bard Co., Tempe, AZ, United States) was released through the guidewire in the innominate artery. A covered stent-graft (10 mm × 40 mm; Fluency, Bard Co.) was released through the guidewire in the left carotid artery. Another 10 mm × 40 mm covered stent-graft (Fluency, Bard Co.) was released through the guidewire in the left subclavian artery. After confirming the correct position by angiography, we recovered the original diameter of the modified stent-graft. The expanding balloons were used to expand the fenestrations. Angiography showed that all the stents were in the correct positions without obvious endoleak, and the three branches of the aortic arch were unobstructed. The operation lasted approximately 4.5 h: 1.5 h for stent-graft modification and 3 h for the operation (Figure 2). The radiation time was approximately 38 min, and the total radiation dose was 1029 mGy. Intraoperative blood loss was approximately 200 mL without transfusion.
The patient recovered well after the surgery and returned to the general ward. There was no temporary bypass during the operation, and no neurological complications occurred after the surgery. The patient received long-term antiplatelet treatment with aspirin (100 mg/d) and clopidogrel (50 mg/d) to prevent in-stent restenosis, and she was discharged from the hospital 10 d after the surgery.
The CTA examination performed 3 mo after the surgery showed that the stents were in the correct position without displacement, and all three branches of the aortic arch were unobstructed. An intramural hematoma had formed. However, there was a small endoleak outside of the aortic stent-graft (Figure 2). Fortunately, the 1st year follow-up CTA examination after surgery indicated that the endoleak had completely disappeared. The intramural hematoma was mostly absorbed, and the lumen of the primary aortic dissection aneurysm had not enlarged further (Figure 2).
In recent years, TEVAR has achieved rapid development and has been widely applied to various descending aortic diseases. Compared with traditional open surgery, TEVAR is safer and leads to fewer perioperative complications[4]. Because of the anatomical complexity of the aortic arch, there are still many difficulties to be solved when we apply TEVAR to treat TAAD[5]. At present, the most common TEVAR techniques include the chimney technique, branched stent technique, and in situ fenestration and pre-fenestration techniques. The chimney technique is simple, but postoperative endoleak and mid- to long-term retrograde ascending aortic dissection are common[6]. The branched stent technique is less likely to cause endoleak because there is no gap between the stents. However, because only single-branched stents are available, hybrid surgery is still needed for three-branch lesions[7]. The in situ fenestration technique can reconstruct anatomical structure, but it is not applicable to all anatomical conditions. For cases of a Myla type Ⅲ aortic arch, in situ fenestration would be more difficult. Temporary bypass during the surgery is needed because blood flow in the aortic arch is blocked. In addition, the frequent operation of the carotid artery and rupture of the covered stent-graft can cause many neurological complications[8]. The pre-fenestration technique can avoid these disadvantages, but it is very important to locate accurately the positions of the fenestrations. If the positions are imprecise, serious complications or even surgical failure can occur.
In our case, the patient received triple pre-fenestration under the guidance of a 3D-printed aortic model. We successfully completed triple pre-fenestration without blocking carotid blood flow. No temporary bypass was established during the operation, and no neurological complications were observed postoperatively. Singh et al[9] first used the pre-fenestration technique in the treatment of aortic disease in 1996. The pre-fenestration technique is theoretically suitable for all anatomical conditions, and the mid- and long-term patency rates of this technique are higher than those of other techniques. At present, there is no commercial fenestrated stent-graft in China. Customized fenestrated stents are expensive and require a long customization period, which makes them difficult to use in emergency cases. Therefore, it is an ideal choice to perform pre-fenestration on commercial stent-grafts during operations[10,11]. For lesions of the aortic arch, accurate pre-fenestration positioning is highly required. In the past, surgeons could only design fenestrated positions based on computerized tomography results and measured linear distances. If the position was not accurate, it would increase the difficulty and time cost of the operation. Although enlarging the diameter of the fenestration was convenient during the operation, it was also prone to cause endoleak. Therefore, accurate positioning is the most important technique in pre-fenestration, especially in triple pre-fenestration[12].
The 3D printing technique enables surgeons to more intuitively understand the relative spatial relationship between the aortic dissection or aneurysm and branches of the aortic arch. Some hospitals use 3D-printed models in individual cases to guide fenestration[13,14]. However, they only use 3D-printed models to understand spatial relationships. We simulated the process of stent release in the aorta with professional software and designed a 3D-printed model according to simulation results. Afterwards, we released the stent-graft in the 3D-printed model to determine accurately the pre-fenestration positions. Temporarily reducing the diameter of the stent was also vital for the success of the operation. In this case, the diameter of the stent was reduced to 60%. The stent could be easily adjusted because it was smaller than the aorta. This technique improved the safety of the surgery. There was a gap between the aortic arch and the stent so that the blood supply of the aortic arch would not be blocked until the stent was completely released. There was adequate blood supply in the carotid arteries, and no bypass was needed, which could reduce neurological complications.
The pre-fenestration technique under the guidance of the 3D-printed aortic model provided a new solution for type A aortic dissection The guidance of 3D printing could accurately locate the fenestration positions. Reducing the diameter improved surgical safety. The patient had no neurological complications or endoleak at the 1 year postoperative follow-up. However, the long-term results of the operation need further research.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Falconi M, Jha NK S-Editor: Zhang L L-Editor: Filipodia P-Editor: Xing YX
| 1. | Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2417] [Cited by in RCA: 2319] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 2. | Joseph G, Canaud L. Commentary: Combining Ascending Aorta and Aortic Arch TEVAR. J Endovasc Ther. 2017;24:81-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Kopp R, Katada Y, Kondo S, Sonesson B, Hongo N, Tse L, Tsilimparis N, Crawford S, Panneton JM, Kölbel T, Xiong J, Guo W, Kasprzak PM; members of the AARCHIF registry. Multicenter Analysis of Endovascular Aortic Arch In Situ Stent-Graft Fenestrations for Aortic Arch Pathologies. Ann Vasc Surg. 2019;59:36-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Cheng D, Martin J, Shennib H, Dunning J, Muneretto C, Schueler S, Von Segesser L, Sergeant P, Turina M. Endovascular aortic repair vs open surgical repair for descending thoracic aortic disease a systematic review and meta-analysis of comparative studies. J Am Coll Cardiol. 2010;55:986-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 295] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 5. | Horton JD, Kölbel T, Haulon S, Khoynezhad A, Green RM, Borger MA, Mussa FF. Endovascular Repair of Type A Aortic Dissection: Current Experience and Technical Considerations. Semin Thorac Cardiovasc Surg. 2016;28:312-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Donas KP, Eisenack M, Panuccio G, Austermann M, Osada N, Torsello G. The role of open and endovascular treatment with fenestrated and chimney endografts for patients with juxtarenal aortic aneurysms. J Vasc Surg. 2012;56:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Tsilimparis N, Detter C, Law Y, Rohlffs F, Heidemann F, Brickwedel J, von Kodolitsch Y, Debus ES, Kölbel T. Single-center experience with an inner branched arch endograft. J Vasc Surg 2019; 69: 977-985. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 8. | Glorion M, Coscas R, McWilliams RG, Javerliat I, Goëau-Brissonniere O, Coggia M. A Comprehensive Review of In Situ Fenestration of Aortic Endografts. Eur J Vasc Endovasc Surg. 2016;52:787-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Singh A, Mafeld S, Williams R, McCaslin J. Physician-Modified Fenestrated Endografts for Managing the Ruptured or Symptomatic Aortic Aneurysm: Technique Overview and Clinical Outcomes. Vasc Endovascular Surg. 2018;52:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Bisdas T, Donas KP, Bosiers MJ, Torsello G, Austermann M. Custom-made vs off-the-shelf multibranched endografts for endovascular repair of thoracoabdominal aortic aneurysms. J Vasc Surg. 2014;60:1186-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Michel M, Becquemin JP, Clément MC, Marzelle J, Quelen C, Durand-Zaleski I; WINDOW Trial Participants. Editor's choice - thirty day outcomes and costs of fenestrated and branched stent grafts vs open repair for complex aortic aneurysms. Eur J Vasc Endovasc Surg. 2015;50:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Starnes BW, Tatum B, Singh N. Procedural and perioperative results in patients treated with fenestrated endovascular aneurysm repair planned by automated software in a physician-sponsored investigational device exemption trial of physician-modified endografts. J Vasc Surg. 2018;68:1297-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Huang J, Li G, Wang W, Wu K, Le T. 3D printing guiding stent graft fenestration: A novel technique for fenestration in endovascular aneurysm repair. Vascular. 2017;25:442-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Zhu J, Dai X, Noiniyom P, Luo Y, Fan H, Feng Z, Zhang Y, Hu F. Fenestrated Thoracic Endovascular Aortic Repair Using Physician-Modified Stent Grafts (PMSGs) in Zone 0 and Zone 1 for Aortic Arch Diseases. Cardiovasc Intervent Radiol. 2019;42:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |