Published online Jan 6, 2021. doi: 10.12998/wjcc.v9.i1.163
Peer-review started: April 2, 2020
First decision: November 3, 2020
Revised: November 13, 2020
Accepted: November 29, 2020
Article in press: November 29, 2020
Published online: January 6, 2021
Processing time: 273 Days and 22.3 Hours
Hypoglycemia due to non-insulin-producing tumors is referred to as non-islet cell tumor hypoglycemia (NICTH). As NICTH is a rare lesion, the natural course of NICTH is not well understood. We report a case of NICTH that was observed 30 years before the onset of hypoglycemia.
A 50-year-old man was diagnosed with an abnormal right chest shadow during a routine X-ray examination, but no further examination was undertaken because the lesion appeared benign. Thirty years after the tumor discovery, the patient was admitted to the hospital with symptoms of severe hypoglycemia, which was diagnosed as NICTH based on a complete examination. The tumor was resected and found to be a solitary fibrous mass (15.6 cm × 13.7 cm × 10.4 cm); thereafter, the patient’s blood glucose levels normalized and he completely recovered.
NICTH can have an acute onset, even if the tumor has been present and asymptomatic over a long time period.
Core Tip: Since hypoglycemia due to non–insulin-producing tumors is a rare lesion, so the natural course of non-islet cell tumor hypoglycemia (NICTH) is not well understood. Here we describe a rare case of NICTH that was caused by a tumor that had been asymptomatic for 30 years. To our knowledge, this is the longest reported latency period before the onset of severe hypoglycemia. The sudden-onset of severe hypoglycemia in the patient described in this report indicates that NICTH can have an acute onset even when the tumor has been present for a longer time.
- Citation: Matsumoto S, Yamada E, Nakajima Y, Yamaguchi N, Okamura T, Yajima T, Yoshino S, Horiguchi K, Ishida E, Yoshikawa M, Nagaoka J, Sekiguchi S, Sue M, Okada S, Fukuda I, Shirabe K, Yamada M. Late-onset non-islet cell tumor hypoglycemia: A case report. World J Clin Cases 2021; 9(1): 163-169
- URL: https://www.wjgnet.com/2307-8960/full/v9/i1/163.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i1.163
Hypoglycemia due to non–insulin-producing tumors is referred to as non-islet cell tumor hypoglycemia (NICTH)[1,2]. NICTH is a rare lesion, but constitutes the second commonest cause of hypoglycemia after insulinoma; approximately 25% of hypoglycemic cases occur due to NICTH[3,4]. Moreover, NICTH is typically induced due to the overconsumption of glucose or overexpression of insulin-like growth factor II (IGF-II) by tumors[5-7]. Moreover, high-molecular-weight IGF-II, known as big IGF-II, has potent insulin-like activity that leads to hypoglycemia[8]. However, big IGF-II fails to build a complex with both IGF-binding protein-3 (IGFPB-3) and its acid-labile subunit, whereas it binds easily to insulin receptors to induce glucose uptake in muscles and adipose tissues, thus leading to hypoglycemia[9]. Tumors of mesenchymal or hepatic origin are usually described as NICTH. Solitary fibrous tumor (SFT) is another rare mesenchymal tumor, wherein approximately 4%-6% of SFT cases develop NICTH[2].
During a routine chest X-ray examination, a 50-year-old man was diagnosed with an abnormal shadow in the right chest. As there were no signs to suggest the mass was malignant, the patient decided to forego further examination or treatment.
Ten years after the initial discovery, a routine chest X-ray revealed an increase in the size of the previously detected shadow, and the patient was advised a biopsy and tumor removal. However, the patient refused further examination or treatment in the absence of any symptoms. Thirty years after the initial discovery, the patient was hospitalized with symptoms of severe hypoglycemia. Prior to the acute episode necessitating hospitalization, the patient had no history of hypoglycemic symptoms, such as sweating, palpitations, and loss of consciousness. Moreover, there was no history of an increase in weight for the preceding 6 mo. As the patient’s hypoglycemia was resistant to treatment by frequent oral glucose supplementation, he was hospitalized for further management.
The patient had no medical records or family history of hypoglycemia.
The patient was on treatment with azelnidipine for hypertension and rosuvastatin for hyperlipidemia.
Clinical examination revealed no signs, except attenuation of respiratory sounds over the right chest.
Laboratory test results (Table 1) after hospitalization showed a slight increase in C-reactive protein (CRP) levels to 2.11 mg/dL. The anti-insulin antibody test result was negative, and fasting blood glucose was 48 mg/dL, accompanied by a low immunoreactive insulin (IRI) level of < 5.0 µU/mL. Interestingly, there was no change in the levels of insulin-counterregulatory hormones, such as cortisol, growth hormone (GH), and norepinephrine (Table 2).
| Clinical values (normal range) | |
| WBC (/μL) | 6800 (4000-9600) |
| Hb (g/dL) | 14.7 (13.2-17.3) |
| Platelets × 104 (/μL) | 21.9 (16-35) |
| Total protein (g/dL) | 7.3 (6.3-7.9) |
| Albumin (g/dL) | 3.7 (3.9-5.0) |
| Total bilirubin (mg/dL) | 0.6 (0.3-1.2) |
| AST (U/L) | 18 (13-33) |
| ALT (U/L) | 10 (8-42) |
| LDH (U/L) | 221 (119-229) |
| ALP (U/L) | 229 (115-359) |
| γ-GTP (U/L) | 16 (10-47) |
| ChE (U/L) | 257 (213-501) |
| AMY (U/L) | 78 (49-136) |
| Blood urea nitrogen (mg/dL) | 5 (8-20) |
| Creatinine (mg/dL) | 0.60 (0.65-1.07) |
| Na (mEq/L) | 144 (137-145) |
| K (mEq/L) | 4.0 (3.5-4.8) |
| Cl (mEq/L) | 107 (100-107) |
| T-Cho (mg/dL) | 206 (128-219) |
| TG (mg/dL) | 44 (30-149) |
| HbA1c (%) | 5.4 (4.6-6.2) |
| Glycoalbumin (%) | 15.8 (11.0-16.0) |
| Insulin antibody | Negative |
| CEA (ng/mL) | 2.2 (0-5.0) |
| SCC (ng/mL) | 1.0 (0-1.5) |
| NSE (ng/mL) | 10.6 (0-12) |
| CYFRA (ng/mL) | 2.1 (< 3.5) |
| ProGRP (pg/mL) | 51.8 (< 80) |
| SLX (U/mL) | 19.4 (0-38.0) |
| Blood glucose (mg/dL) | 130 (80-110) |
| Immunoreactive insulin (μg/mL) | 6.8 (1.0-21.74) |
| Serum C-peptide (ng/mL) | 1.50 (1.1-3.3) |
| Free T3 (pg/mL) | 2.30 (1.88-3.18) |
| Free T4 (ng/dL) | 0.93 (0.70-1.48) |
| Adrenocorticotropic hormone (pg/mL) | 77.0 (7.2-63.3) |
| Cortisol (μg/dL) | 11.0 (3.0-19.6) |
| Human growth hormone (ng/mL) | < 0.07 (< 2.10) |
| IGF-1 (ng/mL) | 92 |
| Clinical values (normal range) | |
| Blood glucose (mg/dL) | 48 (80-110) |
| Immunoreactive insulin (μg/mL) | < 5.0 (1.0-21.74) |
| Serum C-peptide (ng/mL) | 0.02 (1.1-3.3) |
| Free T3 (pg/mL) | 2.48 (1.88-3.18) |
| Free T4 (ng/dL) | 1.16 (0.70-1.48) |
| Adrenocorticotropic hormone (pg/mL) | 36.9 (7.2-63.3) |
| Cortisol (μg/dL) | 11.0 (3.0-19.6) |
| Human growth hormone (ng/mL) | 0.49 (< 2.10) |
| Glucagon (pg/mL) | 188 (71-174) |
| Epinephrine (ng/mL) | 0.18 (< 0.10) |
| Norepinephrine (ng/mL) | 0.78 (0.1-0.5) |
| Dopamine (ng/mL) | 0.02 (< 0.03) |
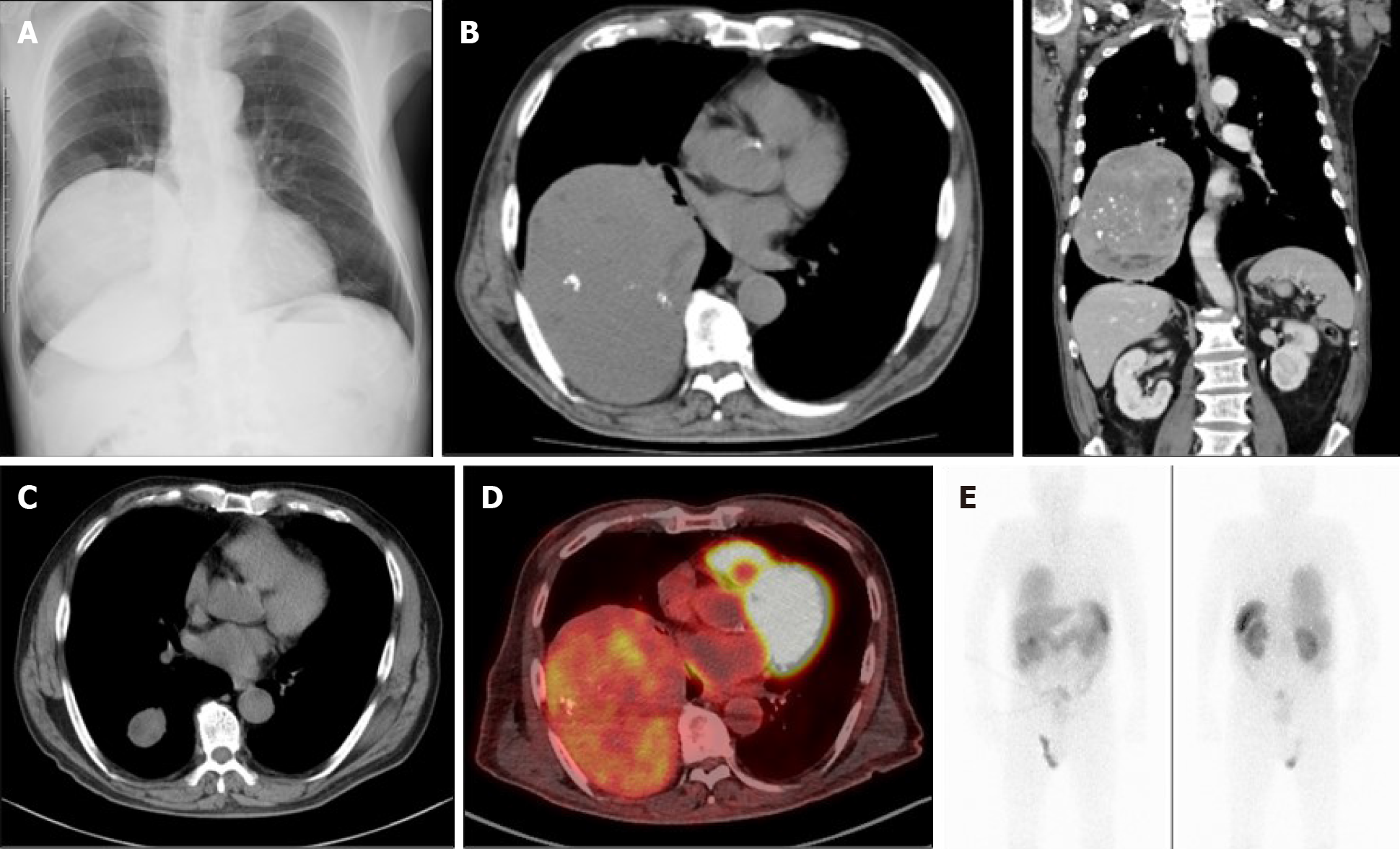
Chest X-ray and computed tomography (CT) scanning showed a giant solid tumor (11 cm × 14 cm × 15 cm) in the right lower chest region (Figure 1A and B). A fluorine-18 fluoro-2-deoxy-D-glucose (FDG) positron emission tomography showed uneven accumulation of the maximum standardized uptake value (SUVmax 3.1) indicating possible characteristics of a malignancy (Figure 1D). Interestingly, the mass showed a propensity for accumulation by octreotide scintigraphy, which was characteristic of a neuroendocrine tumor (Figure 1E).
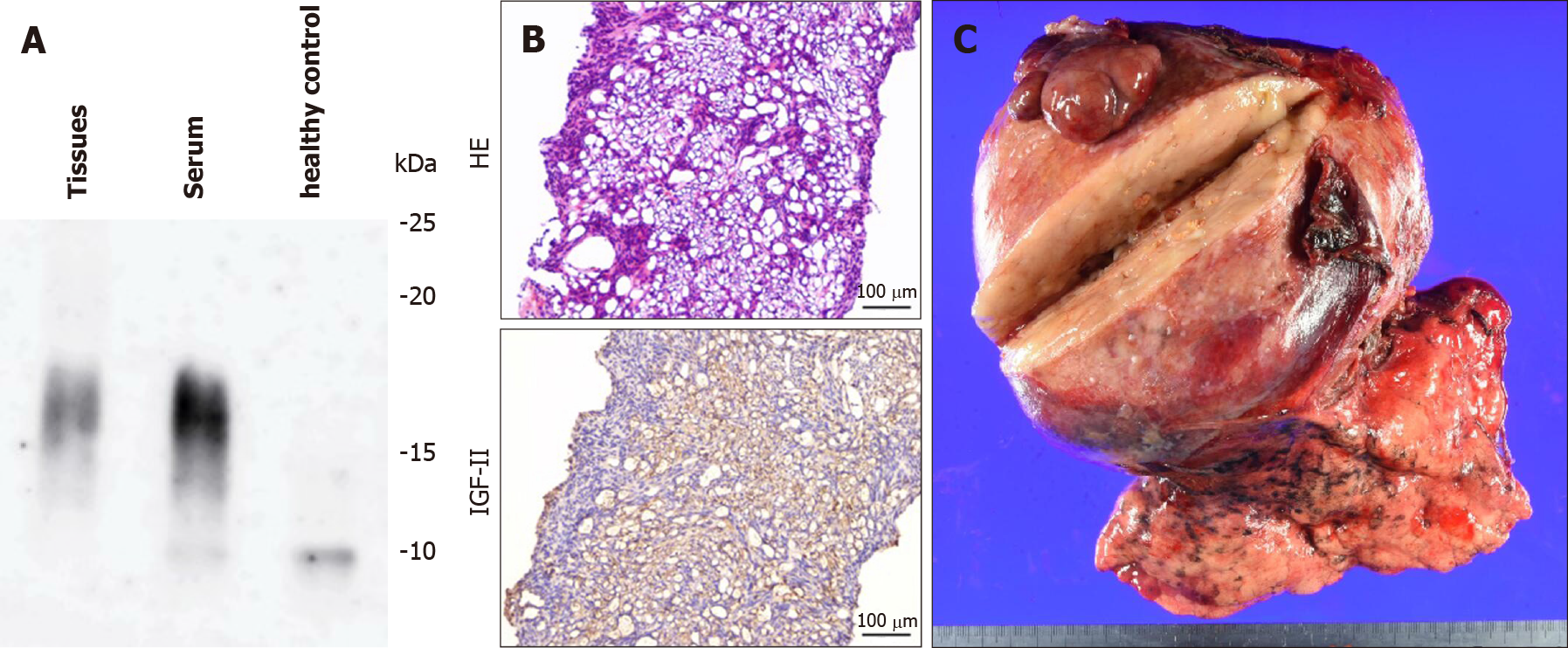
From the biochemical and histological findings, we suspected an NICTH. To confirm this diagnosis, we undertook a core biopsy of the tumor. Immunoblots of serum IGF-II and tumoral tissue IGF-II from tumor biopsies were conducted[10], and a high-molecular-weight form of IGF-II was identified in both types of samples on Western blotting (Figure 2A). Furthermore, immunohistochemical staining for IGF-II in tumoral tissue showed numerous immunopositive tumor cells (Figure 2B).
Based on the findings from the examination and investigations, the patient was diagnosed with NICTH.
Because of problematic symptoms that were refractory to clinical treatment, we obtained written informed consent to carry out tumor resection. The resected mass was shown to be an SFT (15.6 cm × 13.7 cm × 10.4 cm; Figure 2C).
Postoperatively, the patient’s blood glucose levels quickly normalized, and the patient recovered completely.
Hypoglycemia is a usual feature that is observed during the treatment of NICTH. However, in the present case, NICTH was mainly diagnosed on the basis of a hypoglycemic attack[8]. Another symptom that implicated the tumor as an NICTH was its size, which exceeded 10 cm[2,8]. There are no reports in the literature whether an increase in the tumor size induces hypoglycemia; however, in our case, the size of a tumor could be related to the occurrence of hypoglycemia. Moreover, this assumption is supported by the theory that partial resection of the tumor could reduce the incidence of hypoglycemic episodes[11]. In the present case, we observed the progression of the tumor over 30 years, wherein the increase in tumor size eventually caused hypoglycemia. Interestingly, the initial phenomenon of a hypoglycemic attack without any increase of body weight possibly implies that sudden-onset NICTH could occur during the tumor progression.
Besides the overexpression of big IGF-II, NICTH of hepatic origin could be attributed to irregular of gluconeogenesis[2], or an increase of glucose consumption by big tumors[12]. Notably, the tumor, in the present case, showed an increase in the FDG uptake, indicating glucose uptake by the tumor. The histopathological examination diagnosed the tumor as an SFT with no evidence of malignancy; therefore, the sign of FDG uptake could indicate the possible development of malignancy, and increased glucose uptake by the tumor may have induced hypoglycemia through big IGF-II. Indeed, insulin-like effects of big IGF-II are reported to lead to increased glucose uptake in insulin-sensitive tissues, especially muscle and fat[8], although they may induce glucose uptake in the tumor itself[1].
NICTH is supposed to be induced by big IGF-II insulin-like activity; however, there are reports that IGF-II might regulate other insulin-counterregulatory hormones, such as GH and IGF-II, which could lead to hypoglycemia[2]. Furthermore, IGF-II could downregulate the expression of IGF-II[1]. Interestingly, the initial pathological phenomenon of a hypoglycemic attack without previous weight gain and potentially implies the sudden-onset occurrence of NICTH, to immediately exceed the threshold during tumor progression.
The complete treatment of NICTH is total resection of tumor, which could be difficult because of the characteristics of the tumor itself, metastasis, location, size, and so on[1,2,7]. In the present case, the total resection of the tumor could be undertaken despite the inconveniences of the patient’s age and treatment preference. Therefore, we considered other treatment options: Introduction of intravenous hyper-alimentation, enteral tube feeds, local therapies (e.g., embolization, radiation), systemic therapies (e.g., chemotherapy, targeted antitumor therapy such as imatinib), glucocorticoids, rh GH, glucagon, octreotide, diazoxide, or bendrofluazide[2,3]. However, we found none of the options to be sustainable for self-management. This was especially because the patient showed an accumulation of octreotide, demonstrating tumor characteristics typical of a neuroendocrine tumor. Nonetheless, a high concentration of octreotide is not a definitive indicator for NICTH even if octreotide is accumulated within the tumor[13]. Considering all of these factors, we finally obtained informed consent from the patient to undertake lung resection.
In conclusion, we describe a case of NICTH that was observed for 30 years, which is most likely the longest reported duration up to the onset of severe hypoglycemia thus far. The sudden-onset severe hypoglycemia in the present case indicates that NICTH could occur immediately with IGF-II levels above threshold during the tumor progression even over a longer time course.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Avtanski D S-Editor: Gao CC L-Editor: A P-Editor: Liu JH
| 1. | Schutt RC, Gordon TA, Bhabhra R, Cathro HP, Cook SL, McCartney CR, Weiss GR. Doege-Potter syndrome presenting with hypoinsulinemic hypoglycemia in a patient with a malignant extrapleural solitary fibrous tumor: a case report. J Med Case Rep. 2013;7:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Bodnar TW, Acevedo MJ, Pietropaolo M. Management of non-islet-cell tumor hypoglycemia: a clinical review. J Clin Endocrinol Metab. 2014;99:713-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 3. | de Groot JW, Rikhof B, van Doorn J, Bilo HJ, Alleman MA, Honkoop AH, van der Graaf WT. Non-islet cell tumour-induced hypoglycaemia: a review of the literature including two new cases. Endocr Relat Cancer. 2007;14:979-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Takayama S, Nagai N, Hirata Y. Incidence of insulin autoimmune syndrome in Japan during the three-year period from 1979 to 1981. J Jap Diab Soc. 1983;26:143-146. [DOI] [Full Text] |
| 5. | Daughaday WH, Emanuele MA, Brooks MH, Barbato AL, Kapadia M, Rotwein P. Synthesis and secretion of insulin-like growth factor II by a leiomyosarcoma with associated hypoglycemia. N Engl J Med. 1988;319:1434-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 219] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Ma RC, Tong PC, Chan JC, Cockram CS, Chan MH. A 67-year-old woman with recurrent hypoglycemia: non-islet cell tumour hypoglycemia. CMAJ. 2005;173:359-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Han G, Zhang Z, Shen X, Wang K, Zhao Y, He J, Gao Y, Shan X, Xin G, Li C, Liu X. Doege-Potter syndrome: A review of the literature including a new case report. Medicine (Baltimore). 2017;96:e7417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Fukuda I, Hizuka N, Ishikawa Y, Yasumoto K, Murakami Y, Sata A, Morita J, Kurimoto M, Okubo Y, Takano K. Clinical features of insulin-like growth factor-II producing non-islet-cell tumor hypoglycemia. Growth Horm IGF Res. 2006;16:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Phillips LS, Robertson DG. Insulin-like growth factors and non-islet cell tumor hypoglycemia. Metabolism. 1993;42:1093-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Hikichi M, Kiriyama Y, Hayashi T, Ushimado K, Kobayashi N, Urano M, Kuroda M, Utsumi T. A Hypoglycemia-inducing Giant Borderline Phyllodes Tumor Secreting High-molecular-weight Insulin-Like Growth Factor II: Immunohistochemistry and a Western Blot Analysis. Intern Med. 2018;57:237-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Takeuchi S, Goda T, Taguchi J, Douhata Y, Honma R, Ariga S, Ohhara Y, Shimizu Y, Kinoshita I, Fukuda I, Nagashima Y, Akita H. Late Onset of Non-islet Cell Tumor Hypoglycemia Managed via Multidisciplinary Treatment in a Patient with a Solitary Fibrous Tumor. Intern Med. 2018;57:2431-2436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Unger RH. The riddle of tumor hypoglycemia. Am J Med. 1966;40:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Perros P, Simpson J, Innes JA, Teale JD, McKnight JA. Non-islet cell tumour-associated hypoglycaemia: 111In-octreotide imaging and efficacy of octreotide, growth hormone and glucocorticosteroids. Clin Endocrinol (Oxf). 1996;44:727-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |