Published online Jan 6, 2021. doi: 10.12998/wjcc.v9.i1.102
Peer-review started: July 16, 2020
First decision: September 24, 2020
Revised: October 6, 2020
Accepted: November 12, 2020
Article in press: November 12, 2020
Published online: January 6, 2021
Processing time: 169 Days and 3.1 Hours
Nonalcoholic fatty liver disease (NAFLD) affects more than one-quarter of the global population. Due to the lack of approved chemical agents, many patients seek treatment from traditional Chinese medicine (TCM) formulas. A variety of systematic reviews have been published regarding the effectiveness and safety of TCM formulas for NAFLD.
To critically appraise available systematic reviews and sort out the high-quality evidence on TCM formulas for the management of NAFLD.
Seven databases were systematically searched from their inception to 28 February 2020. The search terms included “non-alcoholic fatty liver disease,” “Chinese medicines,” “systematic review,” and their synonyms. Systematic reviews involving TCM formulas alone or in combination with conventional medications were included. The methodological quality and risk of bias of eligible systematic reviews were evaluated by using A Measure Tool to Assess Systematic Reviews 2 (AMSTAR 2) and Risk of Bias in Systematic Review (ROBIS). The quality of outcomes was assessed by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system.
Seven systematic reviews were ultimately included. All systematic reviews were conducted based on randomized controlled trials and published in the last decade. According to the AMSTAR 2 tool, one systematic review was judged as having a moderate confidence level, whereas the other studies were rated as having a low or extremely low level of confidence. The ROBIS tool showed that the included systematic reviews all had a high risk of bias due to insufficient consideration of identified concerns. According to the GRADE system, only two outcomes were determined as high quality; namely, TCM formulas with the HuoXueHuaYu principle were better than conventional medications in ultrasound improvement, and TCM formulas were superior to antioxidants in alanine aminotransferase normalization. Other outcomes were downgraded to lower levels, mainly because of heterogeneity among studies, not meeting optimal information sample size, and inclusion of excessive numbers of small sample studies. Nevertheless, the evidence quality of extracted outcomes should be further downgraded when applying to clinical practice due to indirectness.
The quality of available systematic reviews was not satisfactory. Researchers should avoid repeatedly conducting systematic reviews in this area and focus on designing rigorous randomized controlled trials to support TCM formula applications.
Core Tip: Several systematic reviews have reported the efficacy of traditional Chinese medicine (TCM) formulas for nonalcoholic fatty liver disease. This overview critically appraised currently available systematic reviews. Based on high-quality evidence, TCM formulas may benefit ultrasound improvement and alanine aminotransferase normalization. Nevertheless, the quality of evidence should be further downgraded when applying to clinical practice due to indirectness. The included systematic reviews were generally of poor quality, possibly due to the unsatisfactory quality of the available randomized controlled trials (RCTs). Hence, further emphasis should be placed on designing rigorous RCTs instead of repeatedly conducting systematic reviews.
- Citation: Dai L, Zhou WJ, Zhong LLD, Tang XD, Ji G. Chinese medicine formulas for nonalcoholic fatty liver disease: Overview of systematic reviews. World J Clin Cases 2021; 9(1): 102-117
- URL: https://www.wjgnet.com/2307-8960/full/v9/i1/102.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i1.102
Nonalcoholic fatty liver disease (NAFLD) is a frequently encountered chronic hepatic disease in gastrointestinal outpatients. According to the latest systematic review, the prevalence of NAFLD in Asia has risen to 29.62%[1]. In mainland China, this number is 29.88%, along with a steadily increasing trend in the last decade[2,3]. Apart from lifestyle modifications, conventional pharmacotherapies for NAFLD include insulin sensitizers, antioxidants, cytoprotective drugs, and lipid lowering agents[4,5]. However, uncertain clinical efficacy and potential adverse events still limit the clinical application of these substances[4-7]. Hence, many patients seek traditional Chinese medicine (TCM) treatment, hoping to introduce TCM formulas as an element of NAFLD treatment.
TCM formula is a combination of various herbal medicines based on specific therapeutic principle, and has been employed in clinical practice for a thousand years. Many researchers have published works regarding TCM formulas for treating NAFLD, both in domestic and international academic journals. Accordingly, systematic reviews on this topic are also common[8-10]. Nonetheless, the results of these systematic reviews have not been consistent. Various systematic reviews with different conclusions may cause confusion among clinical practitioners regarding their clinical decisions. Systematic reviews are high-level clinical evidence that can fundamentally affect the recommendation of an intervention[11]. Therefore, it is crucial to assess the quality of systematic reviews and select accurate and high-quality bodies of evidence to guide clinical practice.
In 2019, the National Administration of Traditional Chinese Medicine of China launched a program to enhance the evidence-based capacity for TCM. As a part of this program, our study group conducted this overview of TCM formulas for NAFLD. The aims of this overview were to comprehensively evaluate eligible systematic reviews, summarize the corresponding results, and set clear directions for future research.
The protocol of this overview was registered in the PROSPERO database (CRD42020184746). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was adopted to complete this overview[12]. The corresponding checklist is shown in Supplement 1. The data sources of this overview were based on available systematic reviews; hence, ethical approval was waived.
Seven mainstream databases were comprehensively searched including PubMed, EMBASE, Cochrane Library, Chinese Biomedical Literature Database (SinoMed), China National Knowledge Infrastructure (CNKI), Chinese Scientific Journal Database (VIP), and Wanfang. Systematic reviews published in English or Chinese were filtered from their inception to 28 February 2020. We used subject terms combined with free-text to perform the database search. The key terms included “nonalcoholic fatty liver disease,” “Chinese medicines,” and “systematic review.” The detailed search strategy for each database is shown in Supplement 2.
We included systematic reviews of controlled trials regardless of randomization. Eligible participants were adults with a clear diagnosis of NAFLD. Lifestyle modification should be the fundamental intervention. Qualified experimental treatments were TCM formulas alone or in combination with conventional medications. The comparators should be conventional medications or placebo control. Exclusion criteria included participants with hepatic steatosis induced by other reasons, simple literature reviews, and duplicate studies. In addition, systematic reviews involving agents made from active components of herbal medicine were also excluded.
After eligibility confirmation of the included systematic reviews, the following information was extracted: authors, titles, year of publication, study size, details of methodological information, details of interventions, data analysis methods, outcomes, adverse effects, and funding information. The aim of this overview was to evaluate the effect of TCM formulas on NAFLD; hence, the prescribed outcomes included hepatic function [alanine aminotransferase (ALT)], aspartate aminotransferase and gamma-glutamyl transpeptidase), blood lipid profiles (triglyceride, total cholesterol, low-density lipoprotein cholesterol and high-density lipoprotein cholesterol), radiologic improvement rate, global improvement rate, and adverse events. Two authors (LD, WJZ) independently completed the database search, study selection, and data extraction. A third author (GJ) was consulted to solve discrepancies when necessary. Given that this overview was based on available systematic reviews, no statistical analysis was conducted.
A Measure Tool to Assess Systematic Reviews 2 (AMSTAR 2) and Risk of Bias in Systematic Review (ROBIS) were utilized to appraise the methodological quality and risk of bias of the included systematic reviews, respectively.
AMSTAR 2 is an updated tool based on the classical AMSTAR[13]. The application scope of AMSTAR 2 has been extended to systematic reviews based on both randomized controlled studies (RCTs) and nonrandomized studies of the effects of interventions. It comprises 16 items, 7 of which are identified as critical domains including protocol registration, adequacy of literature search, justification of excluded studies, risk of bias evaluation of individual studies, appropriateness of data synthesis methods, impact of risk of bias on results and likelihood of publication bias. AMSTAR 2 provides four overall confidence levels. A higher confidence level indicates better methodological quality.
ROBIS is a specialized tool for evaluating the risk of bias of systematic reviews[14]. This tool consists of three phases. Phase 1 is intended for relevance to the target question. Phase 2 includes four domains: study eligibility criteria, identification and selection of studies, data collection and study appraisal, and synthesis and findings. Phase 3 is the risk of bias in the review. For phase 1, the results of the assessment are shown as “yes,” “partial,” or “no.” For phases 2 and 3, the results of the assessment are presented as “high risk,” “low risk,” or “unclear risk.”
Two authors (LD, LZ) individually employed the above tools to appraise the included systematic reviews. Disagreement was settled after discussion with an additional author (GJ).
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system was utilized to evaluate the evidence quality of the results from the included systematic reviews[15]. Eight dimensions were assessed to determine the level of evidence: risk of bias, inconsistency, indirectness, imprecision, publication bias, effect magnitude, dose-response gradient, and plausible confounding factors. Two authors (LD, LZ), who have completed GRADE training, individually conducted the quality of evidence evaluation. A third author (XDT) was consulted if a consensus could not be reached. The reasons should be indicated clearly when the level of evidence was downgraded or upgraded. The final quality of evidence was labeled “high,” “moderate,” “low,” or “very low.”
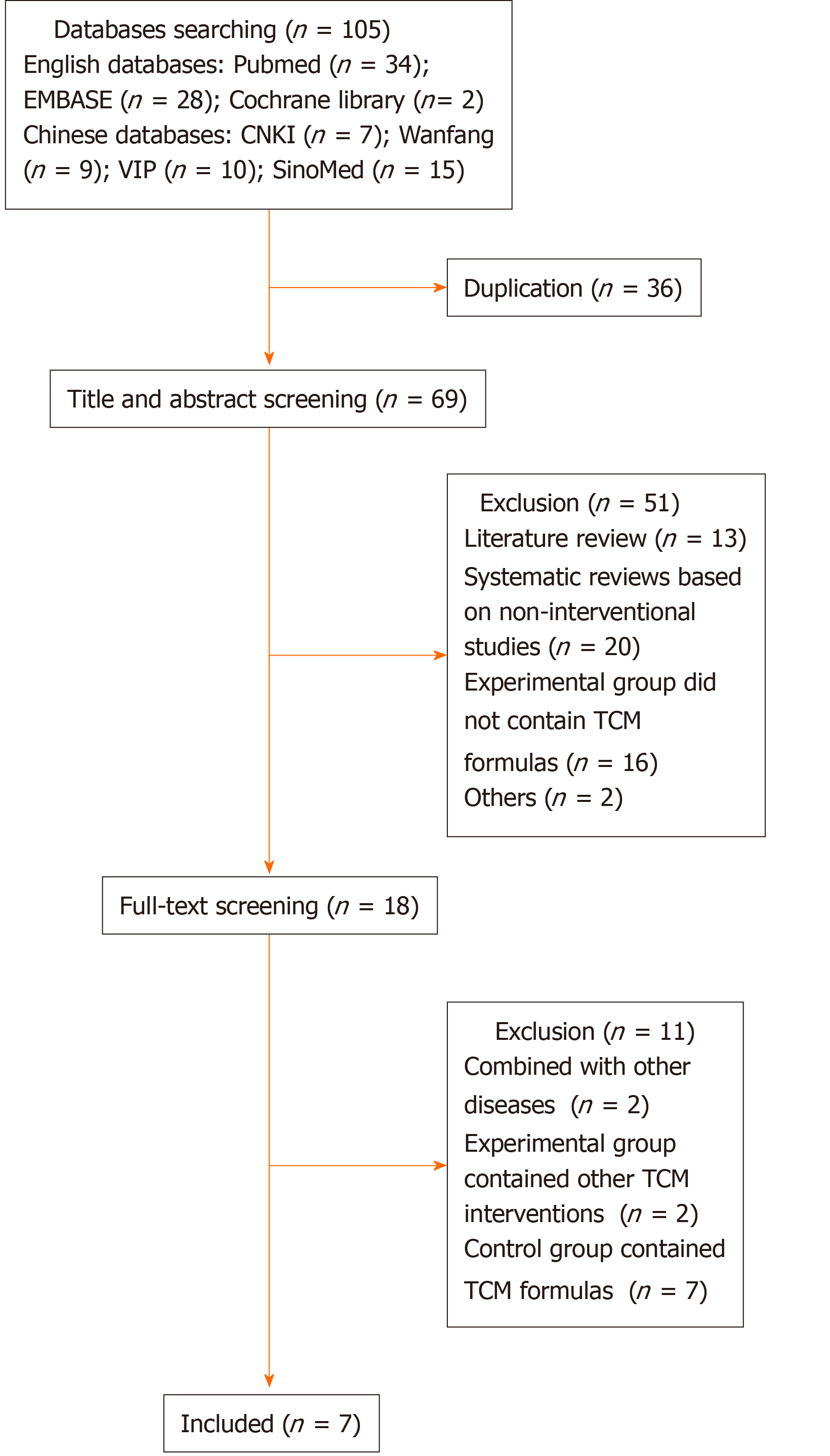
A total of 105 records were found after performing database searching. After eliminating duplicate articles, 69 articles were screened for titles and abstracts. Most records were excluded for various reasons. Full-text screening further filtered out 11 records. Finally, seven systematic reviews were included[8,9,16-20]. The detailed literature search flowchart is shown in Figure 1. The general characteristics of the included systematic reviews are presented in Table 1.
| Ref. | Included study design, n | Total sample size | Intervention | Comparator | Evaluation of methodological quality | Outcomes |
| Cai et al[9], 2019 | RCT, 13 | 1429 | TCM formulas based on HuoXueHuaYu principle | Conventional medications | Cochrane Risk of Bias Tool | Ultrasound improvement rate, blood lipid profiles (TC, TG), hepatic function (ALT, AST), global improvement rate |
| Shi et al[8], 2012 | RCT, 62 | 5904 | TCM formulas, alone or in combination with conventional medications | Placebo or conventional medications | Not mentioned | ALT normalization rate, blood lipids normalization rate, hepatic steatosis disappearance rate |
| He et al[16], 2010 | RCT, 11 | 1078 | TCM formulas, alone | Conventional medications | Jadad Scale | Hepatic function (ALT, AST, GGT), blood lipid profiles (TC, TG, HDL-C) |
| Li et al[17], 2011 | RCT, 22 | 2442 | TCM formulas, alone | Conventional medications | Schulz and Jadad Criteria | Cure rate, global improvement rate, hepatic function (ALT, AST, GGT), blood lipid profiles (TC, TG, HDL-C, LDL-C) |
| Li et al[18], 2014 | RCT, 17 | 1552 | TCM formulas, alone or in combination with polyene phosphatidyl choline | Polyene phosphatidyl choline | Not mentioned | Global improvement rate |
| Yang et al[19], 2019 | RCT, 19 | 1490 | TCM formulas based on JianPiHuaTan principle, alone or in combination with conventional medications | Conventional medications | Jadad Scale | Global improvement rate, hepatic function (ALT, AST), blood lipid profiles (TC, TG), adverse events |
| Zhang et al[20], 2014 | RCT, 10 | 1395 | TCM formulas alone | Placebo or conventional medications | Jadad Scale | Global improvement rate, adverse events |
All included systematic reviews were published in the last decade and performed on RCTs. The sample sizes ranged from 1395 to 5904. Two studies involved placebo as the comparator[8,20]. The Jadad scale, referred to by three systematic reviews, was the most commonly utilized risk of bias tool[16,19,20]. The Cochrane risk of bias tool was only employed in one systematic review[9]. Four studies conducted subgroup analysis[8,17-19], and two studies involved sensitivity analysis[9,19].
The methodological quality assessment using AMSTAR 2 is presented in Table 2. Only one systematic review was judged as moderate confidence. The rest of the included studies were all rated as low or critically low confidence. For the seven core domains, no systematic review reported protocol registration and listing of excluded studies. Two systematic reviews did not present a search strategy[16,17], and the remaining five studies did not consider gray literature[8,9,18-20]. Only one study used a well-recognized tool to comprehensively evaluate the risk of bias[9], and two systematic reviews did not mention relevant assessment tools[8,18]. Most of the included systematic reviews explained the rationale of the chosen meta-analytical methods and analyzed sources of heterogeneity[8,9,18-20]. However, only one systematic review considered the impact of risk of bias when interpreting the results[19]. Publication bias was evaluated in five systematic reviews[8,9,18-20].
| Ref. | Cai et al[9], 2019 | Shi et al[8], 2012 | He et al[16], 2010 | Li et al[17], 2011 | Li et al[18], 2014 | Yang et al[19], 2019 | Zhang et al[20], 2014 |
| Item 1 | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Item 2 | No | No | No | No | No | No | No |
| Item 3 | No | Yes | No | No | No | No | No |
| Item 4 | Partial Yes | Partial Yes | No | No | Partial Yes | Partial Yes | Partial Yes |
| Item 5 | No | Yes | Yes | Yes | No | No | No |
| Item 6 | Yes | Yes | No | Yes | No | No | Yes |
| Item 7 | No | No | No | No | No | No | No |
| Item 8 | No | Partial Yes | No | No | No | No | No |
| Item 9 | Yes | No | Partial Yes | Partial Yes | No | Partial Yes | Partial Yes |
| Item 10 | No | No | No | No | No | No | No |
| Item 11 | Yes | Yes | No | No | Yes | Yes | Yes |
| Item 12 | No | No | No | No | No | Yes | No |
| Item 13 | No | No | No | No | No | Yes | No |
| Item 14 | Yes | No | Yes | No | No | Yes | Yes |
| Item 15 | Yes | Yes | No | No | Yes | Yes | Yes |
| Item 16 | Yes | Yes | No | No | No | No | No |
| Overall quality | Low | Extremely low | Extremely low | Extremely low | Extremely low | Moderate | Low |
The risk of bias evaluation using ROBIS is shown in Table 3. All systematic reviews conformed to the target question and hence were rated as yes in phase 1. Phase 2 contains four domains. For study eligibility criteria, two studies were rated as high risk due to ambiguous inclusion criteria and uncertain restrictions[17,18]. All studies were judged as high risk in study identification. Underlying reasons included not retrieving other data sources and incomplete search terms. Most studies were determined to be high risk in the data collection and study appraisal domain, mainly because of inappropriate risk of bias tools and insufficient study characteristics. Since heterogeneity and risk of bias were not properly managed, four systematic reviews were rated as high risk in the synthesis and findings domain[8,16,17,20]. Phase 3 considers the overall risk of bias of a systematic review. In this overview, as no study comprehensively addressed the concerns identified in phase 2, all included systematic reviews were rated as high risk.
| Ref. | Phase 1 | Phase 2 | Phase 3 | |||
| Relevance | Study eligibility criteria | Identification and selection of studies | Data collection and study appraisal | Synthesis and findings | Risk of bias in the review | |
| Cai et al[9], 2019 | Yes | Low risk | High risk | Low risk | Low risk | High risk |
| Shi et al[8], 2012 | Yes | Low risk | High risk | High risk | High risk | High risk |
| He et al[16], 2010 | Yes | Low risk | High risk | High risk | High risk | High risk |
| Li et al[17], 2011 | Yes | High risk | High risk | High risk | High risk | High risk |
| Li et al[18], 2014 | Yes | High risk | High risk | High risk | Not clear | High risk |
| Yang et al[19], 2019 | Yes | Low risk | High risk | High risk | Low risk | High risk |
| Zhang et al[20], 2014 | Yes | Low risk | High risk | High risk | High risk | High risk |
The outcomes of the included systematic reviews were summarized and reassessed using the GRADE system in Table 4. Only two outcomes were categorized as high quality. Other outcomes were downgraded to different levels, and corresponding reasons were included under the table. Three dimensions were most affected, namely, inconsistency due to heterogeneity, imprecision due to not reaching optimal information sample size and publication bias due to the involvement of too many small sample studies.
| Ref. | Intervention vs comparator | Outcomes | Study numbers and sample size | A | B | C | D | E | Quality of evidence |
| Cai et al[9], 2019 | TCM formulas based on HuoXueHuaYu principle vs conventional medications | Ultrasound improvement rate: OR = 2.33; 95%CI: 1.60, 3.40; P < 0.001 | 7 (590) | 0 | 0 | 0 | 0 | 0 | High |
| TC: MD = -0.38; 95%CI: -0.48, -0.29; P < 0.001 | 5 (358) | 0 | -21 | 0 | -12 | 0 | Very low | ||
| TG: MD = -0.31; 95%CI: -0.37, -0.24; P < 0.001 | 6 (418) | 0 | -21 | 0 | 0 | 0 | Low | ||
| ALT: SMD = -1.69; 95%CI: -2.24, -1.14; P < 0.001 | 6 (418) | 0 | -21 | 0 | 0 | 0 | Low | ||
| AST: MD = -22.53; 95%CI: -33.16, -11.90; P < 0.001 | 5 (354) | 0 | -21 | 0 | -12 | 0 | Very low | ||
| Global improvement rate: OR = 3.55; 95%CI: 2.65, 4.76; P < 0.001 | 12 (1389) | -13 | 0 | 0 | 0 | 0 | Moderate | ||
| Shi et al[8], 2012 | TCM formulas vs placebo | ALT normalization rate: OR = 1.73, 95%CI: 1.34, 2.23; P < 0.001 | 8 (902) | 0 | 0 | 0 | -14 | -15 | Low |
| Blood lipids normalization rate: OR = 1.74, 95%CI: 1.34, 2.26; P < 0.001 | 8 (922) | 0 | 0 | 0 | 0 | -15 | Moderate | ||
| Hepatic steatosis disappearance rate: OR = 2.43, 95%CI: 1.48, 3.97; P < 0.001 | 9 (933) | 0 | -16 | 0 | -14 | -15 | Very low | ||
| TCM formulas vs UDCA | ALT normalization rate: OR = 1.45, 95%CI: 1.05, 1.98; P = 0.023 | 7 (702) | 0 | 0 | 0 | -14 | 0 | Moderate | |
| Blood lipids normalization rate: OR = 1.57, 95%CI: 0.88, 2.80; P = 0.124 | 3 (350) | 0 | 0 | 0 | -14 | 0 | Moderate | ||
| Hepatic steatosis disappearance rate: OR = 1.92, 95%CI: 1.20, 3.07; P = 0.006 | 5 (519) | 0 | 0 | 0 | -14 | -15 | Low | ||
| TCM formulas + UDCA vs UDCA | ALT normalization rate: OR = 1.55, 95%CI: 1.08, 2.23; P = 0.019 | 4 (341) | 0 | 0 | 0 | -14 | -15 | Low | |
| Blood lipids normalization rate: OR = 1.65, 95%CI: 0.71, 3.87; P = 0.247 | 1 (60) | -17,8 | 0 | 0 | -14 | 0 | Low | ||
| Hepatic steatosis disappearance rate: OR = 1.94, 95%CI: 1.28, 2.96; P = 0.002 | 4 (341) | 0 | 0 | 0 | -14 | -15 | Low | ||
| TCM formulas vs insulin sensitizers | ALT normalization rate: OR = 1.67, 95%CI: 0.53, 5.28; P = 0.385 | 1 (80) | -17,8 | 0 | 0 | -14 | 0 | Low | |
| Blood lipids normalization rate: OR = 1.67, 95%CI: 0.53, 5.28; P = 0.385 | 1 (80) | -17,8 | 0 | 0 | -14 | 0 | Low | ||
| Hepatic steatosis disappearance rate: OR = 1.67, 95%CI: 0.53, 5.28; P = 0.385 | 1 (80) | -17,8 | 0 | 0 | -14 | 0 | Low | ||
| TCM formulas + insulin sensitizers vs insulin sensitizers | ALT normalization rate: OR = 3.31, 95%CI: 0.82, 13.42; P = 0.094 | 1 (61) | 0 | 0 | 0 | -14 | 0 | Moderate | |
| Blood lipids normalization rate: OR = 5.51, 95%CI: 0.25, 119.50; P = 0.277 | 1 (61) | 0 | 0 | 0 | -14 | 0 | Moderate | ||
| Hepatic steatosis disappearance rate: OR = 5.51, 95%CI: 0.25, 119.50; P = 0.277 | 1 (61) | 0 | 0 | 0 | -14 | 0 | Moderate | ||
| TCM formulas vs fibrates | ALT normalization rate: OR = 2.36, 95%CI: 1.55, 3.60; P < 0.001 | 5 (681) | 0 | 0 | 0 | -14 | -15 | Low | |
| Blood lipids normalization rate: OR = 2.13, 95%CI: 1.34, 3.39; P = 0.001 | 4 (463) | 0 | 0 | 0 | -14 | -15 | Low | ||
| Hepatic steatosis disappearance rate: OR = 2.35, 95%CI: 1.61, 3.41; P < 0.001 | 7 (781) | 0 | 0 | 0 | -14 | 0 | Moderate | ||
| TCM formulas + fibrates vs fibrates | ALT normalization rate: OR = 1.47, 95%CI: 0.84, 2.56; P = 0.180 | 2 (132) | 0 | 0 | 0 | -14 | 0 | Moderate | |
| Blood lipids normalization rate: OR = 1.83, 95%CI: 0.89, 3.75; P = 0.102 | 2 (132) | 0 | 0 | 0 | -14 | 0 | Moderate | ||
| Hepatic steatosis disappearance rate: OR = 1.80, 95%CI: 0.76, 4.29; P = 0.183 | 1 (70) | -17,8 | 0 | 0 | -14 | 0 | Low | ||
| TCM formulas vs statins | ALT normalization rate: OR = 1.43, 95%CI: 1.03, 2.00; P = 0.035 | 5 (456) | 0 | 0 | 0 | -14 | 0 | Moderate | |
| Blood lipids normalization rate: OR = 1.26, 95%CI: 0.85, 1.87; P = 0.249 | 4 (364) | 0 | 0 | 0 | -14 | -15 | Low | ||
| Hepatic steatosis disappearance rate: OR = 1.76, 95%CI: 1.30, 2.37; P < 0.001 | 8 (764) | 0 | 0 | 0 | -14 | -15 | Low | ||
| TCM formulas + statins vs statins | ALT normalization rate: OR = 1.51, 95%CI: 1.11, 2.05; P = 0.009 | 6 (571) | 0 | 0 | 0 | -14 | 0 | Moderate | |
| Blood lipids normalization rate: OR = 1.32, 95%CI: 0.91, 1.93; P = 0.148 | 5 (504) | 0 | 0 | 0 | -14 | 0 | Moderate | ||
| Hepatic steatosis disappearance rate: OR = 2.13, 95CI: 1.42, 3.19; P < 0.001 | 6 (601) | 0 | 0 | 0 | -14 | 0 | Moderate | ||
| TCM formulas vs antioxidants | ALT normalization rate: OR = 1.48, 95%CI: 1.15, 1.92; P = 0.003 | 8 (652) | 0 | 0 | 0 | 0 | 0 | High | |
| Blood lipids normalization rate: OR = 1.53, 95%CI: 0.94, 2.51; P = 0.087 | 3 (257) | 0 | 0 | 0 | -14 | -15 | Low | ||
| Hepatic steatosis disappearance rate: OR = 1.81, 95%CI: 1.27, 2.58; P < 0.001 | 7 (585) | 0 | 0 | 0 | -14 | 0 | Moderate | ||
| TCM formulas + antioxidants vs antioxidants | ALT normalization rate: OR = 1.62, 95%CI: 1.06, 2.46; P = 0.025 | 4 (267) | 0 | 0 | 0 | -14 | 0 | Moderate | |
| Blood lipids normalization rate: OR = 1.26, 95%CI: 0.95, 2.94; P = 0.075 | 2 (143) | 0 | 0 | 0 | -14 | 0 | Moderate | ||
| Hepatic steatosis disappearance rate: OR = 1.77, 95%CI: 1.10, 2.84; P = 0.018 | 4 (257) | 0 | 0 | 0 | -14 | 0 | Moderate | ||
| He et al[16], 2010 | TCM formulas vs conventional medications | ALT: MD = -9.55; 95%CI: -12.45, -6.65; P < 0.001 | 11 (1078) | 0 | -21 | 0 | 0 | -19 | Very low |
| AST: MD = -9.40; 95%CI: -12.96, -5.85; P < 0.001 | 11 (1078) | 0 | -21 | 0 | 0 | -19 | Very low | ||
| GGT: MD = -18.31; 95%CI: -27.06, -9.56; P < 0.001 | 11 (1078) | 0 | -21 | 0 | 0 | -19 | Very low | ||
| TC: MD = -1.12; 95%CI: -1.80, -0.44; P < 0.001 | 11 (1078) | 0 | -21 | 0 | 0 | -19 | Very low | ||
| TG: MD = -0.39; 95%CI: -0.64, -0.15; P = 0.002 | 11 (1078) | 0 | -21 | 0 | 0 | -19 | Very low | ||
| HDL-C: MD = 0.21; 95%CI: 0.14, 0.28; P < 0.001 | 11 (1078) | 0 | -21 | 0 | 0 | -19 | Very low | ||
| Li et al[17], 2011 | TCM formulas vs conventional medications | Cure rate: RR = 1.48, 95%CI: 1.12, 1.94; P = 0.005 | 16 (1644) | 0 | -16 | 0 | 0 | -19 | Low |
| Global improvement rate: RR = 1.29, 95%CI: 1.16, 1.43; P < 0.001 | 20 (2118) | -13 | -21 | 0 | 0 | -19 | Very low | ||
| ALT: MD = -18.90; 95%CI: -26.34, -11.46; P < 0.001 | 18 (1935) | 0 | -21 | 0 | 0 | -19 | Very low | ||
| AST: MD = -10.59; 95%CI: -15.61, -5.58; P < 0.001 | 14 (1480) | 0 | -21 | 0 | 0 | -19 | Very low | ||
| GGT cannot be evaluated due to data mistake | \ | \ | \ | \ | \ | \ | \ | ||
| TC: MD = -0.68; 95%CI: -1.14, -0.21; P = 0.004 | 17 (1885) | 0 | -21 | 0 | 0 | -19 | Very low | ||
| TG: MD = -0.48; 95%CI: -0.92, -0.03; P = 0.036 | 17 (1885) | 0 | -21 | 0 | 0 | -19 | Very low | ||
| HDL-C: MD = 0.07; 95%CI: -0.17, 0.37; P = 0.561 | 8 (731) | 0 | -21 | 0 | 0 | -19 | Very low | ||
| LDL-C: MD = -0.59; 95%CI: -0.80, -0.37; P < 0.001 | 7 (776) | 0 | -21 | 0 | 0 | -19 | Very low | ||
| Li et al[18], 2014 | TCM formulas vs PPC | Global improvement rate: RR = 1.20, 95%CI: 1.12, 1.28; P value not reported | 11 (982) | -13 | 0 | 0 | 0 | -19 | Low |
| TCM formulas + PPC vs PPC | Global improvement rate: RR = 1.31, 95%CI: 1.20, 1.43; P value not reported | 7 (600) | -13 | 0 | 0 | 0 | -19 | Low | |
| Yang et al[19], 2019 | TCM formulas based on JianPiHuaTan principle vs conventional medications | Global improvement rate: RR = 1.30, 95%CI: 1.16, 1.46; P < 0.001 | 17 (1344) | -13 | -21 | 0 | 0 | -15 | Very low |
| ALT: MD = -8.55; 95%CI: -12.76, -4.34; P < 0.001 | 15 (1151) | 0 | -21 | 0 | 0 | -19 | Very low | ||
| AST: MD = -3.60; 95%CI: -5.83, -1.37; P = 0.002 | 13 (979) | 0 | -16 | 0 | 0 | -19 | Low | ||
| TC: MD = -0.88; 95%CI: -1.15, -0.61; P < 0.001 | 13 (1008) | 0 | 0 | 0 | 0 | -19 | Moderate | ||
| TG: MD = -0.47; 95%CI: -0.65, -0.30; P < 0.001 | 14 (1088) | 0 | -21 | 0 | 0 | -19 | Very low | ||
| Adverse events: OR = 5.62, 95%CI: 2.02, 15.59; P < 0.001 | 4 (292) | -13 | -16 | 0 | -14 | -19 | Very low | ||
| TCM formulas based on JianPiHuaTan principle + conventional medications vs conventional medications | Global improvement rate: RR = 1.33, 95%CI: 1.14, 1.55; P < 0.001 | 2 (146) | -13 | 0 | 0 | 0 | 0 | Moderate | |
| Zhang et al[20], 2014 | TCM formulas vs conventional medications | Global improvement rate: RR = 1.51, 95%CI: 1.41, 1.62; P < 0.01 | 10 (1395) | -13 | 0 | 0 | 0 | 0 | Moderate |
| Adverse events: No meta-analysis conducted | 5 (618) | \ | \ | \ | \ | \ | \ |
For efficacy evaluation, based on high-quality evidence, one systematic review reported that TCM formulas based on the HuoXueHuaYu principle resulted in a better radiologic improvement rate than conventional medications evaluated by ultrasound (odds ratio [OR] = 2.33; 95% confidence interval [CI]: 1.60, 3.40; P < 0.001). The other systematic review suggested that TCM formulas were better than antioxidants in ALT normalization (OR = 1.48, 95%CI: 1.15, 1.92; P = 0.003). However, both radiologic improvement and ALT normalization are considered as surrogate outcomes for NAFLD treatment. The quality of evidence should be further downgraded when applying to clinical practice. For safety assessment, only two systematic reviews reported relevant contents. One study indicated that more adverse events were found in patients who received TCM formulas according to the JianPiHuaTan principle, while the evidence level was rated as very low. The other study only conducted a descriptive analysis. Mild adverse events were reported in patients who received TCM formulas.
This overview critically assessed published systematic reviews regarding TCM formulas for NAFLD and re-sorted the evidence according to the GRADE system. Both AMSTAR 2 and ROBIS indicated that the quality of available systematic reviews was not satisfactory. Only one systematic review was rated as having a moderate confidence level, and no systematic review was judged as having a low risk of bias. Among all outcomes, only two were defined as high quality. The results indicated that TCM formulas based on the HuoXueHuaYu principle had better efficacy for radiologic improvement than conventional medications, and TCM formulas showed a better effect on ALT normalization than antioxidants.
Based on the AMSTAR 2 tool, two critical items were consistently ignored by all included systematic reviews, namely, protocol registration and excluded studies. Prospective protocol registration, which serves as the foundation of a successful systematic review, could enhance the transparency of the systematic review and prevent unnecessary, repetitive works[21,22]. In addition, registration is also an item listed in the PRISMA checklist. Accordingly, the PROSPERO database, an open and international database launched in 2011, was established for systematic review registration. Interestingly, the records included in our systematic review, even those published after 2011, did not report registration information. One underlying reason may be the lack of awareness of standard procedures for conducting a systematic review. For the latter item, it is quite understandable that, unlike Cochrane reviews, systematic reviews published in other platforms (even top medical journals) generally would not include a listing of excluded studies due to word count limitations[23-25]. Nevertheless, we still suggest that authors of systematic reviews indicate excluded studies in case readers need to comprehensively appraise the review.
Another considerable factor contributing to the low quality of the included systematic reviews is the choice of risk of bias evaluation tool. Half of the included studies employed the Jadad scale. Indeed, this is one of most frequently applied tools for appraising methodological quality[26]. However, it does not cover dimensions such as allocation concealment, blinding of outcome assessors, and selective outcome reporting. The Cochrane Risk of Bias Tool may be a more appropriate choice for RCTs[27], but it was only utilized in one systematic review. Hence, there is still a need to promote the understanding of evidence-based medicine in relevant research.
Gray literature, including academic dissertations, conference abstracts, and clinical trial registries, is an important source of evidence in systematic reviews. It has been reported that gray literature can account for up to 75% of studies included in a meta-analysis[28]. Given the fact that approximately 50% of clinical trials may not be officially published[29,30], the involvement of gray literature could reduce publication bias and contribute to a comprehensive evaluation of evidence[31-34]. However, in our overview, only one study included a gray literature search[19]. The inefficient search strategy may lead to potential bias in the pooled results. Further education should be arranged for researchers, aiming to improve the recognition and usage of gray literature.
Our overview included seven systematic reviews; however, only two high-quality outcomes were extracted. Moreover, the two outcomes were not general suggestions, focusing only on two subcategories, namely, TCM formulas based on the HuoXueHuaYu principle vs conventional medications and TCM formulas vs antioxidants. The low quality of the included systematic reviews may be one reason for the lack of high-quality outcomes, but more importantly, the inherently unsatisfactory quality of the included RCTs should be addressed. Various studies have reported that the overall quality of RCTs regarding TCM formulas is poor[35-38]. Hence, the fervent desire to conduct systematic reviews based on RCTs should be relaxed, and the quality of fundamental clinical trials should be improved in advance. Eventually, systematic reviews could serve as valuable summaries of high-quality studies.
According to the efficacy evaluation, TCM formulas seemed to be beneficial for NAFLD treatment. However, this statement should be treated with caution. Two “high-quality” outcomes (radiologic improvement and ALT normalization) were found, whereas the evaluation of “high quality” was only for the outcomes themselves, not the clinical recommendations. In general, clinical recommendation for NAFLD treatment should be established on the foundation of key clinical outcomes including hepatic histological improvement and liver-related composite events[39]. Surrogate endpoints, such as hepatic fat and enzymes, are applicable for early phase NAFLD trials. Therefore, if we make a recommendation for TCM formulas treating NAFLD, then according to GRADE system[40], the quality of evidence of these two outcomes should be further downgraded at least one level due to indirectness. Actually, this overview did not find any outcomes involving liver histology or adjudicated events among extracted information. Clinical recommendations regarding TCM formulas should be carefully made.
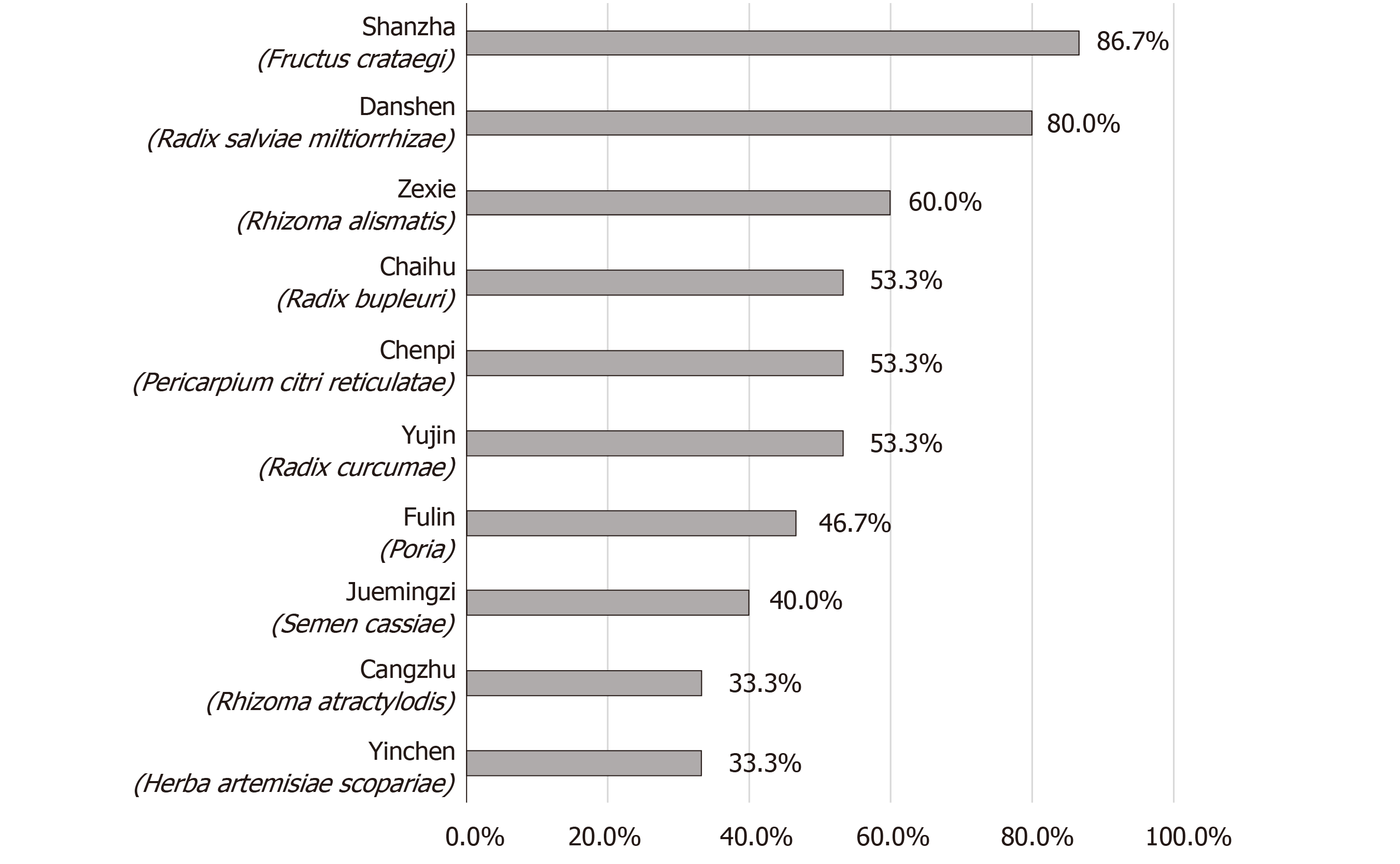
Nevertheless, the unsatisfactory results could not deny the therapeutic potential of TCM formulas. Based on the two relatively high-quality outcomes, we summarized herbs the 10 most commonly used herbs among included RCTs (Figure 2). These herbs may serve as the candidate drug pool for NAFLD treatment. For instance, preclinical experiments found that Shanzha (Crataegus pinnatifida) could activate the peroxisome proliferator-activated receptor alpha (PPARα) pathway to improve lipid metabolism[41]. In addition, network pharmacology approach also indicated that Danshen (Radix salviae miltiorrhizae) may possess excellent hepatoprotective effects, and the potential targets contained PPARα, cytochrome P450 1A2, and matrix metalloproteinase-2[42]. Other future research directions may involve the exploration of active components, the development of innovative TCM formulas, and retrospective real-world data analysis.
To the best of our knowledge, this is the first overview of available systematic reviews concerning the efficacy of TCM formulas for NAFLD. Our overview provides a critical appraisal of published studies using generally acknowledged tools, and suggests that future research should focus on designing rigorous high-quality RCTs instead of repeatedly conducting systematic reviews. Indeed, there were also several limitations. First, the enrolled systematic reviews may have included duplicate clinical studies, which may interfere with the interpretation of the results. Second, our overview evaluated the research status in a specific area; hence, we did not evaluate the reporting quality of the included systematic reviews. This may not intuitively reflect the researchers’ understanding of the PRISMA statement. Third, due to language restrictions, we only searched English and Chinese databases. The data sources may have been insufficient.
In conclusion, the available systematic reviews were generally of poor methodological quality and possessed a high risk of bias. Although two high-quality outcomes were extracted, caution is still necessary in the clinical application of TCM formulas for NAFLD management. Future research should focus on designing rigorous RCTs rather than repeatedly conducting meaningless systematic reviews.
Nonalcoholic fatty liver disease (NAFLD) is a common chronic hepatic disease in clinical practice, affecting approximate one-third of the population in Asia. Conventional pharmacotherapies contain insulin sensitizers, antioxidants, cytoprotective drugs, and lipid lowering agents. However, the uncertain clinical efficacy and risk of adverse events still trouble clinical application. Hence, many patients introduce traditional Chinese medicine (TCM) formulas to the management of NAFLD. TCM formulas for NAFLD treatment have always been a popular research topic. Many relevant systematic reviews have been published in recent decades, but the results have not been consistent. Based on the different conclusions, it is not realistic to establish a standardized clinical pathway of TCM formulas for NAFLD. Therefore, this overview was conducted to critically assess the quality of available systematic reviews, summarize the results, and determine future research directions.
Various clinical and basic studies have reported the effectiveness of TCM formulas for NAFLD. Correspondingly, a number of systematic reviews have been published. It is well known that systematic reviews are high-level clinical evidence and can fundamentally affect the recommendation of an intervention. However, systematic reviews with different conclusions cause doctors to feel confused about their clinical decisions. By objectively evaluating the available systematic reviews, it is possible to sort out the high-quality evidence regarding TCM formulas and recognize future research issues in this area.
The aim of this overview was to critically appraise the available systematic reviews using well-acknowledged tools, and summarize high-quality evidence regarding TCM formulas for treating NAFLD. In addition, based on extracted outcomes, clinicians and researchers can determine directions for further research and prevent unnecessary duplications.
Seven English and Chinese databases were comprehensively searched including PubMed, EMBASE, Cochrane Library, Chinese Biomedical Literature Database (SinoMed), China National Knowledge Infrastructure (CNKI), Chinese Scientific Journal Database (VIP) and Wanfang. The search terms included “nonalcoholic fatty liver disease,” “Chinese medicines,” “systematic review,” and their synonyms. The eligibility of systematic reviews was determined by agreement of two authors or discussion with a third individual. The following information was extracted: authors, titles, year of publication, study size, details of methodological information, details of interventions, data analysis methods, outcomes, adverse effects, and funding information. The methodological quality and risk of bias of the included systematic reviews were assessed by A Measure Tool to Assess Systematic Reviews 2 (AMSTAR 2) and Risk of Bias in Systematic Review (ROBIS), respectively. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system was employed to appraise the evidence quality of the outcomes of systematic reviews.
Seven systematic reviews were included in total. All systematic reviews were published in the recent decade and conducted based on randomized controlled trials (RCTs). The methodological quality assessment using AMSTAR 2 showed that only one study was in moderate confidence. The remaining systematic reviews were judged as low or critically low confidence. The main reasons for downgrading were protocol registration, adequacy of literature search, justification of excluded studies, and impact of risk of bias on results. The risk of bias assessment employing ROBIS indicated that all systematic reviews were in the high-risk category in overall appraisal (Phase 3). The outcomes of included systematic reviews were assessed by the GRADE system. Most outcomes were downgraded to different levels. The main reasons contained inconsistency due to heterogeneity, imprecision due to inadequate sample size and publication bias due to excessive inclusion of small sample studies. Only two high-quality outcomes were found, namely TCM formulas based on the HuoXueHuaYu principle induced a better ultrasonic improvement rate than conventional medications, and TCM formulas had better efficacy than antioxidants in alanine aminotransferase (ALT) normalization. However, the quality of evidence should be downgraded when applying to clinical practice due to indirectness.
This overview critically evaluated available systematic reviews regarding TCM formulas for treating NAFLD by using well-established tools such as AMSTAR 2, ROBIS, and the GRADE system. The quality of included systematic reviews was not satisfactory. No systematic review was judged as a high confidence level or a low risk of bias. Protocol registration, literature adequacy, and risk of bias analysis were the main shortages. Only two high-quality outcomes were recognized. The corresponding outcomes were TCM formulas based on the HuoXueHuaYu principle showed better ultrasonic improvement than conventional medications, and TCM formulas were superior to antioxidant in ALT normalization. However, when making clinical recommendations, the quality of these outcomes should be further downgraded at least one level due to indirectness. According to the results, although various systematic reviews were published, it is still not sufficient to support the application of TCM formulas to NAFLD in clinical practice. The major reason is the unsatisfactory quality of primary clinical trials. Future emphasis should be paid to designing rigorous RCTs rather than repeatedly conducting systematic reviews.
TCM formulas for NAFLD have always been a popular topic in the gastrointestinal area. Based on available evidence, it is still not possible to establish a recommendation regarding TCM formulas in NAFLD management. Researchers should understand the essence of evidence-based medicine and avoid conducting unnecessary systematic reviews. It would be more valuable to design high-quality RCTs to lay a solid foundation.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bader El Din NG, Balaban YH, Reggiani G S-Editor: Fan JR L-Editor: Filipodia P-Editor: Xing YX
| 1. | Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, Fujii H, Wu Y, Kam LY, Ji F, Li X, Chien N, Wei M, Ogawa E, Zhao C, Wu X, Stave CD, Henry L, Barnett S, Takahashi H, Furusyo N, Eguchi Y, Hsu YC, Lee TY, Ren W, Qin C, Jun DW, Toyoda H, Wong VW, Cheung R, Zhu Q, Nguyen MH. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4:389-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 703] [Article Influence: 117.2] [Reference Citation Analysis (0)] |
| 2. | Wu Y, Zheng Q, Zou B, Yeo YH, Li X, Li J, Xie X, Feng Y, Stave CD, Zhu Q, Cheung R, Nguyen MH. The epidemiology of NAFLD in Mainland China with analysis by adjusted gross regional domestic product: a meta-analysis. Hepatol Int. 2020;14:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 3. | Zhou F, Zhou J, Wang W, Zhang XJ, Ji YX, Zhang P, She ZG, Zhu L, Cai J, Li H. Unexpected Rapid Increase in the Burden of NAFLD in China From 2008 to 2018: A Systematic Review and Meta-Analysis. Hepatology. 2019;70:1119-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 421] [Article Influence: 70.2] [Reference Citation Analysis (0)] |
| 4. | European Association for the Study of the Liver (EASL). European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3180] [Article Influence: 353.3] [Reference Citation Analysis (4)] |
| 5. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4949] [Article Influence: 707.0] [Reference Citation Analysis (9)] |
| 6. | Musso G, Cassader M, Paschetta E, Gambino R. Thiazolidinediones and Advanced Liver Fibrosis in Nonalcoholic Steatohepatitis: A Meta-analysis. JAMA Intern Med. 2017;177:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 342] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 7. | Miller ER 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1820] [Cited by in RCA: 1627] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 8. | Shi KQ, Fan YC, Liu WY, Li LF, Chen YP, Zheng MH. Traditional Chinese medicines benefit to nonalcoholic fatty liver disease: a systematic review and meta-analysis. Mol Biol Rep. 2012;39:9715-9722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Cai Y, Liang Q, Chen W, Chen M, Chen R, Zhang Y, Xiao Y, Chen L. Evaluation of HuoXueHuaYu therapy for nonalcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trial. BMC Complement Altern Med. 2019;19:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Liu ZL, Xie LZ, Zhu J, Li GQ, Grant SJ, Liu JP. Herbal medicines for fatty liver diseases. Cochrane Database Syst Rev. 2013;(8):CD009059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | OCEBM Levels of Evidence Working Group. The Oxford levels of evidence 2 [cited 1 July 2020]. In: Oxford Centre for Evidence-Based Medicine, 2011 [Internet]. Available from: https://www.cebm.net/index.aspx?o=5653. |
| 12. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18665] [Cited by in RCA: 17536] [Article Influence: 1096.0] [Reference Citation Analysis (1)] |
| 13. | Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, Henry DA. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3100] [Cited by in RCA: 5697] [Article Influence: 712.1] [Reference Citation Analysis (0)] |
| 14. | Whiting P, Savović J, Higgins JP, Caldwell DM, Reeves BC, Shea B, Davies P, Kleijnen J, Churchill R; ROBIS group. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 718] [Cited by in RCA: 1327] [Article Influence: 132.7] [Reference Citation Analysis (0)] |
| 15. | Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4091] [Cited by in RCA: 5706] [Article Influence: 407.6] [Reference Citation Analysis (0)] |
| 16. | He M, Jiang J. Meta-analysis of effect of TCM on main biochemical indexes of non-alcoholic fatty liver disease. Zhonghua Zhongyiyao Zazhi. 2010;25:1214-1220. |
| 17. | Li L, Su DM, Han HX. Traditional Chinese medicine for non-alcoholic steatohepatitis: a systematic review. honguo Xunzheng Yixue Zazhi. 2011;11:195-203. [DOI] [Full Text] |
| 18. | Li YH, Zhu HJ. Study on effect of oral Chinese medicine compared with polyene phosphatidylcholine group treatment for non-alcoholic fatty liver disease. Shiyong Yaowu Yu Linchuang. 2014;17:1016-1018. [DOI] [Full Text] |
| 19. | Yang HC, Sun JG. Systemic evaluation of the efficacy and safety of invigorating spleen and resolving phlegm method in the treatment of nonalcoholic fatty liver disease. Xiandai Zhongxiyijiehe Zazhi. 2019;28:267-273. [DOI] [Full Text] |
| 20. | Zhang J, Zhang HH. Systematic review of treating nonalcoholic fatty liver with taking TCM and screening of active components. Zhongguo Weisheng Chanye. 2014;11:43-45. [DOI] [Full Text] |
| 21. | PLoS Medicine Editors. Best practice in systematic reviews: the importance of protocols and registration. PLoS Med. 2011;8:e1001009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Stewart L, Moher D, Shekelle P. Why prospective registration of systematic reviews makes sense. Syst Rev. 2012;1:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 229] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 23. | Brown TJ, Brainard J, Song F, Wang X, Abdelhamid A, Hooper L; PUFAH Group. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: systematic review and meta-analysis of randomised controlled trials. BMJ. 2019;366:l4697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 177] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 24. | Sobieraj DM, Weeda ER, Nguyen E, Coleman CI, White CM, Lazarus SC, Blake KV, Lang JE, Baker WL. Association of inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: a systematic review and meta-analysis. JAMA. 2018;319:1485-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 230] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 25. | Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1634] [Cited by in RCA: 1920] [Article Influence: 320.0] [Reference Citation Analysis (0)] |
| 26. | Olivo SA, Macedo LG, Gadotti IC, Fuentes J, Stanton T, Magee DJ. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther. 2008;88:156-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 605] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 27. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24844] [Article Influence: 1774.6] [Reference Citation Analysis (3)] |
| 28. | McAuley L, Pham B, Tugwell P, Moher D. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet. 2000;356:1228-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 488] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 29. | Riveros C, Dechartres A, Perrodeau E, Haneef R, Boutron I, Ravaud P. Timing and completeness of trial results posted at ClinicalTrials.gov and published in journals. PLoS Med. 2013;10:e1001566; discussion e1001566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 185] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 30. | Scherer RW, Meerpohl JJ, Pfeifer N, Schmucker C, Schwarzer G, von Elm E. Full publication of results initially presented in abstracts. Cochrane Database Syst Rev. 2018;11:MR000005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 31. | Benzies KM, Premji S, Hayden KA, Serrett K. State-of-the-evidence reviews: advantages and challenges of including grey literature. Worldviews Evid Based Nurs. 2006;3:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 32. | Conn VS, Valentine JC, Cooper HM, Rantz MJ. Grey literature in meta-analyses. Nurs Res. 2003;52:256-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 213] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 33. | Hopewell S, McDonald S, Clarke M, Egger M. Grey literature in meta-analyses of randomized trials of health care interventions. Cochrane Database Syst Rev. 2007;(2):MR000010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 243] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 34. | Mahood Q, Van Eerd D, Irvin E. Searching for grey literature for systematic reviews: challenges and benefits. Res Synth Methods. 2014;5:221-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 332] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 35. | He J, Du L, Liu G, Fu J, He X, Yu J, Shang L. Quality assessment of reporting of randomization, allocation concealment, and blinding in traditional Chinese medicine RCTs: a review of 3159 RCTs identified from 260 systematic reviews. Trials. 2011;12:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Shi C, Tian J, Ren D, Wei H, Zhang L, Wang Q, Yang K. Methodological reporting of randomized trials in five leading Chinese nursing journals. PLoS One. 2014;9:e113002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Wang G, Mao B, Xiong ZY, Fan T, Chen XD, Wang L, Liu GJ, Liu J, Guo J, Chang J, Wu TX, Li TQ; CONSORT Group for Traditional Chinese Medicine. The quality of reporting of randomized controlled trials of traditional Chinese medicine: a survey of 13 randomly selected journals from mainland China. Clin Ther. 2007;29:1456-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 38. | Zhang J, Chen X, Zhu Q, Cui J, Cao L, Su J. Methodological reporting quality of randomized controlled trials: a survey of seven core journals of orthopaedics from Mainland China over 5 years following the CONSORT statement. Orthop Traumatol Surg Res. 2016;102:933-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Rinella ME, Tacke F, Sanyal AJ, Anstee QM; participants of the AASLD/EASL Workshop. Report on the AASLD/EASL Joint Workshop on Clinical Trial Endpoints in NAFLD. Hepatology. 2019;70:1424-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 40. | Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Falck-Ytter Y, Jaeschke R, Vist G, Akl EA, Post PN, Norris S, Meerpohl J, Shukla VK, Nasser M, Schünemann HJ; GRADE Working Group. GRADE guidelines: 8. Rating the quality of evidence--indirectness. J Clin Epidemiol. 2011;64:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1045] [Cited by in RCA: 1366] [Article Influence: 97.6] [Reference Citation Analysis (0)] |
| 41. | Kuo DH, Yeh CH, Shieh PC, Cheng KC, Chen FA, Cheng JT. Effect of shanzha, a Chinese herbal product, on obesity and dyslipidemia in hamsters receiving high-fat diet. J Ethnopharmacol. 2009;124:544-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Hong M, Li S, Wang N, Tan HY, Cheung F, Feng Y. A biomedical investigation of the hepatoprotective effect of radix salviae miltiorrhizae and network pharmacology-based prediction of the active compounds and molecular targets. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |