Published online May 6, 2020. doi: 10.12998/wjcc.v8.i9.1745
Peer-review started: January 14, 2020
First decision: February 26, 2020
Revised: March 26, 2020
Accepted: April 17, 2020
Article in press: April 17, 2020
Published online: May 6, 2020
Processing time: 102 Days and 12.7 Hours
Biliary hamartomas (BH) are a rare benign disease caused by malformation of the intrahepatic bile ducts. BH are occasionally diagnosed, but often lack obvious clinical symptoms. They are usually diagnosed by biopsy and imaging tests in clinical practice. Few studies have reported the association of BH with portal hypertension.
A 40-year-old man was repeatedly admitted to our hospital due to hematochezia. The source of bleeding was considered to be gastroesophageal varices and portal hypertensive gastropathy by endoscopy. He had no history of hepatitis virus infection, alcohol abuse, drug-induced liver injury, or autoimmune liver disease. He underwent magnetic resonance imaging, which showed rounded, irregular, low-signal-T1 and high-signal-T2 lesions diffusely distributed on the liver, that were not communicated with the biliary system on magnetic resonance cholangiopancreatography. According to the imaging examination, the patient was considered to have a diagnosis of BH with portal hypertension.
Based on the present case report, BH may be a potential etiology of portal hypertension.
Core tip: Biliary hamartomas (BH) are a rare benign disease caused by malformation of the intrahepatic bile ducts. BH are occasionally diagnosed, but often lack obvious clinical symptoms. Herein, we report a patient diagnosed with BH by imaging tests who presented with recurrent variceal bleeding, which suggested the possibility of BH as a potential cause of portal hypertension.
- Citation: Li QQ, Guo XZ, Li HY, Qi XS. Portal hypertension in a patient with biliary hamartomas: A case report. World J Clin Cases 2020; 8(9): 1745-1751
- URL: https://www.wjgnet.com/2307-8960/full/v8/i9/1745.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i9.1745
Biliary hamartomas (BH), also known as von Meyenburg complexes, are usually considered a benign disease caused by congenital bile duct malformation[1,2]. They are clinically rare with a prevalence of 0.6% on biopsy[3]. Microscopical images often show bile duct-like structures covered by a single layer of columnar epithelium. Dilated lumens contain bile and are surrounded by fibrous stroma[2,4]. Except for liver biopsy, BH can often be detected by computed tomography (CT) and magnetic resonance imaging (MRI) images, which often appear as multiple irregularly shaped lesions with a diameter of about 10 mm[5].
Most patients with BH are usually asymptomatic. Some patients accidentally present with mild symptoms, such as abdominal pain, fever, or liver dysfunction[1,6,7]. Herein, we report a patient with BH who presented with variceal bleeding and underwent endoscopic variceal therapy.
On September 20, 2018, a 40-year-old man presented with dark red colored bloody stool for one day.
The patient presented with dark red colored bloody stool for one day. He had been diagnosed with esophageal and gastric varices on endoscopy, and then underwent endoscopic variceal ligation and repeated gastric glue tissue adhesive injection for variceal bleeding at our department.
He had no history of hepatitis virus infection, alcohol abuse, drug-induced liver injury, or autoimmune liver disease.
At admission, laboratory tests showed that hemoglobin was 55 g/L, red blood cell count was 1.90 × 1012/L (reference range: 4.3-5.8 × 1012/L), hematocrit was 16.3% (reference range: 40%-50%), white blood cell count was 3.0 × 109/L (reference range: 3.5-9.5 × 109/L), platelet count was 21 × 109/L (reference range: 125-350 × 109/L), prothrombin time was 16.8 s (reference range: 11.5-14.5 s), and activated partial thromboplastin time was 36.7 s (reference range: 28.0-40.0 s). Other biochemical indices showed no obvious abnormalities. He received intravenous infusion of proton pump inhibitors and vasoconstrictors and a transfusion of suspended red blood cells and fresh frozen plasma.
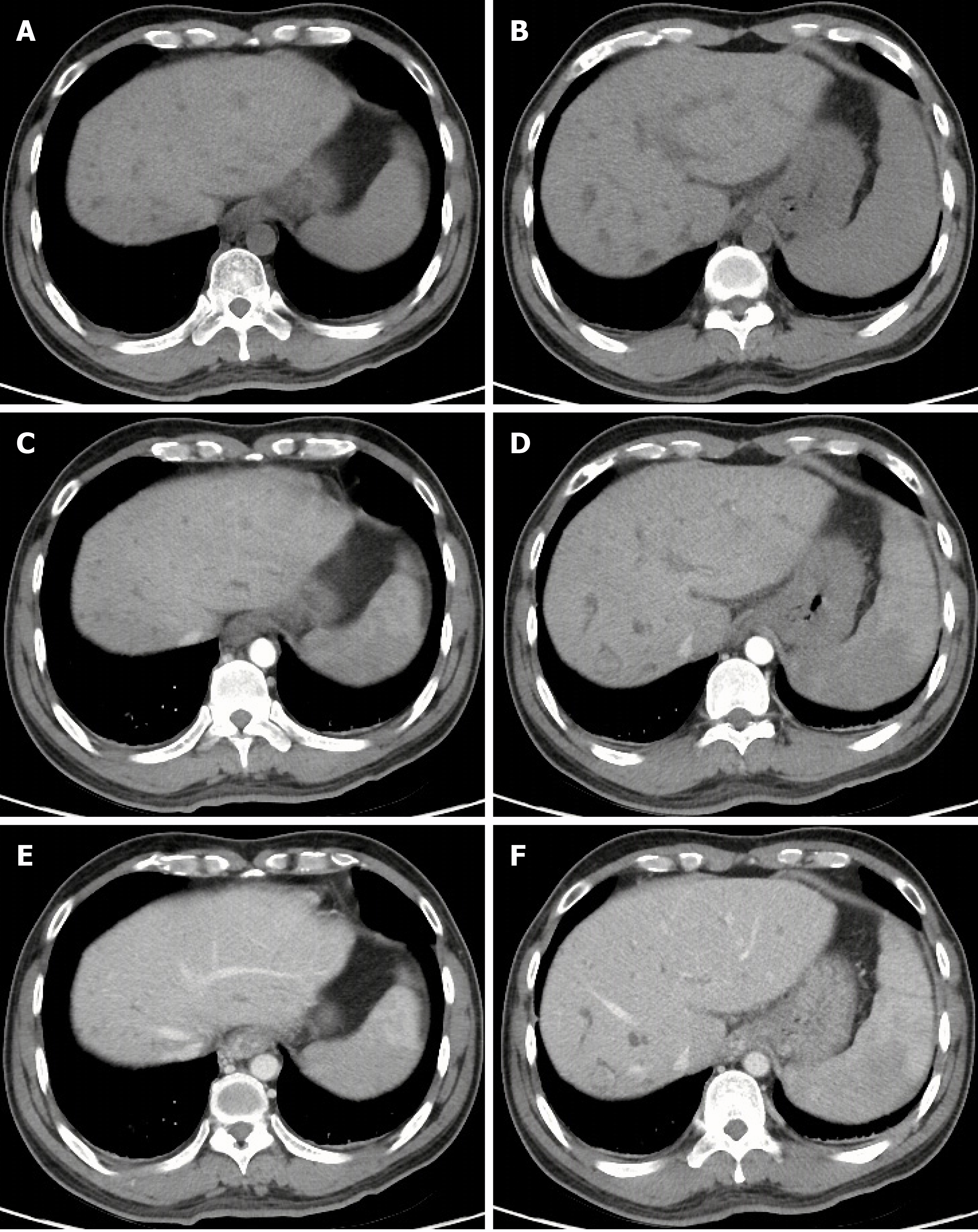
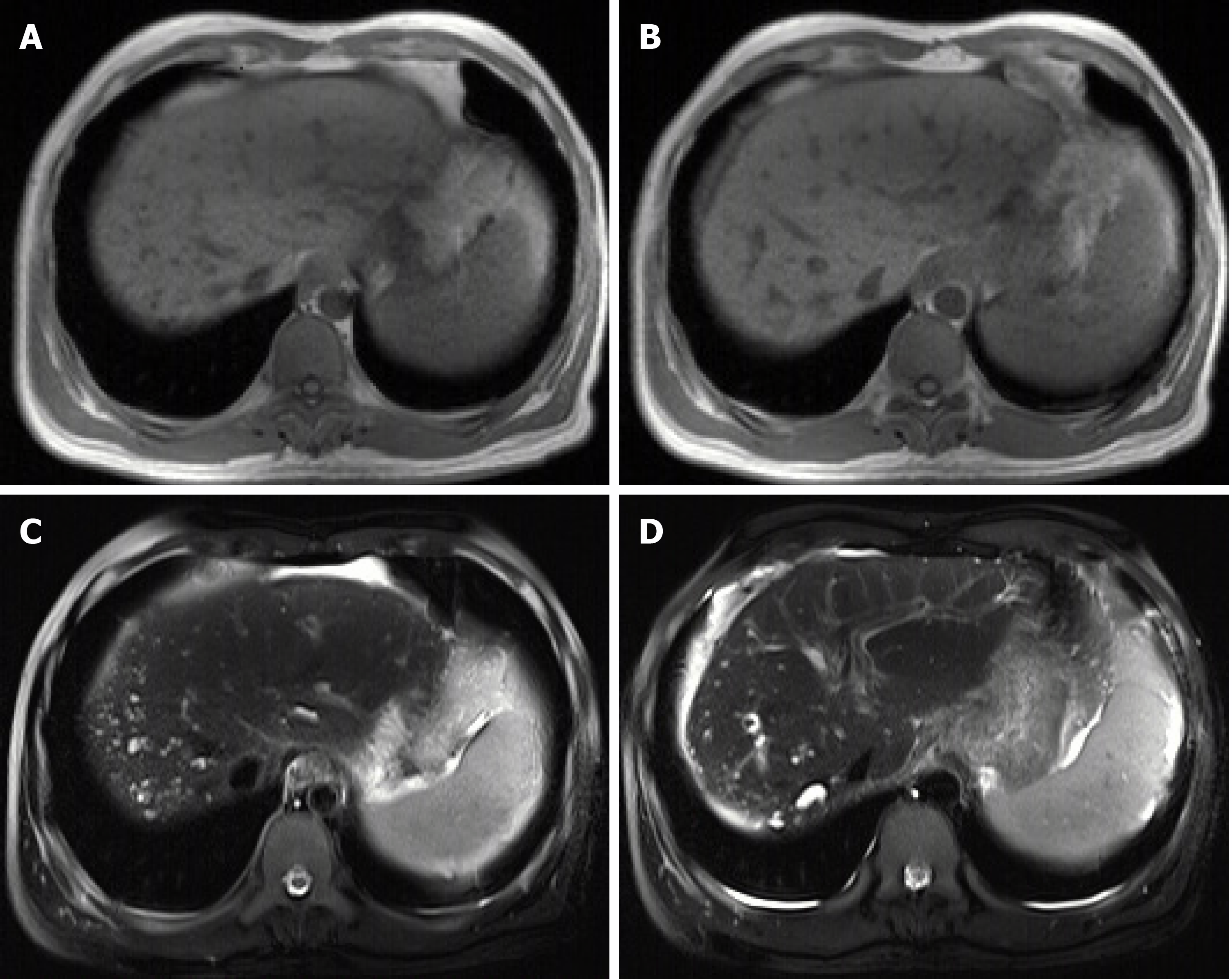
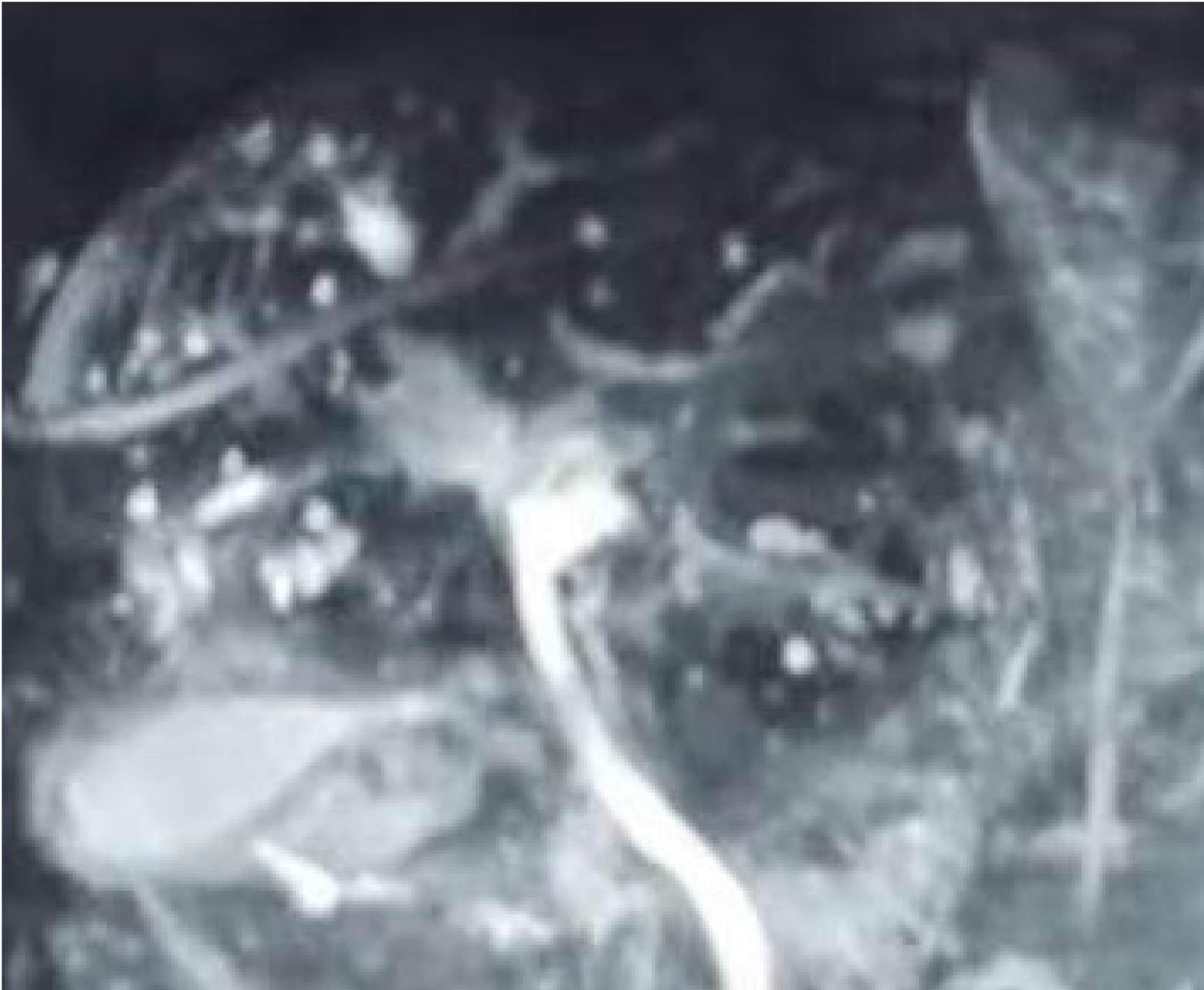
On September 21, 2018, the patient underwent upper gastrointestinal endoscopy, which showed mild esophageal varices, portal hypertensive gastropathy, and a removing tissue glue at the gastric fundus which was considered the major source of gastrointestinal bleeding. Thus, our endoscopist did not perform endoscopic variceal therapy on this patient. Contrast-enhanced CT scans showed multiple, rounded, low density areas on the liver, which were not significantly enhanced at the arterial and portal vein phases (Figure 1). MRI and magnetic resonance cholangiopancreatography (MRCP) were then performed. MRI showed rounded, irregular, low-signal-T1 and high-signal-T2 lesions diffusely distributed on the liver which were not significantly enhanced (Figure 2). MRCP showed that cystic high-signal lesions were diffusely distributed on the liver but were not communicated with the biliary system (Figure 3). The patient refused liver biopsy.
A diagnosis of BH was considered.
He continued to receive intravenous infusion of proton pump inhibitors, vasoconstrictors and nutritional fluids. Following treatment, he was stable and discharged on oral propranolol.
On December 5, 2018, the patient was again admitted to our department due to melena for 5 h. Upper gastrointestinal endoscopy showed mild gastric varices and portal hypertensive gastropathy which was considered the major source of gastrointestinal bleeding.
On February 11, 2019, the patient underwent upper gastrointestinal endoscopy, which showed mild esophageal varices and several gastric varices with red color sign. He then underwent endoscopic glue tissue adhesive injection.
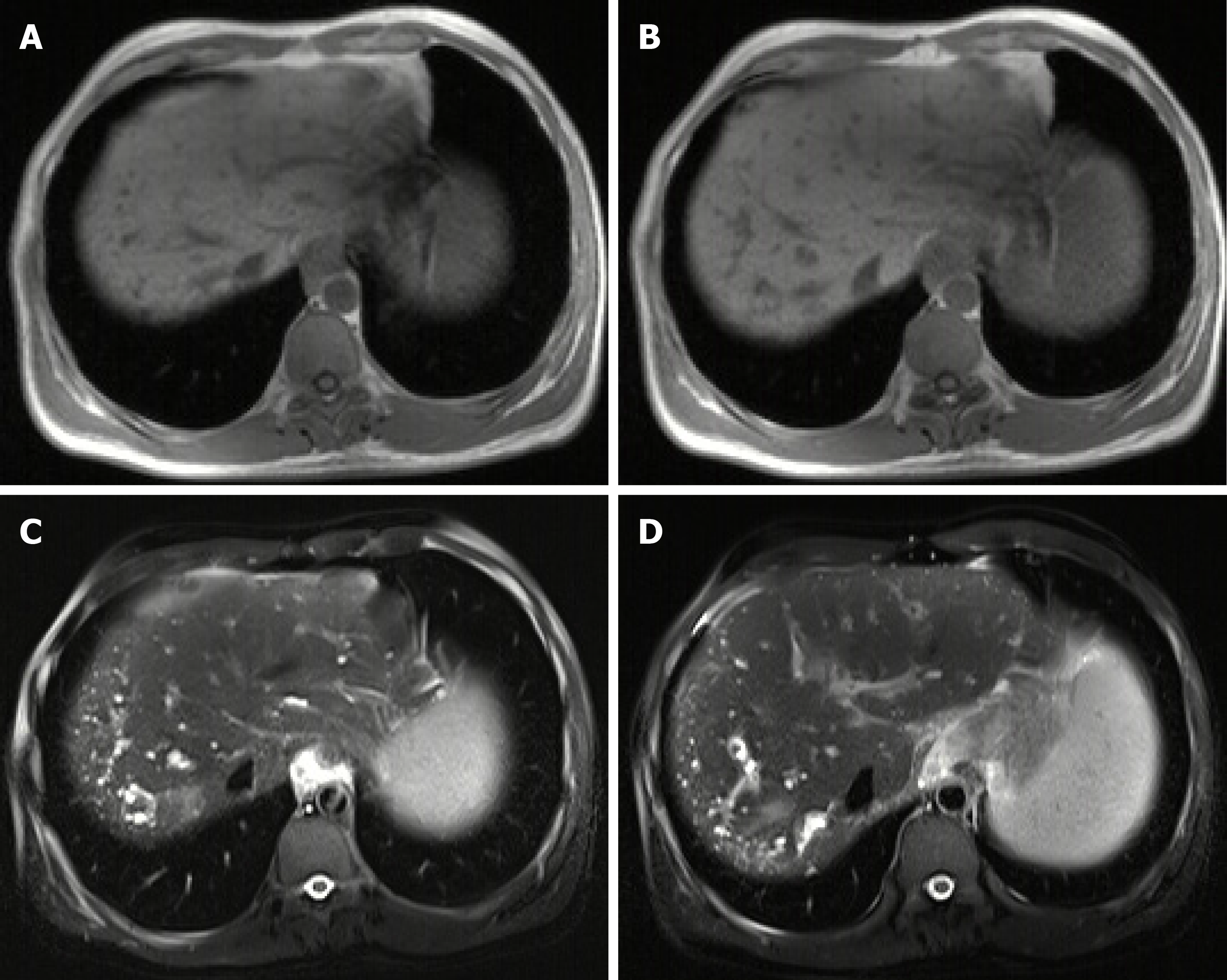
On July 4, 2019, the patient underwent upper gastrointestinal endoscopy. At this time, endoscopic variceal therapy was not necessary. He underwent MRI and MRCP again. No significant changes in liver lesions were seen when compared with the previous images (Figures 4 and 5). A diagnosis of BH was still considered.
Congenital malformations of the intrahepatic bile duct include many diseases, such as BH, Caroli disease, autosomal dominant polycystic kidney disease, and congenital hepatic fibrosis, which have malformations at different levels of the biliary tree. Of these, BH refers to malformations of small interlobular bile ducts[8,9]. BH are characterized as multiple low-density lesions with an irregular outline on plain CT scans with a diameter of 10-15 mm. They are not usually enhanced[9]. Generally, MRI is considered to be superior to CT in the diagnosis of BH[10,11]. On MRI scans, BH lesions are characterized as low signal intensity on T1-weighted images and high signal intensity on T2-weighted images[12,13]. In a few cases, the rim of lesions can be enhanced, which is considered to be the surrounding compressed liver parenchyma[14,15]. BH should be distinguished from several other diseases. First, simple hepatic cysts, a benign disease, are characterized as round homogeneous low-density lesions with typically regular outlines without enhancement on CT scans. Hepatic cysts are larger in size than BH[10]. Second, Caroli disease is a congenital malformation of larger intrahepatic bile ducts. Multiple low-density lesions can be seen on CT scans. The classical imaging presentation is the central dots of the cystic bile duct surrounding the branches of the veins. MRCP is usually a good diagnostic modality for Caroli disease, which can show that the cystic bile duct communicates with the biliary system[16,17]. Third, polycystic liver disease is inherited and often associated with autosomal dominant polycystic kidney disease. Multiple cystic lesions are often observed in both liver and kidney[18,19]. Due to a large number of cysts, the incidence of intracapsular hemorrhage is increased, and abnormal signals can be seen on the images[10]. Positive family history is also one of the major diagnostic criteria[20]. Fourth, cystic metastases are characterized as an enhancement of peripheral viable tissue around the lesion on contrast-enhanced CT and MRI scans[10]. Fifth, abscesses are inflammatory lesions characterized as double target signs on CT, which consist of a hypodense pus area, a hyperdense ring of granulation tissue, and a hypodense zone of inflammatory edema[21]. In addition, abscesses can be readily differentiated from BH by clinical symptoms, such as fever and abdominal pain[22].
Due to the small diameter of lesions, BH may not lead to obvious clinical symptoms. Only a few studies have reported that BH might progress to cholangiocarcinoma[23]. However, our patient had another rare but fatal portal hypertension-related complication, and presented with bleeding from gastroesophageal varices and hypersplenism. Of course, the etiology of portal hypertension in this case needs to be differentiated from liver cirrhosis which is often produced by viral hepatitis, alcohol abuse, or autoimmune factors. However, the patient refused to undergo liver biopsy. In the absence of liver histological analyses, we would like to emphasize that our case does not have any potential cause of chronic liver diseases nor abnormal liver function. Therefore, we believe that portal hypertension was mainly related to the presence of BH. Indeed, Yoshida et al[24] reported a similar case and thought that portal veins were compressed by the cystic bile duct, thereby leading to the development of portal hypertension. Besides, BH can be found in either normal liver or congenital hepatic fibrosis. The latter could be one of the potential causes of portal hypertension development[2,25]. Komatsu et al[26] reported a patient with congenital hepatic fibrosis and BH who did not have any significant abnormality in liver function, but repeatedly developed gastroesophageal varices and underwent endoscopic variceal therapy.
In conclusion, we report a patient with BH presenting with severe portal hypertension-related complications, which suggested the possibility of BH as a potential etiology of portal hypertension. However, this conclusion should be cautiously interpreted due to the absence of liver histology.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Gangl A, Garbuzenko DV S-Editor: Dou Y L-Editor: Webster JR E-Editor: Liu MY
| 1. | Quentin M, Scherer A. The "von Meyenburg complex". Hepatology. 2010;52:1167-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Desmet VJ. Congenital diseases of intrahepatic bile ducts: variations on the theme "ductal plate malformation". Hepatology. 1992;16:1069-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 340] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 3. | Thommesen N. Biliary hamartomas (von Meyenburg complexes) in liver needle biopsies. Acta Pathol Microbiol Scand A. 1978;86:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Lin S, Weng Z, Xu J, Wang MF, Zhu YY, Jiang JJ. A study of multiple biliary hamartomas based on 1697 liver biopsies. Eur J Gastroenterol Hepatol. 2013;25:948-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Zheng RQ, Zhang B, Kudo M, Onda H, Inoue T. Imaging findings of biliary hamartomas. World J Gastroenterol. 2005;11:6354-6359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 84] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Shah RN, Forster EM. Von Meyenburg Complexes Found Incidentally in a Patient With Acute Cholangitis. Clin Gastroenterol Hepatol. 2019;17:e140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Jáquez-Quintana JO, Reyes-Cabello EA, Bosques-Padilla FJ. Multiple Biliary Hamartomas, The ''Von Meyenburg Complexes''. Ann Hepatol. 2017;16:812-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Desmet VJ. Ludwig symposium on biliary disorders--part I. Pathogenesis of ductal plate abnormalities. Mayo Clin Proc. 1998;73:80-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 82] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Pech L, Favelier S, Falcoz MT, Loffroy R, Krause D, Cercueil JP. Imaging of Von Meyenburg complexes. Diagn Interv Imaging. 2016;97:401-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Mortelé KJ, Ros PR. Cystic focal liver lesions in the adult: differential CT and MR imaging features. Radiographics. 2001;21:895-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 255] [Article Influence: 10.6] [Reference Citation Analysis (74)] |
| 11. | Cannella R, Giambelluca D, Diamarco M, Caruana G, Cutaia G, Midiri M, Salvaggio G. Congenital Cystic Lesions of the Bile Ducts: Imaging-Based Diagnosis. Curr Probl Diagn Radiol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Tohmé-Noun C, Cazals D, Noun R, Menassa L, Valla D, Vilgrain V. Multiple biliary hamartomas: magnetic resonance features with histopathologic correlation. Eur Radiol. 2008;18:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Mamone G, Carollo V, Cortis K, Aquilina S, Liotta R, Miraglia R. Magnetic resonance imaging of fibropolycystic liver disease: the spectrum of ductal plate malformations. Abdom Radiol (NY). 2019;44:2156-2171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Semelka RC, Hussain SM, Marcos HB, Woosley JT. Biliary hamartomas: solitary and multiple lesions shown on current MR techniques including gadolinium enhancement. J Magn Reson Imaging. 1999;10:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Guo Y, Jain D, Weinreb J. Von Meyenburg Complex: Current Concepts and Imaging Misconceptions. J Comput Assist Tomogr. 2019;43:846-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Yonem O, Bayraktar Y. Clinical characteristics of Caroli's syndrome. World J Gastroenterol. 2007;13:1934-1937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Yonem O, Bayraktar Y. Clinical characteristics of Caroli's disease. World J Gastroenterol. 2007;13:1930-1933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Qian Q, Li A, King BF, Kamath PS, Lager DJ, Huston J, Shub C, Davila S, Somlo S, Torres VE. Clinical profile of autosomal dominant polycystic liver disease. Hepatology. 2003;37:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 131] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Pirson Y. Extrarenal manifestations of autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Lantinga MA, Gevers TJ, Drenth JP. Evaluation of hepatic cystic lesions. World J Gastroenterol. 2013;19:3543-3554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 119] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (2)] |
| 21. | Qian LJ, Zhu J, Zhuang ZG, Xia Q, Liu Q, Xu JR. Spectrum of multilocular cystic hepatic lesions: CT and MR imaging findings with pathologic correlation. Radiographics. 2013;33:1419-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Lardière-Deguelte S, Ragot E, Amroun K, Piardi T, Dokmak S, Bruno O, Appere F, Sibert A, Hoeffel C, Sommacale D, Kianmanesh R. Hepatic abscess: Diagnosis and management. J Visc Surg. 2015;152:231-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 169] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 23. | Parekh V, Peker D. Malignant Transformation in Von-Meyenburg Complexes: Histologic and Immunohistochemical Clues With Illustrative Cases. Appl Immunohistochem Mol Morphol. 2015;23:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Yoshida S, Kurokohchi K, Ueno T, Yoshino M, Shimada M, Masaki T. Hepatic von Meyenburg complex: a trigger of severe portal hypertension. Liver Int. 2009;29:614-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Brancatelli G, Federle MP, Vilgrain V, Vullierme MP, Marin D, Lagalla R. Fibropolycystic liver disease: CT and MR imaging findings. Radiographics. 2005;25:659-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Komatsu M, Tanaka N, Shibata S, Kimura T, Ichikawa Y, Morita S, Joshita S, Nagaya T, Umemura T, Ichijo T, Matsumoto A, Yoshizawa K, Tanaka E. Laparoscopic findings of congenital hepatic fibrosis: A case report and review of the published work. Hepatol Res. 2014;44:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |