Published online May 6, 2020. doi: 10.12998/wjcc.v8.i9.1729
Peer-review started: December 23, 2019
First decision: February 20, 2020
Revised: March 15, 2020
Accepted: April 10, 2020
Article in press: April 10, 2020
Published online: May 6, 2020
Processing time: 130 Days and 3.8 Hours
Intrapancreatic accessory spleen (IPAS) mimics a pancreatic neoplasm on imaging studies, and due to the lack of radiological diagnostic criteria, patients undergo unnecessary distal pancreatectomies. Endoscopic ultrasonography (EUS) is a reliable and efficient diagnostic modality for pancreatic diseases. However, no EUS criteria have been established for IPAS. We present the EUS-elastography image of IPAS, which may minimize the chance of misdiagnosis in the future.
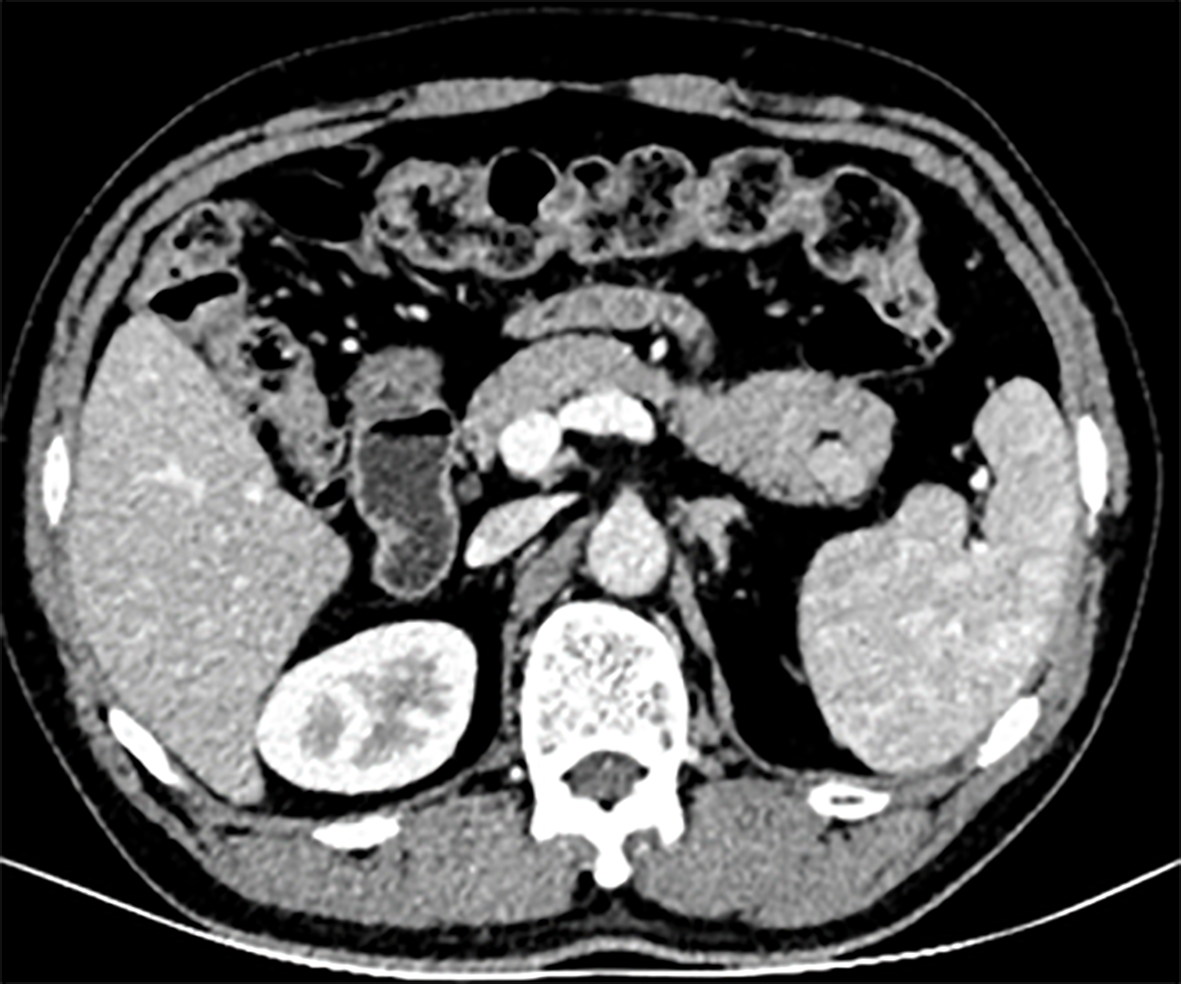
A 50-year-old man was referred for an EUS evaluation after computed tomography showed a hypervascular enhanced mass in the tail of the pancreas, which indicated a neuroendocrine neoplasm. EUS elastography demonstrated that the lesion of interest covered no more than 25% of the region of interest. The patient underwent distal pancreatectomy. However, the resected tissue was evaluated, and the patient was finally diagnosed with IPAS.
IPAS should be considered in patients with suspected pancreatic neuroendocrine tumors of the pancreatic tail before surgery is performed. The differentiation between IPAS and pancreatic neuroendocrine tumors can be demonstrated using EUS-elastography.
Core tip: We report the case of a patient with intrapancreatic accessory spleen evaluated by endoscopic ultrasonography and endoscopic ultrasonography elastography. We present the endoscopic ultrasound-elastography features of intrapancreatic accessory spleen, which may help to minimize the chance of misdiagnosis and prevent patients from undergoing unnecessary surgery in the future.
- Citation: Ge N, Sun SY. Endoscopic ultrasonography elastography in the diagnosis of intrapancreatic ectopic spleen: A case report. World J Clin Cases 2020; 8(9): 1729-1734
- URL: https://www.wjgnet.com/2307-8960/full/v8/i9/1729.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i9.1729
The identification of incidental pancreatic lesions is increasing due to advancements in imaging. Diagnosis remains a challenge for clinicians, with intrapancreatic accessory spleen (IPAS) posing a unique dilemma. IPAS mimics a pancreatic neoplasm on imaging studies, and due to the lack of radiological diagnostic criteria, patients undergo unnecessary distal pancreatectomy. Endoscopic ultrasonography (EUS) is one of the most reliable and efficient diagnostic modalities for pancreatic diseases. However, no EUS criteria have been established for IPAS. The use of EUS-elastography may increase diagnostic accuracy. Herein, we present the EUS-elastography image of IPAS.
A 50-year-old male patient was referred from the surgery department for an EUS evaluation after a computed tomography scan showed a 1.4-cm round hypervascular enhanced mass in the tail of the pancreas, which indicated a neuroendocrine neoplasm (Figure 1).
Initially, the mass was found following a computed tomography examination during his regular medical examination. The patient did not complain of any symptoms.
He has no specific history of past illness.
He has no specific personal or family history.
The patient did not have positive signs on physical examination.
EUS revealed a 1.2 cm × 1.3 cm, round, well-defined homogenous hypoechoic mass in the pancreatic tail with no other endosonographic pancreatic abnormalities (Figure 2).
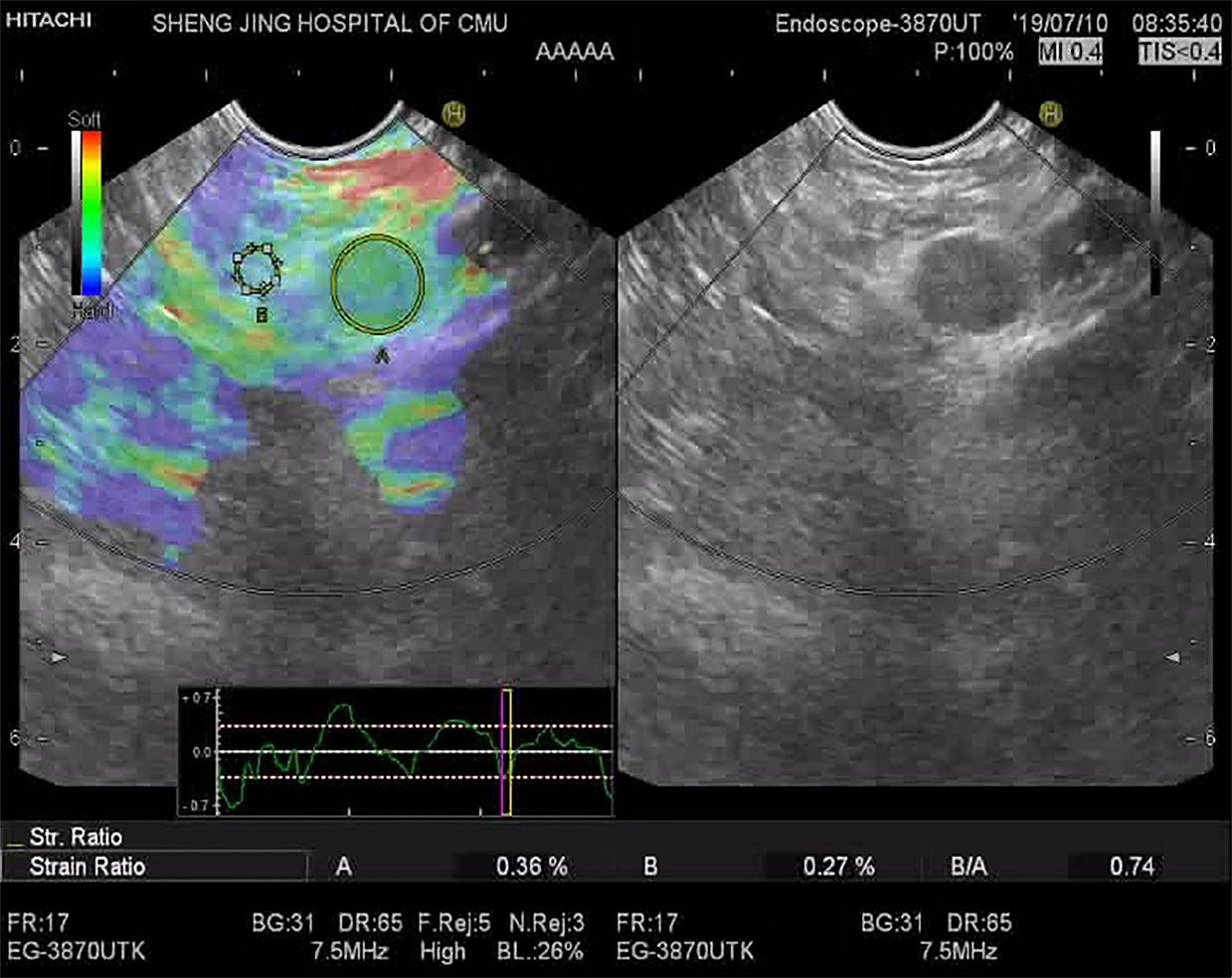
EUS elastography was performed and it was found that the lesion covered no more than 25% of the region of interest. To assess the quality and reproducibility of the elastography image, the image was frozen and the stored cine loop was reviewed frame by frame. A consistent color pattern obtained in a number of consecutive frames indicating a good, reliable technique.
The lesion showed a heterogeneous, predominantly green pattern with slight yellow and blue lines, indicating a soft texture (Figure 3).
Real-time tissue quantitative elastography with strain ratio (SR) measurement was also performed. Region of interest A was placed within the pancreatic tail mass and region of interest B in the adjacent parenchyma. An SR (B/A) of 0.74 indicated the softer elasticity of the lesion compared with the surrounding normal pancreatic tissue (Figure 4).
The patient was finally diagnosed with IPAS.
The patient underwent distal pancreatectomy as initial CT indicated a neuroendocrine neoplasm.
Pathological evaluation of the resected tissue showed a 1.3-cm nodule with a complete capsule in the pancreatic tissue, and the nodule was composed entirely of spleen tissue. The final diagnosis was IPAS. The patient had no adverse effects after surgery.
Accessory spleen is a congenital failure of splenic bud fusion during embryogenesis, which is revealed in about 10%-30% of autopsies[1]. In most cases, accessory spleens are located near the splenic hilum. The second most common site is the pancreatic tail (17%). IPAS can mimic intrapancreatic tumors, such as neuroendocrine neoplasms[2-4]. IPAS is often misdiagnosed as a neuroendocrine neoplasm and treated with distal pancreatectomy. However, asymptomatic IPAS does not require medical treatment, or even surgical resection.
It is important to provide accurate preoperative differential diagnostics to prevent patients undergoing unnecessary surgery.
EUS has high resolution and is considered one of the most reliable and efficient diagnostic modalities for pancreatobiliary diseases[5-7].
Elastography is a new technique for tissue characterization, providing a noninvasive modality for imaging the mechanical properties of tissue[8]. EUS elastography is able to better characterize lesions by tissue stiffness evaluation in various locations which are only accessible from the gastrointestinal tract. Elastography can be adjunct to the EUS examination due to its ease of use, noninvasiveness, and low cost.
EUS elastography for the evaluation of pancreatic tissue was first reported in 2006. The pooled sensitivity and specificity of EUS elastography from 15 studies were 93% and 63%, respectively[9]. Most malignant pancreatic lesions appear stiff in elastography[10]. It is also reported that in patients with small solid pancreatic lesions, EUS elastography can play an important role in ruling out malignancy if the lesion appears soft[11].
Based on qualitative elastographic evaluation, up to 4 well-defined patterns have been described that characterize solid pancreatic lesions and contribute to their classification: A homogeneous green pattern present commonly in the normal pancreas; a heterogeneous, predominantly green pattern with slight yellow and red lines present only in inflammatory pancreatic masses; a heterogeneous, predominantly blue pattern with small green areas and red lines and a geographic appearance present mainly in pancreatic malignant tumors (including pancreatic adenocarcinoma); and a homogeneous blue pattern present only in pancreatic neuroendocrine malignant lesions[12]. Giovannini et al[13], using this qualitative evaluation, reported that the sensitivity and specificity for malignancy were 100% and 67%, respectively. In our case, the lesion showed a heterogeneous, predominantly green pattern with slight yellow and blue lines on qualitative elastographic evaluation, which may exclude pancreatic malignant tumors or neuroendocrine malignant lesions.
SR calculation is based on standard qualitative EUS elastography data[14]. Two different areas (A and B) are selected for quantitative elastographic analysis. Area A is selected including as much of the target lesion as possible without including the surrounding tissues. Area B is selected within a soft reference area outside the target lesion. The SR is calculated as the quotient of B/A. In the past few years, several studies have been conducted to determine the accuracy of SR for detecting malignancies. Different cut-off values have been defined, from 3.7 to 24, with sensitivities ranging from 67% to 98%, and specificity ranging from 45% to 71%[15-20]. In our case, an SR of 0.74 indicated the soft texture of the lesion, which is seldom found in pancreatic malignant tumors or neuroendocrine malignant lesions[12]. When we reviewed the video of the EUS examination, we noticed that this pancreatic tail lesion also had an echogenicity similar to the adjacent spleen tissue.
Recently, Bhutani et al[21] reported that the “bridge sign” in EUS images may differentiate IPAS from a pancreatic neuroendocrine tumor, which could save the patient an EUS-fine needle aspiration, diagnostic confusion, surgery, anxiety, and healthcare dollars. Therefore, when combined with EUS-elastography, IPAS may not be misdiagnosed by EUS in the future. Moreover, unnecessary surgery and other invasive intervention may be avoided, similar to the current irreplaceable role of EUS diagnosis of gastric ectopic pancreas.
IPAS is a relevant differential diagnosis in patients with suspected pancreatic neuroendocrine tumors of the pancreatic tail, which should be considered before surgery is undertaken. Non-invasive differentiation between IPAS and pancreatic neuroendocrine tumors can be provided using EUS-elastography.
Care Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kramer JR, Maurea S S-Editor: Tang JZ L-Editor: Webster JR E-Editor: Xing YX
| 1. | Dodds WJ, Taylor AJ, Erickson SJ, Stewart ET, Lawson TL. Radiologic imaging of splenic anomalies. AJR Am J Roentgenol. 1990;155:805-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 92] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Zhu HX, Lou WH, Kuang TT, Wang DS. Post-splenectomy intrapancreatic accessory spleen mimicking endocrine tumor of the pancreas. Int J Surg Case Rep. 2014;5:1151-1153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Engler CC, Lemke J, Kornmann M, Barth TF, Schmidt SA, Henne-Bruns D. Ectopic spleen and liver hemangioma mimicking metastatic pancreatic neuroendocrine tumor. Int J Surg Case Rep. 2015;17:139-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Kurmann A, Michel JM, Stauffer E, Egger B. Intrapancreatic Accessory Spleen Misdiagnosed as a Nonsecreting Endocrine Tumor: Case Report and Review of the Literature. Case Rep Gastroenterol. 2010;4:210-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Laghi A, Zerunian M, Caruso D. How new technologies could impact on radiology diagnosis and assessment of pancreatic lesions: Future perspectives. Endosc Ultrasound. 2018;7:310-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Sato A, Irisawa A, Bhutani MS, Shibukawa G, Yamabe A, Fujisawa M, Igarashi R, Arakawa N, Yoshida Y, Abe Y, Maki T, Hoshi K, Ohira H. Significance of normal appearance on endoscopic ultrasonography in the diagnosis of early chronic pancreatitis. Endosc Ultrasound. 2018;7:110-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Dong Y, D'Onofrio M, Hocke M, Jenssen C, Potthoff A, Atkinson N, Ignee A, Dietrich CF. Autoimmune pancreatitis: Imaging features. Endosc Ultrasound. 2018;7:196-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Dietrich CF, Bibby E, Jenssen C, Saftoiu A, Iglesias-Garcia J, Havre RF. EUS elastography: How to do it? Endosc Ultrasound. 2018;7:20-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Kitano M, Yoshida T, Itonaga M, Tamura T, Hatamaru K, Yamashita Y. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J Gastroenterol. 2019;54:19-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 214] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 10. | Harmsen FR, Domagk D, Dietrich CF, Hocke M. Discriminating chronic pancreatitis from pancreatic cancer: Contrast-enhanced EUS and multidetector computed tomography in direct comparison. Endosc Ultrasound. 2018;7:395-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Ignee A, Jenssen C, Arcidiacono PG, Hocke M, Möller K, Saftoiu A, Will U, Fusaroli P, Iglesias-Garcia J, Ponnudurai R, Petrone MC, Braden B, Burmester E, Dong Y, Atkinson NS, Dietrich CF. Endoscopic ultrasound elastography of small solid pancreatic lesions: a multicenter study. Endoscopy. 2018;50:1071-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Iglesias-Garcia J, Lariño-Noia J, Domínguez-Muñoz JE. New diagnostic techniques for the differential diagnosis of pancreatic mass: Elastography helps me 100. Endosc Ultrasound. 2017;6:S115-S118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Giovannini M, Hookey LC, Bories E, Pesenti C, Monges G, Delpero JR. Endoscopic ultrasound elastography: the first step towards virtual biopsy? Preliminary results in 49 patients. Endoscopy. 2006;38:344-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 212] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Iglesias-García J, Lariño-Noia J, Domínguez-Muñoz JE. New Imaging Techniques: Endoscopic Ultrasound-Guided Elastography. Gastrointest Endosc Clin N Am. 2017;27:551-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Dawwas MF, Taha H, Leeds JS, Nayar MK, Oppong KW. Diagnostic accuracy of quantitative EUS elastography for discriminating malignant from benign solid pancreatic masses: a prospective, single-center study. Gastrointest Endosc. 2012;76:953-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Lee TH, Cho YD, Cha SW, Cho JY, Jang JY, Jeong SW, Choi HJ, Moon JH. Endoscopic ultrasound elastography for the pancreas in Korea: a preliminary single center study. Clin Endosc. 2013;46:172-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Havre RF, Ødegaard S, Gilja OH, Nesje LB. Characterization of solid focal pancreatic lesions using endoscopic ultrasonography with real-time elastography. Scand J Gastroenterol. 2014;49:742-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Rustemovic N, Opacic D, Ostojic Z, Opacic M, Ledinsky I, Višijić A, Ravić KG, Iveković H, Markoš P. Comparison of elastography methods in patients with pancreatic masses. Endosc Ultrasound. 2014;3:S4. [PubMed] |
| 19. | Kongkam P, Lakananurak N, Navicharern P, Chantarojanasiri T, Aye K, Ridtitid W, Kritisin K, Angsuwatcharakon P, Aniwan S, Pittayanon R, Sampatanukul P, Treeprasertsuk S, Kullavanijaya P, Rerknimitr R. Combination of EUS-FNA and elastography (strain ratio) to exclude malignant solid pancreatic lesions: A prospective single-blinded study. J Gastroenterol Hepatol. 2015;30:1683-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Mayerle J, Beyer G, Simon P, Dickson EJ, Carter RC, Duthie F, Lerch MM, McKay CJ. Prospective cohort study comparing transient EUS guided elastography to EUS-FNA for the diagnosis of solid pancreatic mass lesions. Pancreatology. 2016;16:110-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Bhutani MS, Singh BS, Cazacu IM, Saftoiu A. Differentiating intrapancreatic accessory spleen from a pancreatic neuroendocrine tumor or metastasis by the "bridge sign". Endosc Ultrasound. 2019;8:281-282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |