Published online May 6, 2020. doi: 10.12998/wjcc.v8.i9.1713
Peer-review started: December 30, 2019
First decision: March 5, 2020
Revised: March 26, 2020
Accepted: April 15, 2020
Article in press: April 15, 2020
Published online: May 6, 2020
Processing time: 122 Days and 2.4 Hours
Liver cancer is one of the most common malignant tumors with a high incidence and mortality. Hepatitis-liver cirrhosis-liver cancer is known as the trilogy of liver cancer. At present, due to significant development of imaging interventions, they occupy an irreplaceable position in the field of liver cancer treatment, especially ultrasound-guided ablation. Because patients with liver cancer often present with liver cirrhosis, which leads to morphological deformation of the liver, it is difficult to perform a linear ablation of liver cancer in the areas near the phrenic top and within large blood vessels, among others. The present study reports on two cases of liver cancer that have been subjected to curvilinear ablation. After 1 mo, magnetic resonance imaging showed complete ablation, demonstrating that ultrasound-guided curved ablation is feasible and effective in the treatment of liver cancer.
Two patients were treated at the Liver Disease Department of the Xixi Hospital Affiliated to Zhejiang University of Chinese Medicine in 2019. Because the first liver cancer patient’s tumor was located close to the diaphragm, it was difficult to complete a straight needle ablation procedure in one session. In order to achieve accurate and minimally invasive treatment of this tumor, a curved needle ablation procedure was designed. The second patient presented with a hepatic cyst in front of the tumor. In order not to damage the hepatic cyst, a looper needle ablation technique was used. The procedure was successfully completed in both cases.
Curved ablation is a new technique that can be used to treat tumors situated in a variety of locations, providing new ideas for interventional techniques. Its operation difficulty is higher and further animal experiments are necessary to improve the operation procedure.
Core tip: Ultrasound-guided ablation of liver cancer plays an important role in the treatment of liver cancer. It has the advantages of less trauma and faster recovery, but is difficult to perform with a straight needle in certain portions of the liver tumor. This study reports on two cases of ultrasound-guided curved pathway ablation in order to expand the adaptive range of ultrasound-guided treatment of liver cancer and introduces this technique, thus providing ideas for future clinical treatments.
- Citation: Cao N, Cai HJ, Sun XX, Liu DL, Huang B. Application of curved ablation in liver cancer with special morphology or location: Report of two cases. World J Clin Cases 2020; 8(9): 1713-1720
- URL: https://www.wjgnet.com/2307-8960/full/v8/i9/1713.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i9.1713
Primary liver cancer was the sixth most common cancer in 2018, with incidence and mortality accounting for 6% and 9% of malignant tumors worldwide, respectively. It is the second leading cause of cancer-related death[1,2]. Therefore, early detection, diagnosis, and treatment are important means to reduce liver cancer mortality. In recent years, rapid development in the area of imaging intervention, especially ultrasound intervention, has resulted in more minimally invasive techniques for clinical treatment of liver cancer. However, it is difficult to perform ablation with a straight needle guided by ultrasound in some areas of liver cancer. This study reports on two cases of ultrasound-guided curve ablation and introduces detailed indications and points of consideration in the operation of this technique, thus providing new ideas for clinical treatment of liver cancer (Figure 1).
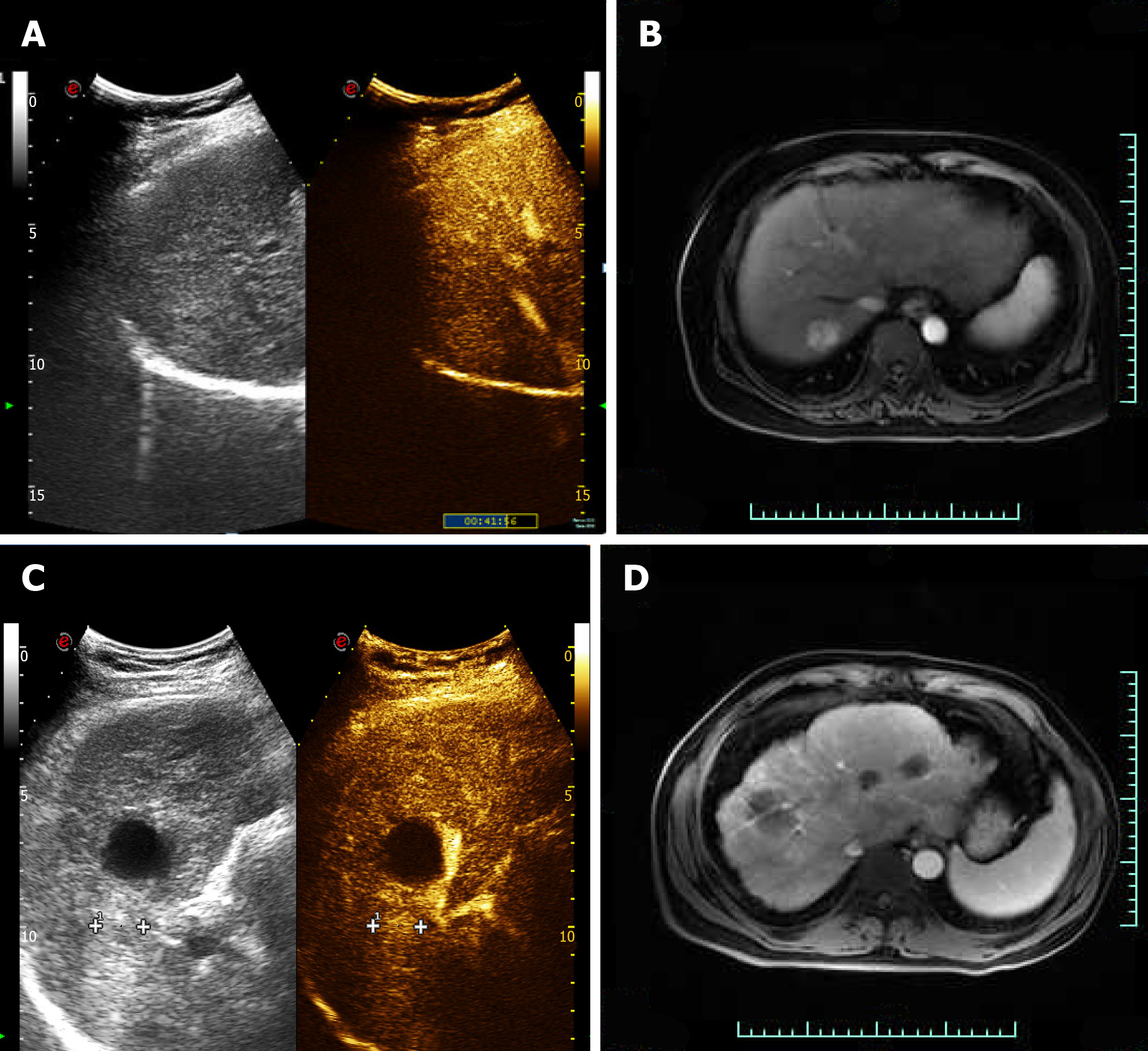
Case 1: A 67-year-old woman was diagnosed with hepatitis and liver cirrhosis for more than five years. In April 2019, enhanced magnetic resonance imaging (MRI) and contrast-enhanced ultrasound (CEUS) showed hypoechoic nodules in the right liver and the patient was diagnosed with liver cancer.
Case 2: A 54-year-old man with abnormal liver function and upper abdominal discomfort presented at our hospital for examination in May 2019.
Case 1: The patient’s history of past illness was unremarkable.
Case 2: The patient underwent a partial hepatectomy procedure for liver cancer five years ago.
Case 1: The family history was unremarkable.
Case 2: The family history was unremarkable.
Case 1: No special physical signs were found and the patient did not have any upper abdominal symptoms.
Case 2: There were no other obvious symptoms except slight tenderness in the upper abdomen.
Case 1: The serum alpha fetoprotein (AFP) level was 2.43 ng/mL.
Case 2: The serum AFP level was 2.08 ng/mL.
Case 1: CEUS of the lesion showed a fast-in and fast-out pattern, while enhanced MRI detected a round long T1 and long T2 signal in the upper posterior segment of the right liver lobe near the liver capsule. The lesion showed a high signal intensity in the diffusion phase, was significantly enhanced in the arterial phase, and was significantly decreased in the portal vein phase (Figure 2).
Case 2: Enhanced MRI and CEUS showed intrahepatic lesions and liver cancer was initially considered (Figure 2).
Pathological results for the two patients revealed a hepatocellular carcinoma.
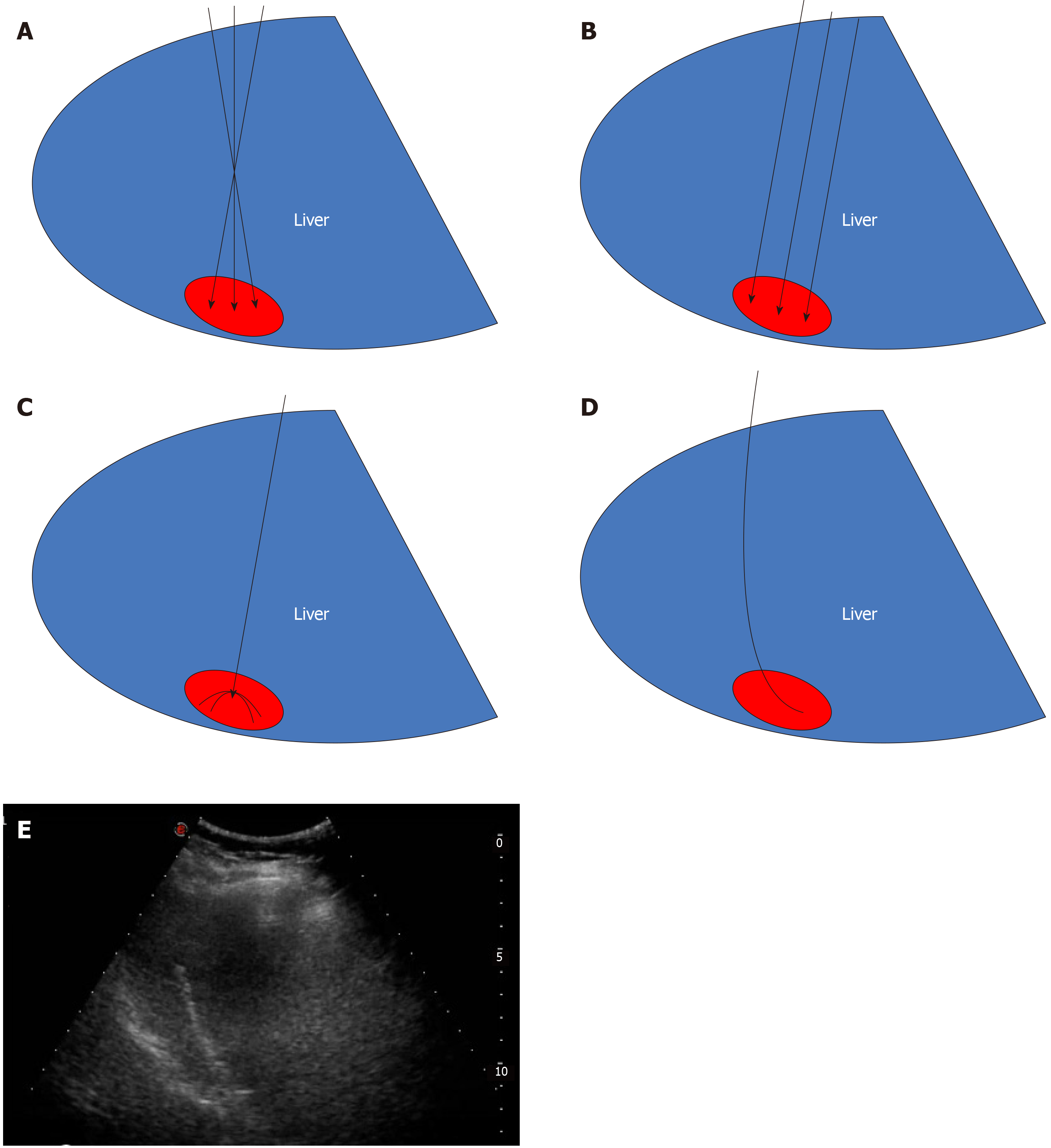
Case 1: Microwave ablation guided by ultrasound was performed with patient’s informed consent. Since the lesion was near the top of the diaphragm, five surgical schemes were prepared. Scheme 1: Multiple ablations with a single needle. This technique may result in bleeding and needle track implantation, may have insufficient safety boundaries, is time-consuming, and has high requirements for the operator. Scheme 2: Concurrent ablation with multiple needles. The ablation range is sufficient in this technique, but its cost is high and liver tissue injury is too much. Scheme 3: Umbrella needle ablation. Because the focus is in close proximity to the diaphragm, lungs, and large blood vessels, tissue can be easily damaged. Scheme 4: Artificial hydrothorax. The needle path might disappear after the artificial hydrothorax, increasing the difficulty of the puncture. Scheme 5: Ablation with a curved needle (designed by the author and commissioned by the manufacturer, Figure 3).
Under general anesthesia, the patient was placed on the left side. The tissue was sterilized and the condition of the lesion and surrounding tissue was evaluated again. A needle was inserted according to the path set prior to the operation and its direction was regulated in real-time under the guidance of ultrasound (Figure 3). The anterior segment of the arc needle was kept parallel to the long axis of the lesion. When the needle tip reached the anterior segment of the lesion, ablation was initiated with the power set at 60 W. After the lesion was completely vaporized and covered, the cold circulation was closed and the needle was pulled out. The ablation range was evaluated by CEUS after the ablation procedure was completed.
Case 2: Since the lesion was located behind the liver cyst, two surgical schemes were prepared. Scheme 1: The cyst in front of the lesion is extracted and the lesion ablated. However, this method has previously hurt the patient twice. Scheme 2: Curved needle ablation, bypassing the anterior cyst and surrounding blood vessels for ablation (Figure 4).
This patient underwent the same procedure as the first patient, following a pre-operatively designed arc path and avoiding surrounding vessels and anterior cysts (Figure 4).
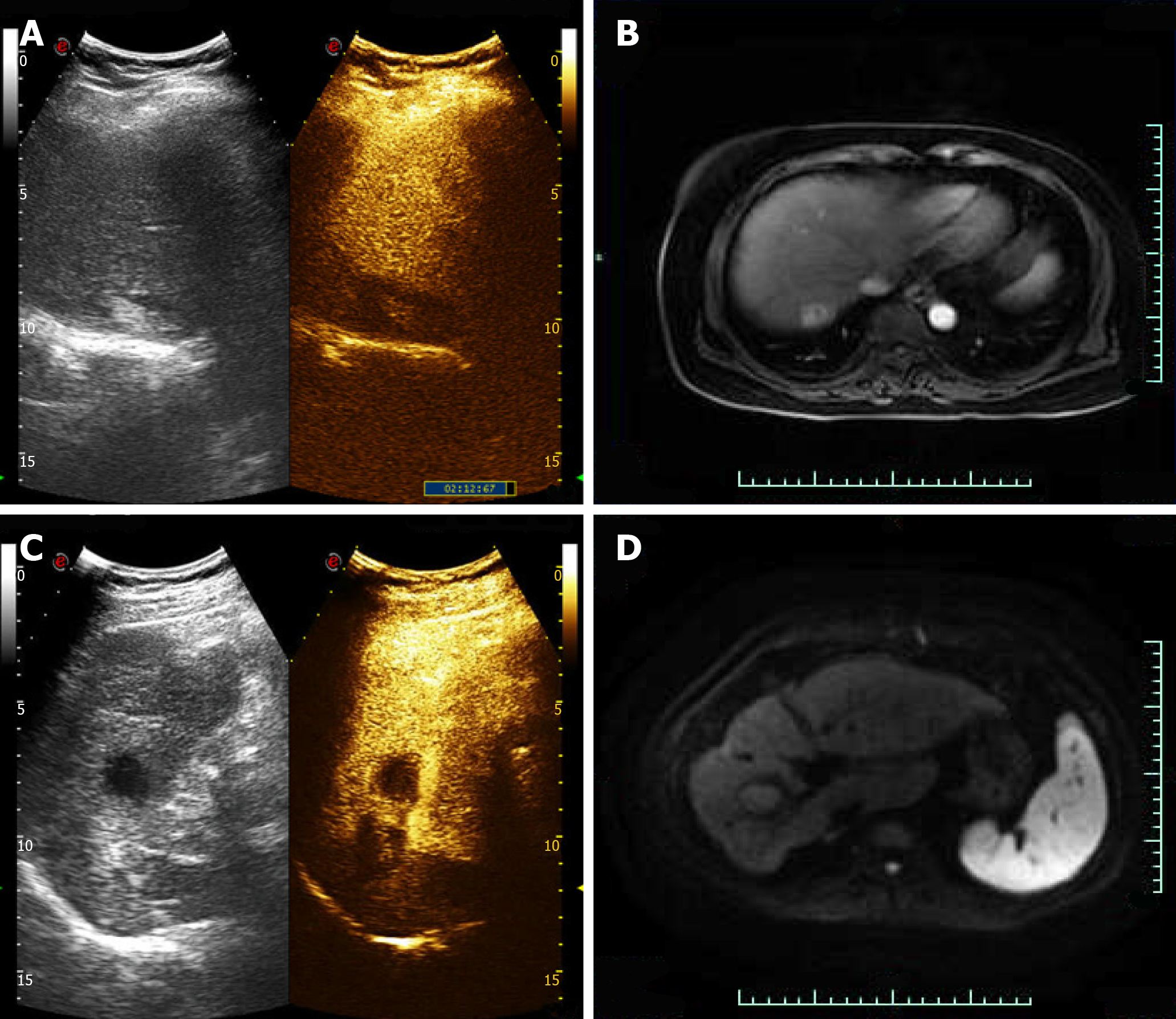
One month after the operation, enhanced MRI results for ablation foci and surrounding areas exhibited no enhancement.
Case 1: One month after the operation, CEUS and enhanced MRI results for ablation foci and surrounding areas exhibited no enhancement (Figure 5).
Case 2: One month after the operation, CEUS and enhanced MRI results for ablation foci and surrounding areas exhibited no enhancement (Figure 5).
The development of liver cancer is slow, usually accompanied by liver cirrhosis that can cause abnormalities within the liver. A long time is needed to become a pathologically advanced liver cancer such as HCC. However, sometimes HCC growth is very fast. At present, the main methods for the treatment of liver cancer are surgery, local ablation, transarterial chemoembolism, radiotherapy, drug therapy, and immunotherapy. Liver cancer patients generally have partial liver function damage, reduction of coagulation function, and surgery intolerance. However, because of the complex liver cancer blood supply, it is difficult for transcatheter arterial chemoembolization to cause complete tumor tissue necrosis. The statistics for II resection specimens show that the complete death rate is only 22%-72%[3]. Chemotherapy, radiotherapy, and immunotherapy have side effects and their cost for patients needing long-term medication is too high[4-6]. Microwave ablation causes less trauma and comes at a lower cost[7,8]. However, it is difficult to perform ablation of the tumors near the gallbladder, diaphragm, and other organs. In order to solve this problem, the curved ablation technique was designed.
The curved ablation method has high requirements for the operator who should have several years of experience in the ablation of liver cancer.
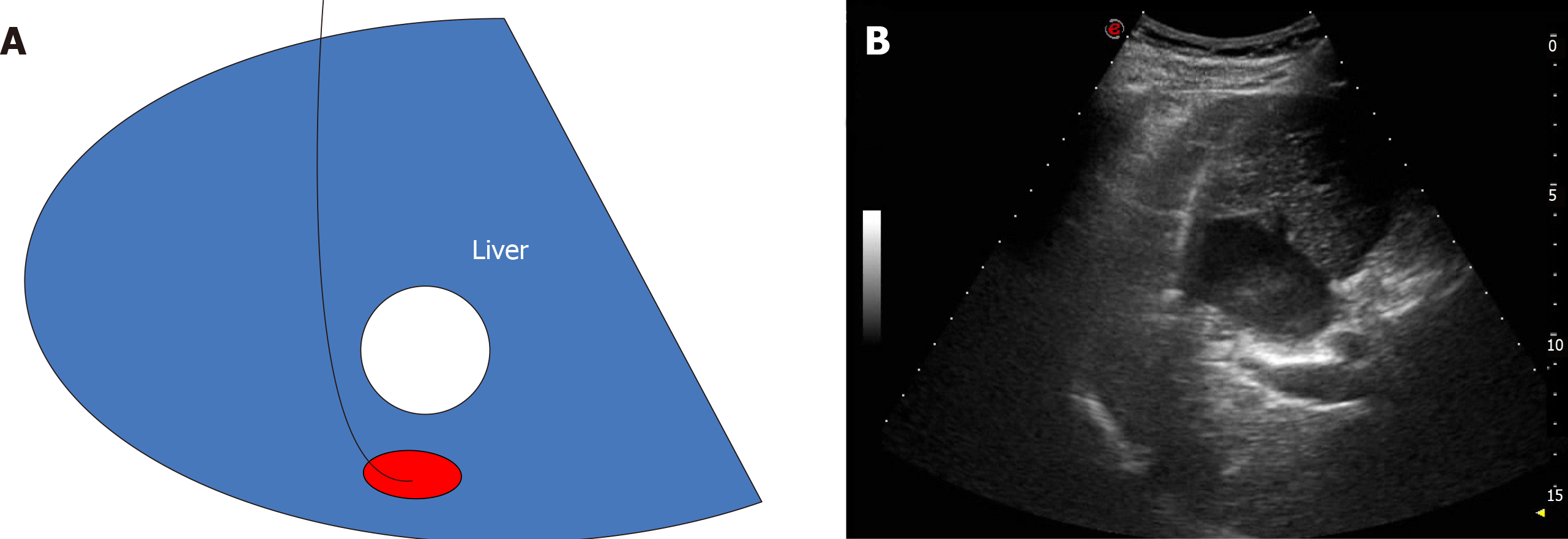
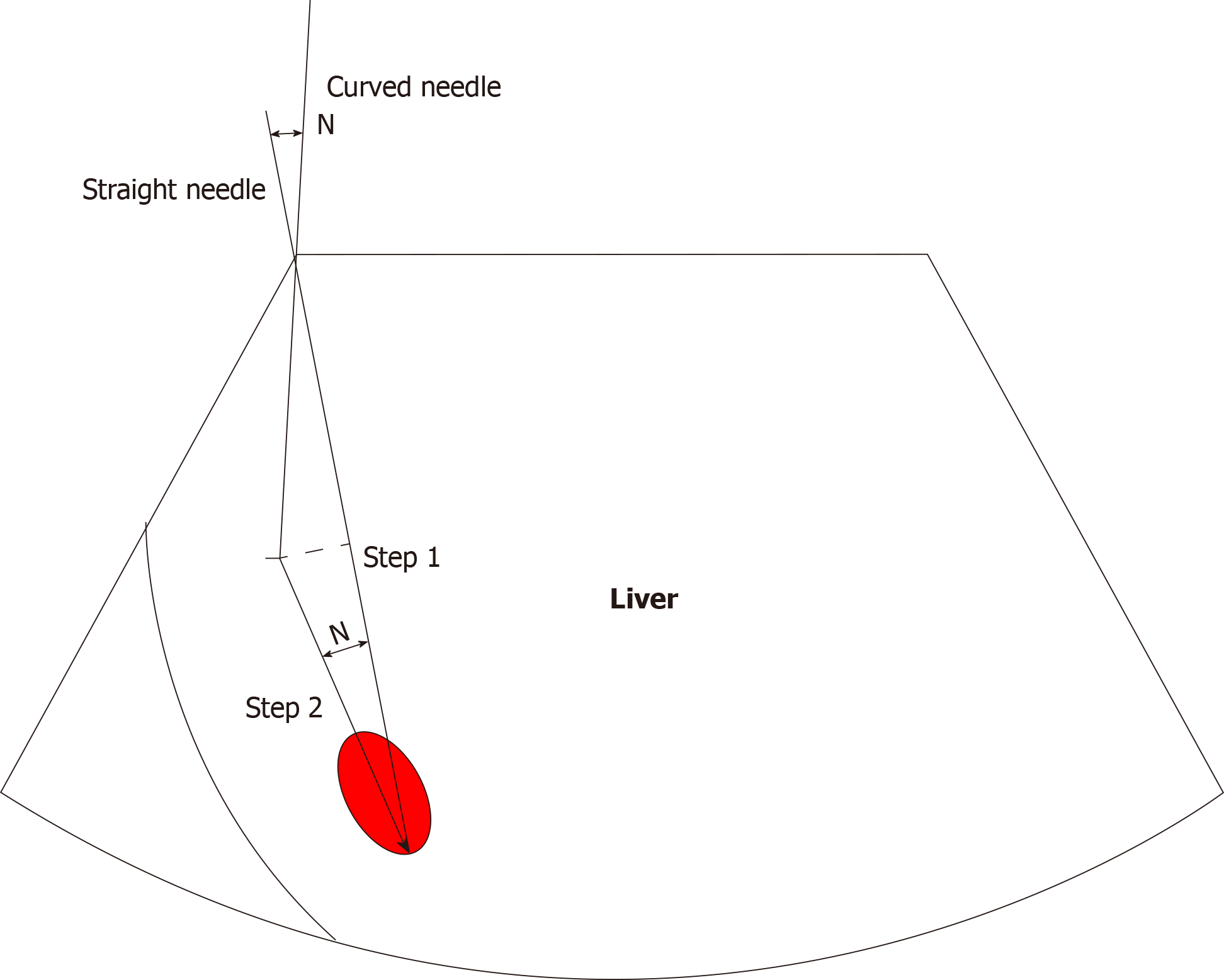
Two cases of curved ablation in this study are summarized and the following conclusions are made: (1) Compared to straight needle ablation, curve ablation is more demanding for operators; (2) General patient state has to be assessed prior to operation; (3) Pre-operation needle approach design involves the following steps: Mark the screen according to the straight needle route; draw angle N between the long axis of the tumor and the path of the straight needle, then measure angle N. The incident angle is offset by N degrees in the direction of the original needle; under the guidance of real-time ultrasound, adjust the direction of the needle, so that the needle advances within the design route until the curved end of the needle is parallel to the long axis of the lesion (Figure 6); (4) The surgeon should work closely with the anesthesiologist to control the patient's breathing as needed and, if necessary, carry out one-lung respiration; (5) If necessary, virtual fusion navigation imaging technology has to be used to evaluate the localization navigation and postoperative ablation range; and (6) The ablation range has to be evaluated by CEUS immediately after operation and by enhanced MRI re-examination 1 mo after operation.
Curved ablation has been demonstrated to be feasible, safe, economical, and effective in these two cases, expanding the adaptive range of ultrasound-guided percutaneous ablation of liver cancer.
The curved ablation technique has special advantages for the ablation of liver cancer at specific sites. Its effectiveness needs to be confirmed by investigating more cases.
Manuscript source: Unsolicited Manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Capasso R, Numata K S-Editor: Wang YQ L-Editor: Wang TQ E-Editor: Wu YXJ
| 1. | El-Serag HB, Mason AC. Risk factors for the rising rates of primary liver cancer in the United States. Arch Intern Med. 2000;160:3227-3230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 279] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55853] [Article Influence: 7979.0] [Reference Citation Analysis (132)] |
| 3. | Zhang B, Zhang B, Zhang Z, Huang Z, Chen Y, Chen M, Bie P, Peng B, Wu L, Wang Z, Li B, Fan J, Qin L, Chen P, Liu J, Tang Z, Niu J, Yin X, Li D, He S, Jiang B, Mao Y, Zhou W, Chen X. 42,573 cases of hepatectomy in China: a multicenter retrospective investigation. Sci China Life Sci. 2018;61:660-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RK, Dinniwell RE, Kassam Z, Ringash J, Cummings B, Sykes J, Sherman M, Knox JJ, Dawson LA. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 593] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 5. | Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 2718] [Article Influence: 339.8] [Reference Citation Analysis (0)] |
| 6. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3315] [Article Influence: 414.4] [Reference Citation Analysis (1)] |
| 7. | Puijk RS, Ruarus AH, Scheffer HJ, Vroomen LGPH, van Tilborg AAJM, de Vries JJJ, Berger FH, van den Tol PMP, Meijerink MR. Percutaneous Liver Tumour Ablation: Image Guidance, Endpoint Assessment, and Quality Control. Can Assoc Radiol J. 2018;69:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Vogl TJ, Nour-Eldin NA, Hammerstingl RM, Panahi B, Naguib NNN. Microwave Ablation (MWA): Basics, Technique and Results in Primary and Metastatic Liver Neoplasms - Review Article. Rofo. 2017;189:1055-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |