Published online May 6, 2020. doi: 10.12998/wjcc.v8.i9.1693
Peer-review started: December 22, 2019
First decision: April 1, 2020
Revised: April 8, 2020
Accepted: April 15, 2020
Article in press: April 15, 2020
Published online: May 6, 2020
Processing time: 127 Days and 1.3 Hours
Nasopharyngeal carcinoma (NPC) is a commonly encountered type of tumor. Fluorouracil (FU) is an effective treatment providing satisfactory oncologic outcomes in nasopharyngeal carcinoma patients. We describe a unique case of colonic perforation in an NPC patient treated with FU. Thus far, only two cases of intestinal perforation associated with FU treatment have been reported. We hope that the analysis of the relationship between the adverse effects of FU and physiological factors will help to reduce the incidence of colonic perforation in patients with nasopharyngeal carcinoma treated with FU.
A 67-year-old female patient suffered from NPC stage pT3N2M0. She had a history of three surgical procedures: Partial enterectomy, partial sigmoidectomy, and sigmoidostomy. After the administration of 2.75 g FU, a bloody stool appeared and the patient developed abdominal pain. Subsequent examination indicated colitis and intestinal perforation.
FU is a commonly used drug in NPC chemotherapy. The most common adverse effect of FU is gastrointestinal reaction, and the colonic perforation found here is thought to be caused by gastrointestinal mucosal injury consequential to the FU treatment. When selecting chemotherapy drugs, their side effects and the physical condition of patients should be considered, particularly in patients with a history of gastrointestinal surgery.
Core tip: Intestinal perforation associated with fluorouracil treatment is very rare. The possible reason of the perforation in the case presented here is that the patient has undergone intestinal surgeries, her physical condition was poor, and the use of fluorouracil has damaged the intestinal mucosa. In general, patients with intestinal perforation require an immediate surgical treatment. However, the use of radiotherapy and chemotherapy in patients after intestinal surgery is challenging.
- Citation: Lu WJ, Li G, Gao L. Colonic perforation in a nasopharyngeal carcinoma patient treated with fluorouracil: A case report. World J Clin Cases 2020; 8(9): 1693-1697
- URL: https://www.wjgnet.com/2307-8960/full/v8/i9/1693.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i9.1693
Fluorouracil (FU) is commonly used in nasopharyngeal carcinoma chemotherapy. A recent article by Fata et al[1] provides a reminder that FU does cause, directly or indirectly, gastrointestinal mucosal damage, and the awareness among oncologists of this problem and resulting complications is necessary. The current report presents a case of colonic perforation induced by FU in a patient with a history of three surgical procedures: Partial enterectomy, partial sigmoidectomy, and sigmoidostomy. Colonic perforation is a rare complication of FU therapy. So far, only two cases of intestinal perforation associated with FU treatment have been reported[2,3], and the uniqueness of the medical history of the patient presented here further highlights the clinical relevance of the current report.
A 67-year-old female patient felt left mandibular lymph node enlargement, accompanied by headache and tinnitus.
The left neck mass was found in May 2019, and the mass gradually increased, but she did not show symptoms of nasal congestion or nosebleed.
Seven years ago, she underwent partial enterectomy, partial sigmoidectomy, and sigmoidostomy.
On admission, the bilateral cervical lymph nodes of the patient were enlarged, and the swollen lymph node under the left jaw was the largest, with a size of 3.2 cm × 2.6 cm. She had no exophthalmos and no difficulty in deglutition or opening the mouth. No tenderness or rebound tenderness was found on abdominal palpation.
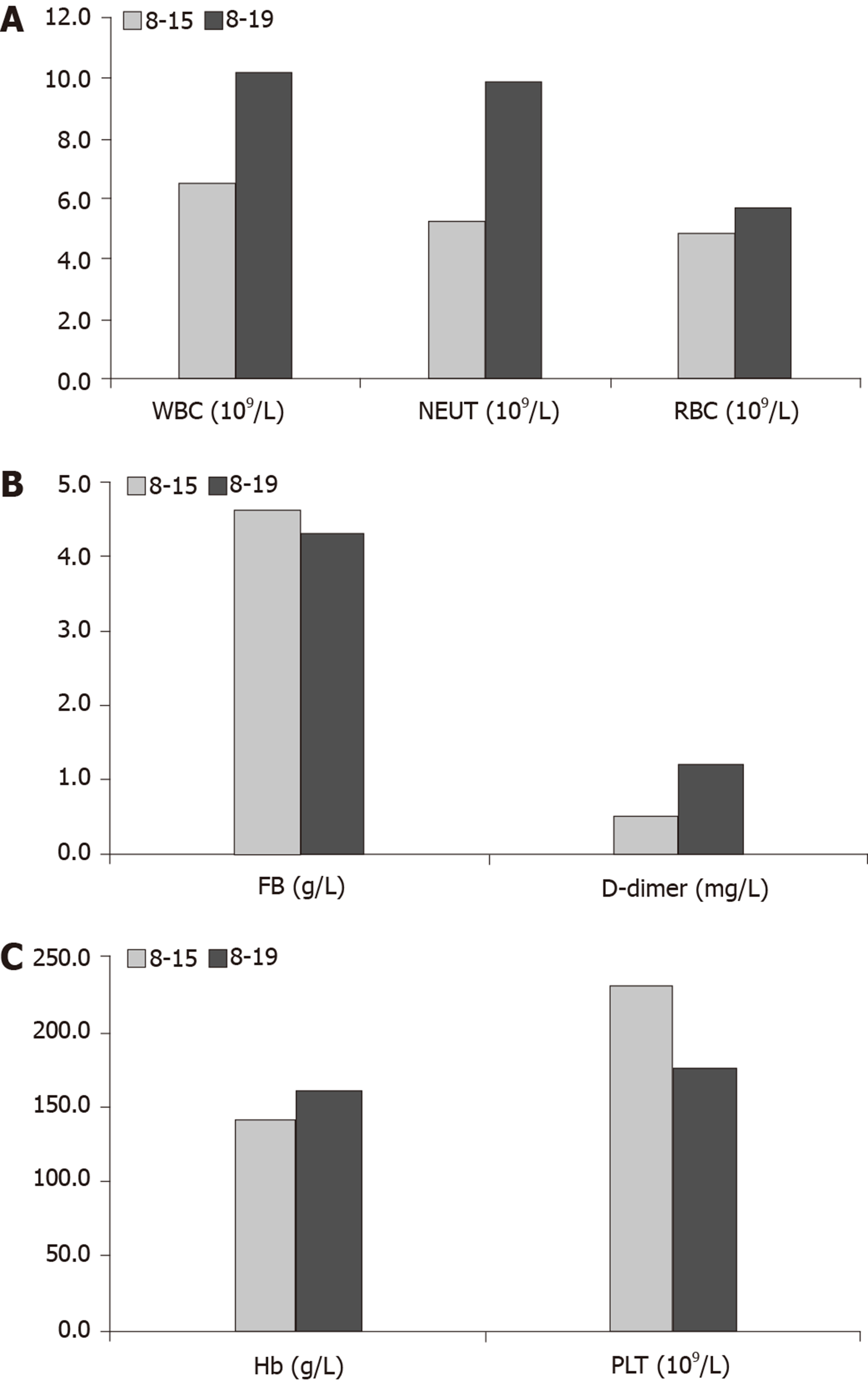
Blood analysis (Figure 1) and urine analysis were normal on August 15, 2019. Chest X-ray, electrocardiogram, and arterial blood gas were also normal.
In August 2019, nasopharyngoscopy identified an undifferentiated non-keratinizing carcinoma. Additionally, magnetic resonance imaging of the nasopharynx, neck, and skull base documented bilateral thickening of the parapharyngeal wall and posterior parapharyngeal wall, and multiple lymph node metastases present bilaterally in the parapharyngeal space and the neck. These findings were consistent with nasopharyngeal carcinoma.
Single-photon emission computed tomography/computed tomography revealed thickened mucous membranes in the bilateral and posterior parietal walls of the nasopharynx, with soft tissue mass shadows.
Based on the combination of imaging, clinical, and pathological results, the nasopharyngeal malignant tumor was diagnosed as pT3N2M0.
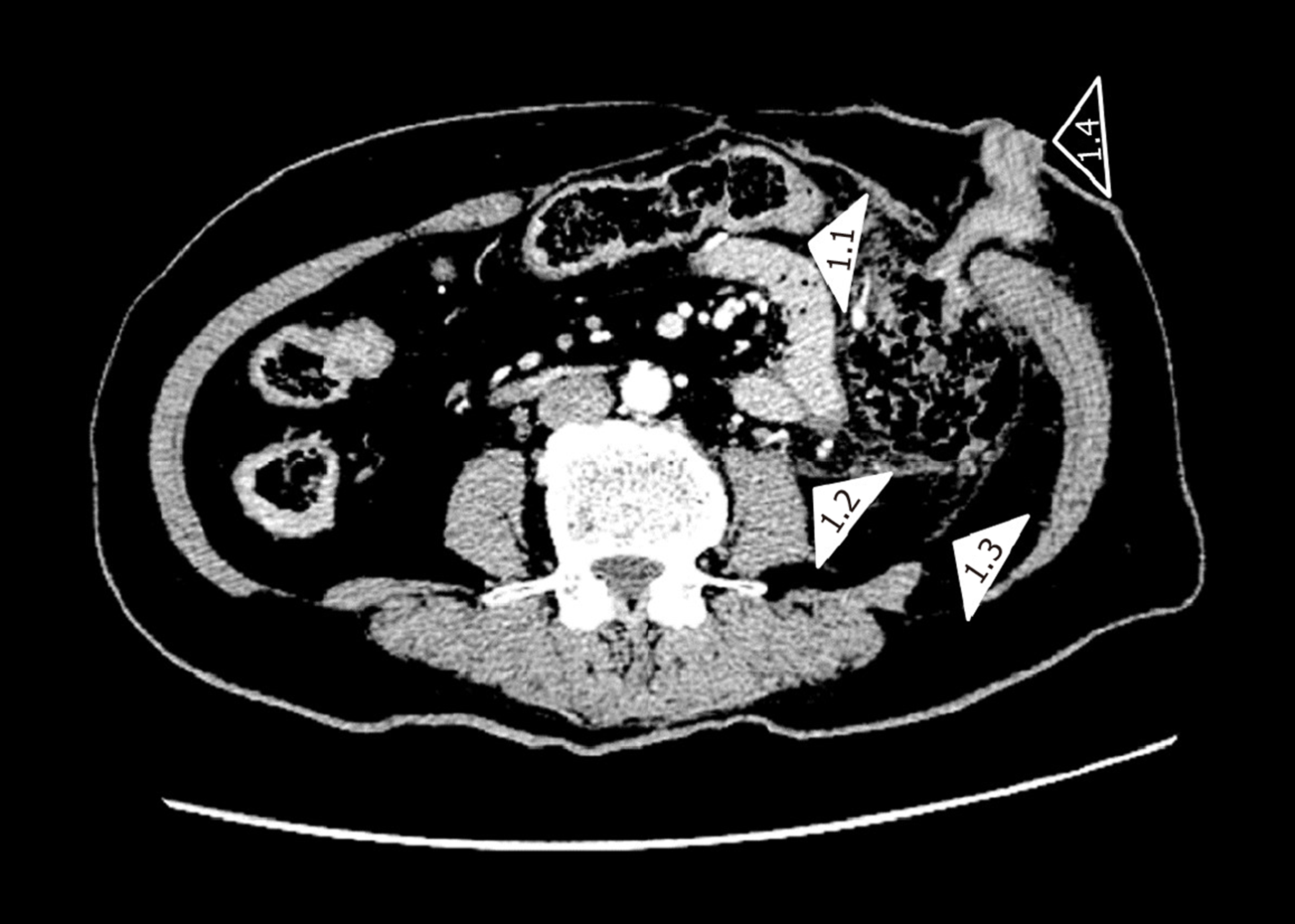
The original treatment plan was to administer the TPF chemotherapy regimen (paclitaxel 180 mg + cisplatin 30 mg + FU 5 g). The first intravenous injection of 0.5 g FU was performed on August 16, 2019, and the remaining 4.5 g was divided into two intravenous doses. On August 19, 2019, after injecting the first of the remaining 2.25 g doses of FU, the patient developed abdominal pain and bloody stool. Of note, cisplatin and paclitaxel were planned to be given to the patient on that day, but they were not administered. Blood tests were performed, which showed an increase in D-dimer, white blood cells, neutrophils, red blood cells, and hemoglobin and a decrease in fibrinogen and platelets compared with the results obtained on August 15 (Figure 1). A computed tomographic scan (Figure 2) of the abdomen showed free intraperitoneal air. Colonoscopy revealed mucosal ulceration and erosion in the sigmoid colon and descending colon. The patient was immediately taken to the operating room for an emergent laparotomy. During the procedure, a perforation was found in the descending colon, and a large amount of exudate flowed out of the perforation. A partial colectomy was performed. Colitis was diagnosed based on pathologic examination of surgical specimens.
After the surgery, the patient was treated with antibiotics. She had diffuse abdominal tenderness to deep palpation. On presentation, her blood pressure was 122/83, pulse 85/min, temperature 37.1 °C, respiratory rate 18/min, and oxygen saturation 97%. The patient's condition gradually stabilized over a period of 24 d, but due to the intestinal damage, the induction chemotherapy had to be postponed. The patient and her family have expressed their understanding regarding the reason for postponing the original treatment plan.
Induction cisplatin-FU chemotherapy prior to definitive radiation improves freedom from distant metastases, disease-free survival, and overall survival in patients with locoregional stage IV nasopharyngeal carcinoma without increasing treatment-related morbidity[4]. In recent years, docetaxel, cisplatin, and FU-based induction chemotherapy has been widely applied in the treatment of locoregionally advanced nasopharyngeal carcinoma[5]. FU can effectively reduce the recurrence of tumors and prolong survival in patients with nasopharyngeal carcinoma, regardless of whether the surgery was performed.
However, the toxic and side effects of FU require careful consideration. The mechanism of adverse effects of FU involves a deficiency of the catabolic pathway, in which the drug competes with uracil, the naturally occurring pyrimidine, as an enzyme substrate. Dihydropyrimidine dehydrogenase (DPD, EC 1.3.1.2) is the initial and rate-controlling enzyme of the catabolism of endogenous pyrimidine and fluoropyrimidine nucleotides. DPD enzyme is expressed in most tissues, including the tumors, and is highly active in the liver and peripheral lymphocytes. The activity of DPD is highly variable among individuals. Low levels of DPD activity have been associated with an increased risk of toxicity during the FU treatment[6]. The most common adverse reaction triggered by FU is gastrointestinal tract response. The patient presented with colonic perforation, acute diffuse peritonitis, and acute infection, and these conditions were, to a large extent, associated with the use of FU. Surgical treatment of colonic perforation resulting from the administration of FU is crucial, and the prognosis depends essentially on the time to diagnosis. It is very important to increase the knowledge about intestinal perforation in order to improve the diagnostic accuracy[7].
Perforations of the colon are thought to be caused by damage to the gastrointestinal mucosa consequential to the treatment with FU. Gastrointestinal perforation is a known adverse event of bevacizumab[8-10], but it occurs only rarely during FU therapy[2,3]. In particular, descending colon perforation is less frequent than gastric and duodenal perforation. Because of the scarcity of reports of descending colon perforation in patients treated with FU, its frequency and mechanism are not completely understood. It is possible that the patient had an enterostomy, and FU may be secondary to the inhibition of the methylation reaction catalyzed by thymidylate synthetase (EC 2.1. 1.45), which transfers a methyl group from N, N’-methylene-tetrahydrofolic acid to deoxyuridylic acid, contributing to the breakup of the gastrointestinal mucosa, which leads to intestinal perforation. Accordingly, we have a reason to believe that the colonic perforation presented in this case report was associated with the FU treatment.
In conclusion, although induction chemotherapy can improve the overall survival, side effects of drugs and the patient's physical condition should be all considered in selecting the optimal treatment modality, particularly in patients with a history of gastrointestinal surgery. The possibility of performing colonoscopy before chemotherapy should be taken into account in these patients.
We thank all the members of our department for their help in this study.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cihan YB, Nakashima T, Noussios G, Shimada S S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Liu JH
| 1. | Fata F, Ron IG, Kemeny N, O'Reilly E, Klimstra D, Kelsen DP. 5-fluorouracil-induced small bowel toxicity in patients with colorectal carcinoma. Cancer. 1999;86:1129-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Liaw CC, Huang JS, Wang HM, Wang CH. Spontaneous gastroduodenal perforation in patients with cancer receiving chemotherapy and steroids. Report of four cases combining 5-fluorouracil infusion and cisplatin with antiemetics dexamethasone. Cancer. 1993;72:1382-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Oguma J, Ozawa S, Kazuno A, Yamasaki Y, Ninomiya Y. Gastrointestinal perforation during neoadjuvant chemotherapy with cisplatin and 5-fluorouracil in patients with esophageal cancer: a report of two cases. Esophagus. 2016;13:374-377. [DOI] [Full Text] |
| 4. | Geara FB, Glisson BS, Sanguineti G, Tucker SL, Garden AS, Ang KK, Lippman SM, Clayman GL, Goepfert H, Peters LJ, Hong WK. Induction chemotherapy followed by radiotherapy versus radiotherapy alone in patients with advanced nasopharyngeal carcinoma: results of a matched cohort study. Cancer. 1997;79:1279-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Zhou R, Zhu J, Chen X, Liu Y, Wang Y, Zhang T. The efficacy and safety of docetaxel, cisplatin and fluorouracil (TPF)-based induction chemotherapy followed by concurrent chemoradiotherapy for locoregionally advanced nasopharyngeal carcinoma: a meta-analysis. Clin Transl Oncol. 2020;22:429-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Wettergren Y, Carlsson G, Odin E, Gustavsson B. Pretherapeutic uracil and dihydrouracil levels of colorectal cancer patients are associated with sex and toxic side effects during adjuvant 5-fluorouracil-based chemotherapy. Cancer. 2012;118:2935-2943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Yang B, Ni HK. Diagnosis and treatment of spontaneous colonic perforation: analysis of 10 cases. World J Gastroenterol. 2008;14:4569-4572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Badgwell BD, Camp ER, Feig B, Wolff RA, Eng C, Ellis LM, Cormier JN. Management of bevacizumab-associated bowel perforation: a case series and review of the literature. Ann Oncol. 2008;19:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Sfakianos GP, Numnum TM, Halverson CB, Panjeti D, Kendrick JE 4th, Straughn JM Jr. The risk of gastrointestinal perforation and/or fistula in patients with recurrent ovarian cancer receiving bevacizumab compared to standard chemotherapy: a retrospective cohort study. Gynecol Oncol. 2009;114:424-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Puthillath A, Mashtare T, Wilding G, Khushalani N, Steinbrenner L, Ross ME, Romano K, Wisniewski M, Fakih MG. A phase II study of first-line biweekly capecitabine and bevacizumab in elderly patients with metastatic colorectal cancer. Crit Rev Oncol Hematol. 2009;71:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |