Published online May 6, 2020. doi: 10.12998/wjcc.v8.i9.1685
Peer-review started: January 16, 2020
First decision: March 27, 2020
Revised: April 8, 2020
Accepted: April 21, 2020
Article in press: April 21, 2020
Published online: May 6, 2020
Processing time: 105 Days and 3.5 Hours
The aberrant expression of the anaplastic lymphoma kinase (ALK) gene in ALK-positive (ALK+) anaplastic large cell lymphoma (ALCL) is usually due to t(2;5)/NPM-ALK. However, rarely, aberrant ALK expression can also result from a rearrangement of the ALK gene with various partner genes. Central nervous system (CNS) metastasis is very rare in ALK+ALCL. Patients with CNS involvement show an inferior prognosis.
Here, we present the case of an 8-year-old girl diagnosed with ALK+ALCL. She presented with fever, skin nodules, leg swelling, and abdominal pain over the preceding 6 mo. She had extensive involvement and showed an extraordinary rare translocation, t(2;17)/CLTC-ALK, as demonstrated by RNA-seq. She underwent chemotherapy as per ALCL99, followed by vinblastine (VBL) maintenance treatment, and achieved complete remission. However, she developed CNS relapse during VBL monotherapy. The patient achieved a durable second remission with high-dose chemotherapy (including methotrexate 8 g/m2) and continuous treatment with alectinib and VBL.
Alectinib showed significant and durable CNS effects in this patient. However, more cases are needed to prove the efficacy and safety of alectinib for pediatric ALK+ALCL patients.
Core tip: Both CLTC-anaplastic lymphoma kinase translocation and central nervous system (CNS) metastasis are very rare in anaplastic large cell lymphoma. This paper reports a rare pediatric case of anaplastic large cell lymphoma with the CLTC-anaplastic lymphoma kinase fusion gene. The patient had an aggressive clinical course and underwent CNS relapse during treatment. The current patient achieved sustained complete remission with chemotherapy and alectinib. Alectinib conferred significant and durable CNS effects in this patient.
- Citation: Yang J, Li J, Gu WY, Jin L, Duan YL, Huang S, Zhang M, Wang XS, Liu Y, Zhou CJ, Gao C, Zheng HY, Zhang YH. Central nervous system relapse in a pediatric anaplastic large cell lymphoma patient with CLTC/ALK translocation treated with alectinib: A case report. World J Clin Cases 2020; 8(9): 1685-1692
- URL: https://www.wjgnet.com/2307-8960/full/v8/i9/1685.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i9.1685
Anaplastic lymphoma kinase (ALK) expression is absent from all normal human postnatal tissues except rare cells in the brain. Expression of the ALK gene in ALK-positive (ALK+) anaplastic large cell lymphoma (ALCL) is usually due to the (2;5)(p23;q35) chromosome translocation, which causes the fusion of the ALK and NPM1 genes, providing a promoter involved in the activation of ALK kinase, which plays a direct causative role in ALCL[1,2].
The aberrant expression of ALK can also result from a rearrangement of the ALK gene with various partner genes[2-4]. The ALK fusion partner determines the intracellular localization of the fusion protein. Immunohistochemistry with specific anti-ALK monoclonal antibodies indicates that NPM1-ALK fusion proteins are characterized by nuclear and cytoplasmic ALK staining, whereas the variant ALK fusion proteins are usually cytoplasmic and rarely show membranous ALK staining. Thus, immunohistochemistry can be used for screening the classical (2;5) translocation instead of molecular tests[1].
CLTC-ALK is a known recurrent fusion gene in ALK-positive diffuse large B-cell lymphoma but very rare in ALCL[2,5-7]. Here, we report a case of ALCL with CLTC-ALK gene fusion arising from t(2;17)(p23;q23). The patient developed central nervous system (CNS) metastasis and achieved complete remission (CR) through chemotherapy and treatment with alectinib, which is a second-generation ALK tyrosine kinase inhibitor (TKI). This study was approved by the Beijing Children’s Hospital Institutional Ethics Committee.
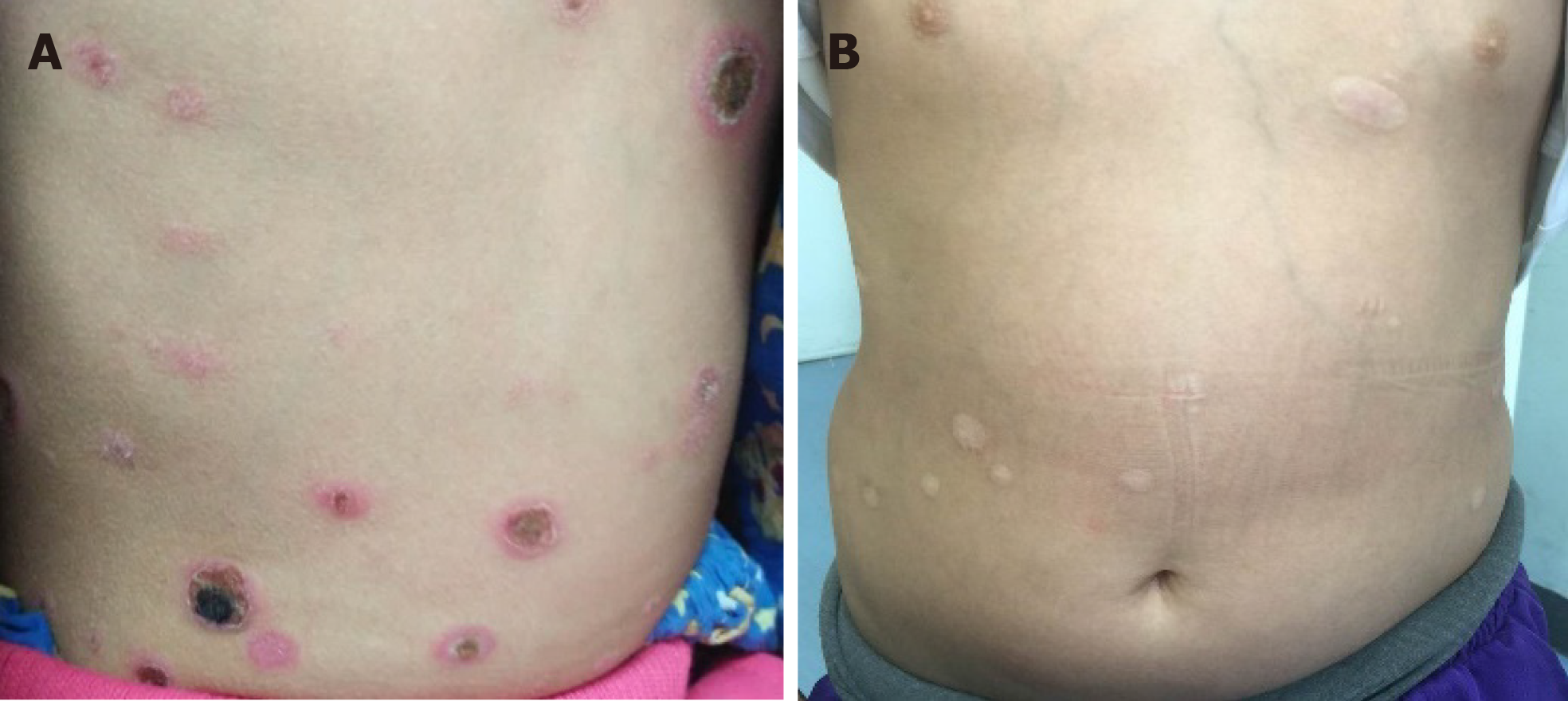
An 8-year-old girl presented with fever, skin nodules with necrosis and ulceration, leg swelling, and abdominal pain over the preceding 6 mo (Figure 1).
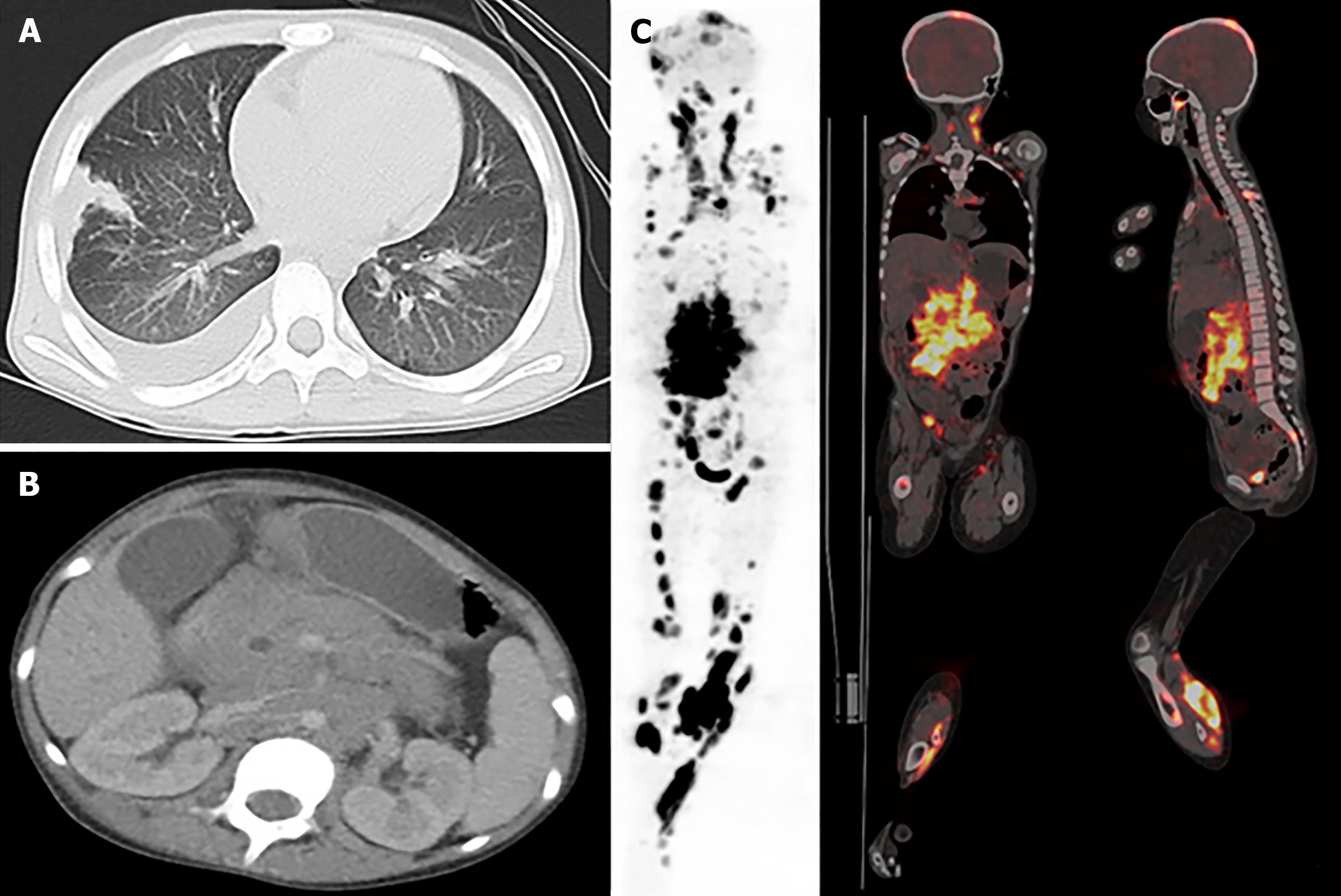
She was admitted to our institution, and magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomography (PET) scans revealed that she had extensive involvement, including the skin, soft tissues, lungs, bones (skull, vertebrae, ribs, ilium, limb bones, and jawbone), bone marrow, lymph nodes (cervical, mediastinal, axillary, pelvic, and inguinal lymph nodes), ovaries, and pancreas, as well as a massive mesenteric tumor (Figure 2).
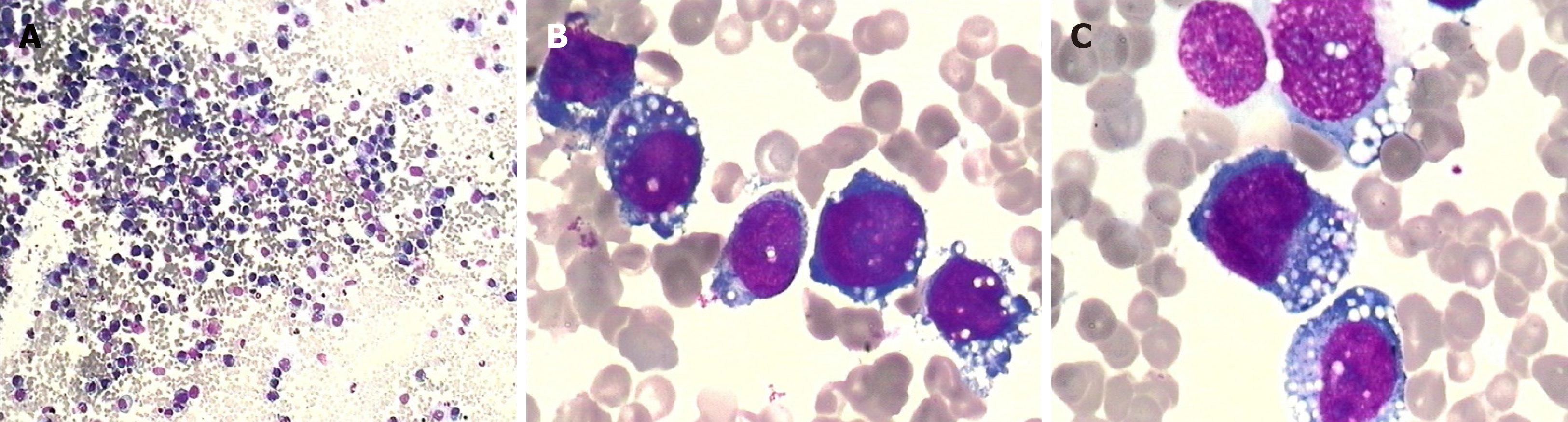
Complete blood count showed mild anemia (hemoglobin 9.9 g/dL), while the white blood cell and platelet counts were normal. Lactose dehydrogenase was slightly elevated (720 IU/L). A bone marrow aspiration smear revealed 86% of atypical lymphocytes, which differed in size and shape. They had eccentric nuclei, prominent nucleoli, and abundant cytoplasm containing prominent vacuoles (Figure 3).
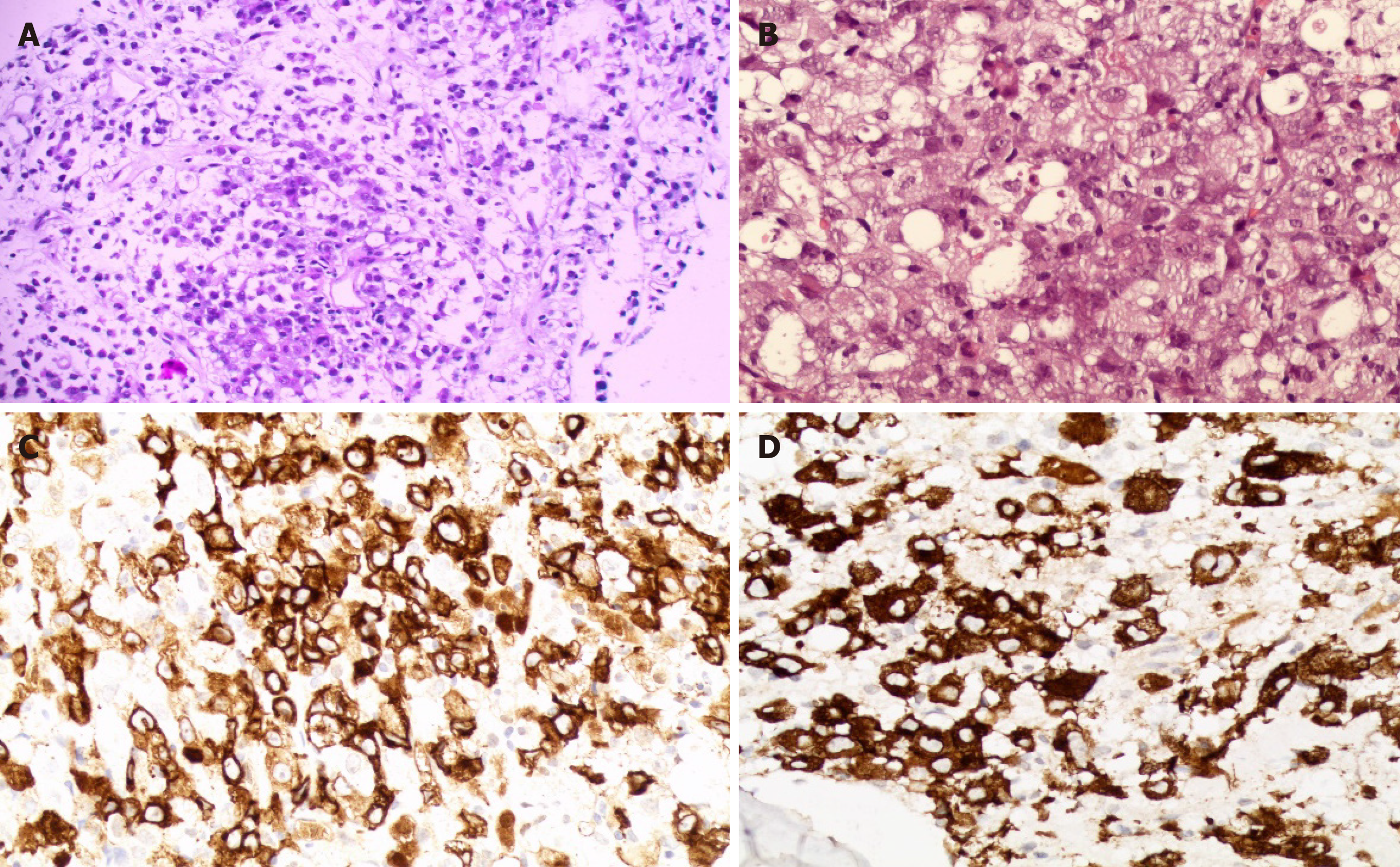
Histopathological examination of the biopsied specimens from inguinal lymph nodes and bone marrow showed a diffuse infiltrate of irregularly shaped tumor cells. Immunophenotypically, the tumor cells were negative for CD3, CD5, CD8, and CD20, and positive for CD30, CD4, CD7, CD2, EMA, TIA-1, Gram-B, and Ki67 (80%+); ALK was strongly positive. Therefore, a diagnosis of ALK+ALCL was made. Rarely, the ALK protein was distributed in a restricted cytoplasmic staining pattern, indicating that the aberrant ALK expression was due to a partner gene other than NPM1 (Figure 4).
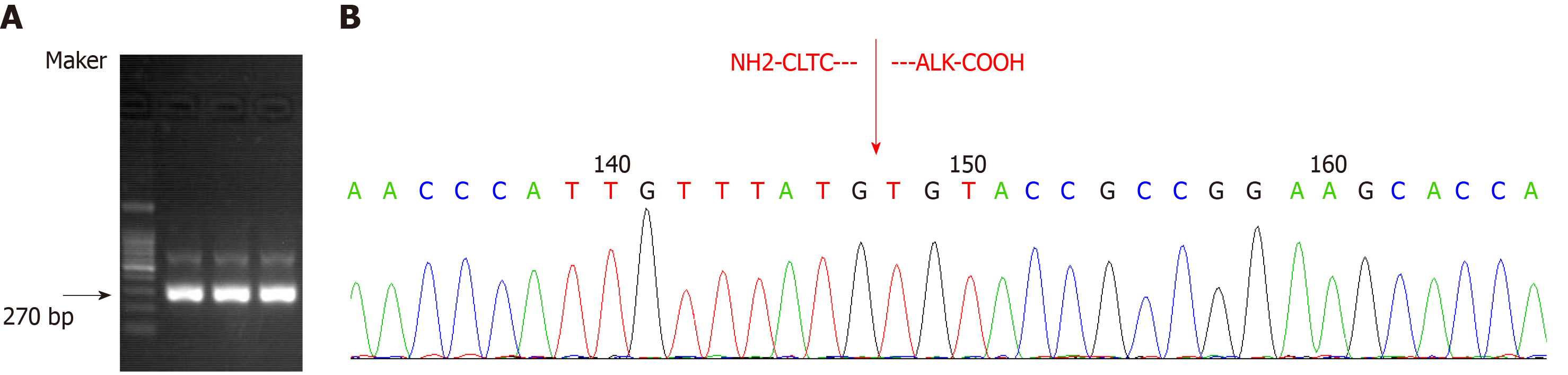
Transcriptome sequencing of bone marrow mononuclear cells (BMMCs) by RNA-seq was performed to identify the fusion gene. Total RNA was extracted with TRIzol reagent. The transcriptome was constructed using the TruSeq Stranded mRNA library kit (Illumina, San Diego, CA, United States), and the library containing 200 bp fragments was paired-end sequenced in duplicate with the Illumina HiSeq X Ten platform. Sequencing data were filtered and quality controlled to obtain clean data and aligned to the hg19 reference genome and its transcriptome. Differential gene expression (log2 fold change > 1 and FDR < 0.01) and its enrichment were obtained. SNP/InDel calling, annotation, and fusion gene analysis were subsequently performed using biostatistical software. A genetic variation of transcriptional fusion was identified with mutation point A in chr17:57768072 and mutation point B in chr2:29448328. We also confirmed the CLTC-ALK fusion by reverse transcription-polymerase chain reaction (RT-PCR). Total RNA was extracted from BMMCs and then reverse transcribed into cDNA. To amplify CLTC-ALK, two rounds of PCR were performed; the amplification conditions and primer sequences were as previously reported[8] (Figure 5).
ALK-positive ALCL with CLTC-ALK fusion gene was found. According to the St Jude Children’s Research Hospital staging system, the disease was stage IV.
The patient underwent chemotherapy as per the ALCL99 protocol[9] and showed a good response. PET-CT, performed after six courses of chemotherapy, indicated that there were only residual tumors in her left lower extremity. We also detected CLTC/ALK expression in her bone marrow (BM) and peripheral blood (PB) samples at follow-up by nested PCR. The outcome was still positive. Then, she received weekly vinblastine (VBL) injections as maintenance treatment, at an initial dose of 6 mg/m2 (maximum, 10 mg) per injection, which was then reduced to 5 mg/m2 for grade 4 neutropenia. Three months later, she achieved CR, and CLTC/ALK was undetectable in her BM and PB samples.
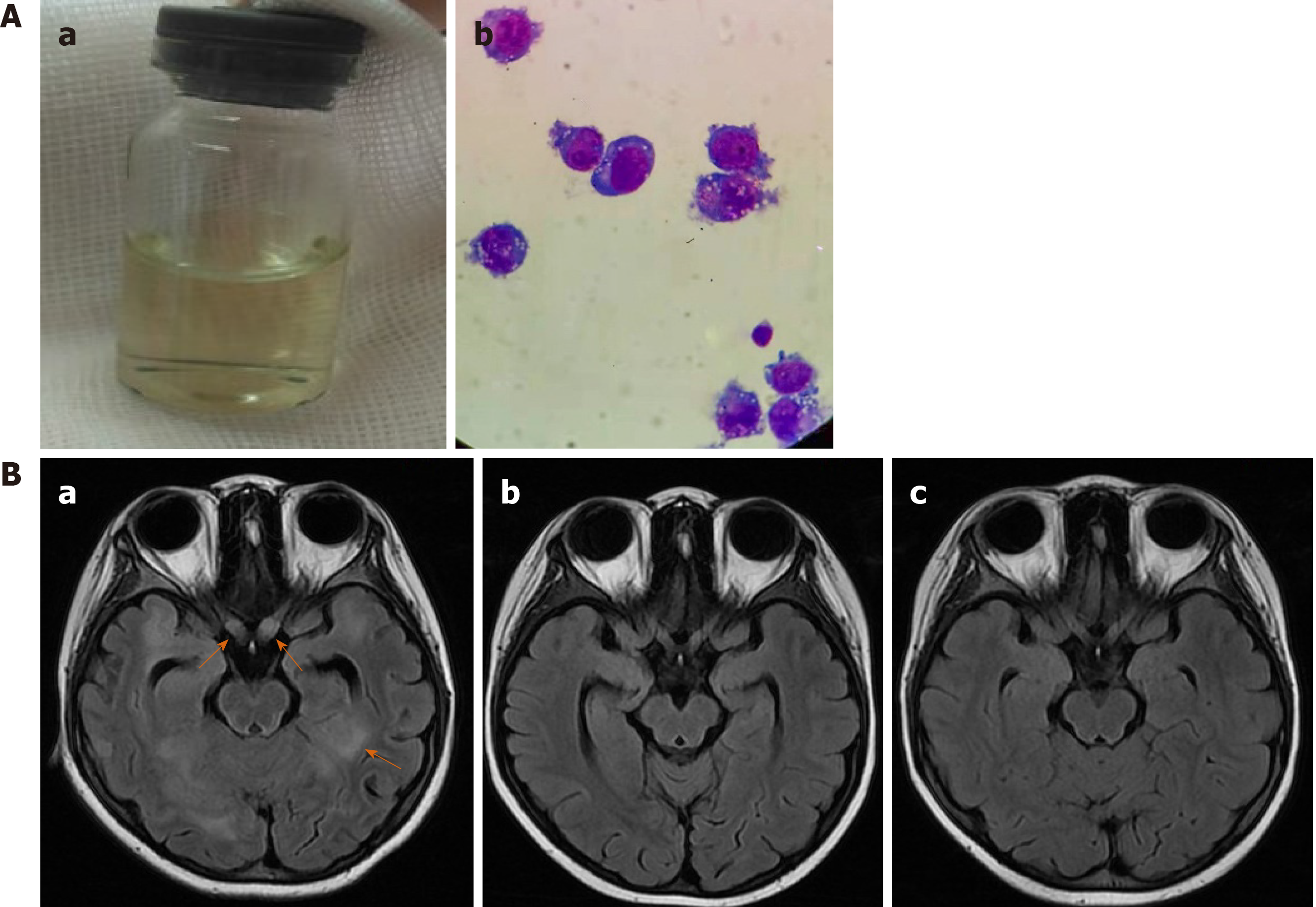
However, 1 mo later, she developed fever and headache and had frequent generalized seizures. The left pupil was dilated and did not react to light or accommodation. The cerebral spinal fluid (CSF) was slightly yellow (Figure 6Aa); CSF glucose level was 0.04 mmol/L, and CSF protein level was 4.5 g/L. A cytospin of CSF revealed approximately 72 tumor cells/high-power field (Figure 6Ab). MRI images of the brain revealed new lesions in the cerebral hemisphere, basal ganglia, thalamus, and brainstem. Intracranial segments of the bilateral optic nerves were enlarged (Figure 6Ba). Besides the CNS, there were no other signs of tumor relapse on CT and MRI scans. CLTC/ALK was positive again in one BM sample.
Two intrathecal triple therapies were administered, but the patient’s condition did not improve. More intensive chemotherapy and ALK-directed kinase inhibitor therapy were considered. She underwent treatment with both chemotherapy of COPADM according to LMB89 group C (including MTX 8 g/m2) and alectinib given orally[10]. There is no information on alectinib use in pediatrics; the dose was extrapolated from the recommended adult dose (600 mg twice daily). Given the patient’s weight (34 kg) and body surface area (1.20 m2), alectinib was commenced at a total daily dose of 750 mg (divided into two doses). Written informed consent was obtained from her parents before commencing alectinib treatment.
Her fever resolved within one day; no seizures occurred thereafter. She received triple intrathecal therapy on the second day of chemotherapy. The CSF was much clearer than before, and no tumor cell was found on CSF cytospin. During the chemotherapy, complication by sepsis due to Escherichia coli and grade 4 neutropenia were observed. After the sepsis was cured, she received three more courses of chemotherapy of COPADM (MTX 8 g/m2), CYVE, and HD-MXT (5 g/m2) sequentially, with simultaneous alectinib. Thereafter, evaluation revealed that the involvement of the brain parenchyma and optic nerves was reduced (Figure 6Bb). She was discharged and started with maintenance treatment of VBL and alectinib at the clinic. The total daily dose of alectinib was reduced to 450-600 mg due to grade 4 neutropenia. The alectinib plasma trough level (Cmin) was monitored, and it ranged from 250 to 427 ng/mL. Two months later, she achieved CR, as shown by brain MRI (Figure 6Bc). CLTC/ALK was negative again in the BM and PB samples. Allogeneic hematopoietic stem cell transplantation was suggested, but it was declined by her parents.
Thus far, she has completed treatment with VBL and alectinib for 17 mo. The treatment was tolerated well, without significant adverse effects, except grades 1-4 neutropenia. She is still in CR, but her vision has not recovered.
Here, we present an extraordinary and rare case of ALCL with the CLTC-ALK fusion gene. The clinical relevance of various translocations has not been confirmed. Five X-ALK variants have been reported to display different proliferative, migratory, and invasive properties, which seem to be due to differential activation of various signaling pathways[11]. This patient’s invasive and refractory clinical process suggests the possibility of a more aggressive property related to the CLTC/ALK variant in ALCL, although more cases are needed to confirm this.
VBL has shown remarkable activity as a single agent even in the treatment of relapsed ALCL[12]. There are limited data on VBL passing the blood–brain barrier (BBB). Besides this patient, there are other reports on CNS progression during VBL therapy for high-risk ALK+ALCL[13]. These cases indicate that the CNS/CSF penetration of VBL is insufficient for the control of high-risk ALCL relapse, and CNS-directed therapy ought to be considered during VBL monotherapy.
The reported incidence of CNS involvement in ALK+ALCL is about 2.6%. Patients with CNS involvement show an inferior prognosis[9]. The first-generation ALK-inhibitor crizotinib has poor CSF penetrance. Alectinib is not a P-glycoprotein substrate; hence, it could penetrate the BBB[14]. The reported CSF penetration rates of crizotinib and alectinib are 0.26% and 86%, respectively[15]. Alectinib has demonstrated potent CNS activity in ALK-positive non-small-cell lung cancer patients[16,17]. It also showed good CNS response in two reported ALK+ALCL patients with CNS involvement[18,19]. However, pediatric dosing data are lacking. A population pharmacokinetic analysis showed that a higher-than-median steady-state alectinib Cmin of ≥ 435 ng/mL is associated with a greater reduction in tumor size[20]. The current patient did not reach this target concentration because of grade 3 or 4 neutropenia. We speculate that this might be related to the co-administration of VBL, which can also cause myelosuppression. Nonetheless, the disease was still well controlled, and no significant adverse events occurred.
In summary, this report describes a rare pediatric ALCL patient with the CLTC-ALK fusion gene. After CNS relapse, she achieved a durable second remission with high-dose chemotherapy and continuous alectinib. Alectinib showed significant and durable CNS effects in this patient. To our knowledge, this is the first published pediatric case report describing the use of alectinib for the treatment of ALK+ALCL. However, our findings are based on a single patient, and more cases are needed to prove the efficacy and safety of alectinib for pediatric patients.
The authors would like to thank the patient and her family.
Manuscript source: Unsolicited Manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cihan YB S-Editor: Ma YJ L-Editor: Wang TQ E-Editor: Wu YXJ
| 1. | Stein H, Foss HD, Dürkop H, Marafioti T, Delsol G, Pulford K, Pileri S, Falini B. CD30(+) anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood. 2000;96:3681-3695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Swerdlow SH, Campo E, Harris NL. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (Revised 4th edition). IARC: Lyon, 2017. |
| 3. | De Paepe P, Baens M, van Krieken H, Verhasselt B, Stul M, Simons A, Poppe B, Laureys G, Brons P, Vandenberghe P, Speleman F, Praet M, De Wolf-Peeters C, Marynen P, Wlodarska I. ALK activation by the CLTC-ALK fusion is a recurrent event in large B-cell lymphoma. Blood. 2003;102:2638-2641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Touriol C, Greenland C, Lamant L, Pulford K, Bernard F, Rousset T, Mason DY, Delsol G. Further demonstration of the diversity of chromosomal changes involving 2p23 in ALK-positive lymphoma: 2 cases expressing ALK kinase fused to CLTCL (clathrin chain polypeptide-like). Blood. 2000;95:3204-3207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Zhang D, Denley RC, Filippa DA, Teruya-Feldstein J. ALK-positive diffuse large B-cell lymphoma with the t(2;17)(p23;q23). Appl Immunohistochem Mol Morphol. 2009;17:172-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Parker BM, Parker JV, Lymperopoulos A, Konda V. A case report: Pharmacology and resistance patterns of three generations of ALK inhibitors in metastatic inflammatory myofibroblastic sarcoma. J Oncol Pharm Pract. 2019;25:1226-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Cools J, Wlodarska I, Somers R, Mentens N, Pedeutour F, Maes B, De Wolf-Peeters C, Pauwels P, Hagemeijer A, Marynen P. Identification of novel fusion partners of ALK, the anaplastic lymphoma kinase, in anaplastic large-cell lymphoma and inflammatory myofibroblastic tumor. Genes Chromosomes Cancer. 2002;34:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 200] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 8. | Wang WY, Gu L, Liu WP, Li GD, Liu HJ, Ma ZG. ALK-positive extramedullary plasmacytoma with expression of the CLTC-ALK fusion transcript. Pathol Res Pract. 2011;207:587-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Le Deley MC, Rosolen A, Williams DM, Horibe K, Wrobel G, Attarbaschi A, Zsiros J, Uyttebroeck A, Marky IM, Lamant L, Woessmann W, Pillon M, Hobson R, Mauguen A, Reiter A, Brugières L. Vinblastine in children and adolescents with high-risk anaplastic large-cell lymphoma: results of the randomized ALCL99-vinblastine trial. J Clin Oncol. 2010;28:3987-3993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Patte C, Auperin A, Michon J, Behrendt H, Leverger G, Frappaz D, Lutz P, Coze C, Perel Y, Raphaël M, Terrier-Lacombe MJ; Société Française d'Oncologie Pédiatrique. The Société Française d'Oncologie Pédiatrique LMB89 protocol: highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood. 2001;97:3370-3379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 364] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 11. | Armstrong F, Duplantier MM, Trempat P, Hieblot C, Lamant L, Espinos E, Racaud-Sultan C, Allouche M, Campo E, Delsol G, Touriol C. Differential effects of X-ALK fusion proteins on proliferation, transformation, and invasion properties of NIH3T3 cells. Oncogene. 2004;23:6071-6082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Brugières L, Pacquement H, Le Deley MC, Leverger G, Lutz P, Paillard C, Baruchel A, Frappaz D, Nelken B, Lamant L, Patte C. Single-drug vinblastine as salvage treatment for refractory or relapsed anaplastic large-cell lymphoma: a report from the French Society of Pediatric Oncology. J Clin Oncol. 2009;27:5056-5061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Ruf S, Hebart H, Hjalgrim LL, Kabickova E, Lang P, Steinbach D, Schwabe GC, Woessmann W. CNS progression during vinblastine or targeted therapies for high-risk relapsed ALK-positive anaplastic large cell lymphoma: A case series. Pediatr Blood Cancer. 2018;65:e27003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Kodama T, Hasegawa M, Takanashi K, Sakurai Y, Kondoh O, Sakamoto H. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol. 2014;74:1023-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 204] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 15. | Wrona A. Management of CNS disease in ALK-positive non-small cell lung cancer: Is whole brain radiotherapy still needed? Cancer Radiother. 2019;23:432-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Lin JJ, Jiang GY, Joshipura N, Ackil J, Digumarthy SR, Rincon SP, Yeap BY, Gainor JF, Shaw AT. Efficacy of Alectinib in Patients with ALK-Positive NSCLC and Symptomatic or Large CNS Metastases. J Thorac Oncol. 2019;14:683-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Gourd E. Alectinib shows CNS efficacy in ALK-positive NSCLC. Lancet Oncol. 2018;19:e520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Tomlinson SB, Sandwell S, Chuang ST, Johnson MD, Vates GE, Reagan PM. Central nervous system relapse of systemic ALK-rearranged anaplastic large cell lymphoma treated with alectinib. Leuk Res. 2019;83:106164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Reed DR, Hall RD, Gentzler RD, Volodin L, Douvas MG, Portell CA. Treatment of Refractory ALK Rearranged Anaplastic Large Cell Lymphoma With Alectinib. Clin Lymphoma Myeloma Leuk. 2019;19:e247-e250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Verheijen RB, Yu H, Schellens JHM, Beijnen JH, Steeghs N, Huitema ADR. Practical Recommendations for Therapeutic Drug Monitoring of Kinase Inhibitors in Oncology. Clin Pharmacol Ther. 2017;102:765-776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 221] [Article Influence: 27.6] [Reference Citation Analysis (0)] |