Published online May 6, 2020. doi: 10.12998/wjcc.v8.i9.1656
Peer-review started: January 30, 2020
First decision: March 18, 2020
Revised: March 27, 2020
Accepted: April 21, 2020
Article in press: April 21, 2020
Published online: May 6, 2020
Processing time: 91 Days and 5.8 Hours
Castleman's disease (CD) is a lymphoproliferative disorder. TAFRO syndrome is classified as a variant of CD based on its key clinical manifestations of thrombocytopenia, anasarca (generalized edema and pleural effusion), fever (pyrexia), reticulin fibrosis in the bone marrow and the proliferation of megakaryocytes, and organomegaly (such as hepatosplenomegaly and multiple lymphadenopathies); TAFRO syndrome is mainly reported in Japanese patients. To our knowledge, this is the first pediatric case report detailing a CD-associated disorder progressing to cirrhosis.
A 10-year old male patient presented with fever and anemia. Six months before hospitalization, he had remarkable abdominal distention. Subsequently, he visited a clinic for a fever that lasted 5 d. The physical findings were marked hepatosplenomegaly and cervical lymphadenopathy. A blood test revealed leukocytosis, microcytic anemia, aspartate aminotransferase-dominant transaminase elevation, high levels of C-reactive protein, polyclonal hypergammaglobulinemia, and high levels of interleukin-6 and vascular endothelial growth factor. Abdominal contrast computed tomography and magnetic resonance imaging suggested cirrhosis, which was confirmed by liver histology. Histological findings in the enlarged hepatic lymph nodes revealed both hyperplasia and atrophy of lymphoid follicles with some vascular hyperplasia and moderate plasmacytosis between the lymphoid follicles, which is compatible with lymph node histology in TAFRO syndrome. Prednisolone was not effective in reducing the patient’s symptoms; therefore, the patient was prescribed tocilizumab. To date, the patient remains free of fever and continues to receive tocilizumab.
We described the clinicopathological features of TAFRO syndrome to highlight the clinical presentation of this rare disease in a pediatric case.
Core tip: Castleman disease (CD) is a lymphoproliferative disorder of unknown cause. TAFRO syndrome is classified as a variant of CD based on its key clinical manifestations of thrombocytopenia, anasarca (generalized edema and pleural effusion), fever (pyrexia), reticulin fibrosis (reticulin fibrosis in the bone marrow and the proliferation of megakaryocytes), and organomegaly (e.g., hepatosplenomegaly and multiple lymphadenopathies). To our knowledge, this is the first pediatric case report detailing a CD-associated disorder progressing to cirrhosis.
- Citation: Kobayashi S, Inui A, Tsunoda T, Umetsu S, Sogo T, Mori M, Shinkai M, Fujisawa T. Liver cirrhosis in a child associated with Castleman's disease: A case report. World J Clin Cases 2020; 8(9): 1656-1665
- URL: https://www.wjgnet.com/2307-8960/full/v8/i9/1656.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i9.1656
Castleman's disease (CD) is a lymphoproliferative disorder with an unknown cause, classified into unicentric (unicentric distribution of the disease) and multicentric (multicentric distribution of the disease) types. While in Western countries, the human herpesvirus 8 (HHV-8) infection-related multicentric CD is common[1,2], majority of the CD cases in Japan are idiopathic[3,4]. The clinical features include anemia, multiple lymphadenopathy, increased inflammatory response, polyclonal hypergammaglobulinemia, and thrombocythemia. Histologically, the hyaline vascular type (HV type) is common in the unicentric type, and multicentric types are further classified into plasma cell type (PC-type) and mixed-type[5]. Patients with the mixed-type idiopathic multicentric Castleman’s disease (MCD), characterized by thrombocytopenia, anasarca (generalized edema and pleural effusion), fever (pyrexia), reticulin fibrosis in the bone marrow and the proliferation of megakaryocytes, and organomegaly (such as hepatosplenomegaly and multiple lymphadenopathies), are considered to have TAFRO syndrome (thrombocytopenia, anasarca, fever, renal impairment or reticulin fibrosis, and organomegaly) and often follow a more severe course[6]. There are no case reports of children with CD or TAFRO syndrome who subsequently develop cirrhosis. We presented this pediatric case to expand our understanding of this disease.
A 10-year-old Japanese boy presented with fever and anemia.
Six months before his hospitalization, the patient experienced remarkable abdominal distention. Subsequently, he visited a clinic for fever that persisted for 5 d. His blood test results were as follows: White blood cell 13.2 × 103/μL (white blood cell normal range: 3.5 × 103/µL-8.5 × 103/µL), hemoglobin 9.8 g/dL (hemoglobin normal range: 11.5-15.0 g/dL), and C-reactive protein (CRP) 8.6 mg/dL (CRP normal range: 0.03 mg/dL or less). Therefore, he was referred to our hospital.
His family history did not reveal anything of significance to his present condition. He was diagnosed with Kawasaki disease when he was 1 year old and treated with intravenous immunoglobulin therapy and antiplatelet drugs. He had a fever of unknown origin when he was 7 years old.
His physical examination revealed no growth disorder. His height and weight were 141.0 cm (+ 1.0 SD) and 34.5 kg (0.0 SD), respectively. His body temperature was 36.3 °C. Redness of the pharynx and bleeding spots in the soft palate were observed, and his lymph nodes were palpable in the cervical region. The patient’s abdomen was slightly distended. Upon investigation, his liver was palpable 10 cm below the right costal margin and his spleen 6 cm below the left costal margin.
Laboratory examinations were performed and are summarized in Table 1. A blood test revealed neutrophil-dominant leukocytosis, microcytic hypochromic anemia, elevation of aspartate aminotransferase (AST)-dominant transaminase, high CRP, polyclonal hypergammaglobulinemia, and high interleukin-6 (IL-6) and vascular endothelial growth factor (VEGF).
| Blood cell count | |
| WBC | 8.49 × 103/μL |
| NET% | 76.1% |
| LYP% | 19.8% |
| MONO% | 2.7% |
| EOS% | 1.3% |
| RBC | 423 × 104/μL |
| Hb | 9.5 g/dL |
| Ht | 31.2% |
| MCV | 73.8 fL |
| MCH | 22.5 pg |
| MCHC | 30.4% |
| PLT | 17 × 104/μL |
| Biochemical and immune serum examination | |
| TP | 8 g/dL |
| Alb | 3.1 g/dL |
| T-bil | 1 mg/dL |
| D-bil | 0.6 mg/dL |
| AST | 95 U/L |
| ALT | 63 U/L |
| LDH | 289 U/L |
| γ-GTP | 132 U/L |
| ALP | 3152 U/L |
| Glu | 97 mg/dL |
| BUN | 7.5 mg/dL |
| Cr | 0.39 mg/dL |
| CRP | 6.83 mg/dL |
| Fe | 24 μg/dL |
| TIBC | 299 μg/dL |
| UIBC | 286 μg/dL |
| Ferritin | 65.9 ng/mL |
| NH3 | 36 μg/dL |
| AFP | 1.3 ng/mL |
| IgG | 2265 mg/dL |
| IgG4 | 191 mg/dL |
| IgA | 469 mg/dL |
| IgM | 399 mg/dL |
| Blood coagulation examination | |
| PT ratio | 60.5% |
| APTT | 32.2 s |
| Fib | 328 mg/dL |
| FDP | 6.6 μg/mL |
| D-dimer | 2.8 μg/mL |
| Cytokine/VEGF | |
| IL-6 | 40 pg/mL |
| VEGF | 49.2 pg/mL |
| Virologic test | |
| HIV antibody | (-) |
| HHV-8 PCR | (-) |
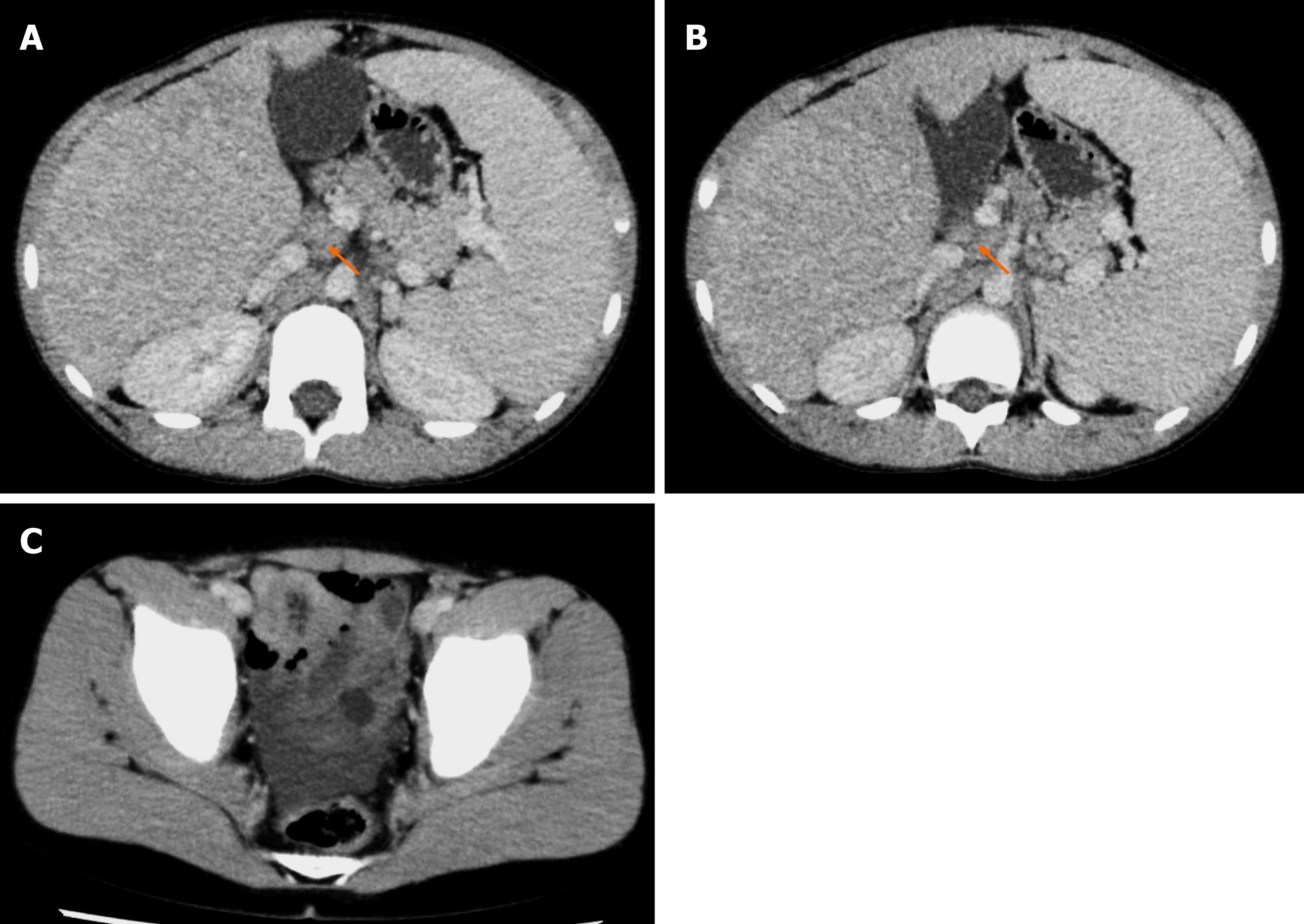
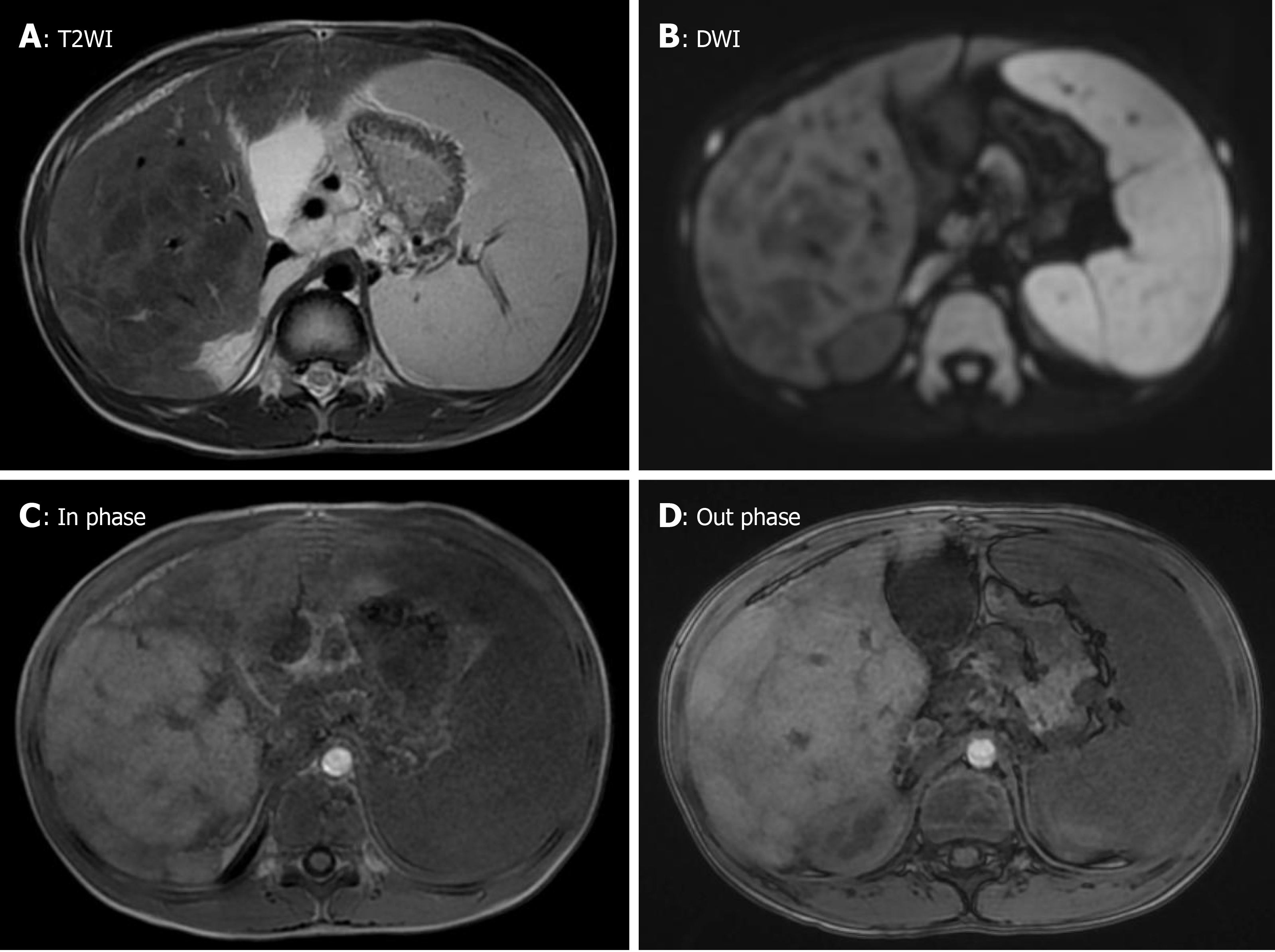
Abdominal ultrasound revealed a rough texture of the liver. Abdominal contrast computed tomography (CT) detected hepatosplenomegaly and heterogeneous reduction of the density of the liver, suggesting advanced cirrhosis. Multiple lymphadenopathies were found in the hepatic portal, and the mesenteric and celiac arteries. A small number of ascites was observed in the pelvis (Figure 1). Multiple nodules were observed in the liver. However, the abdominal enhanced magnetic resonance imaging (MRI) did not show washout of the contrast agent in the nodules during the late phase of the contrast enhancement, which suggested regenerative nodules (Figure 2).
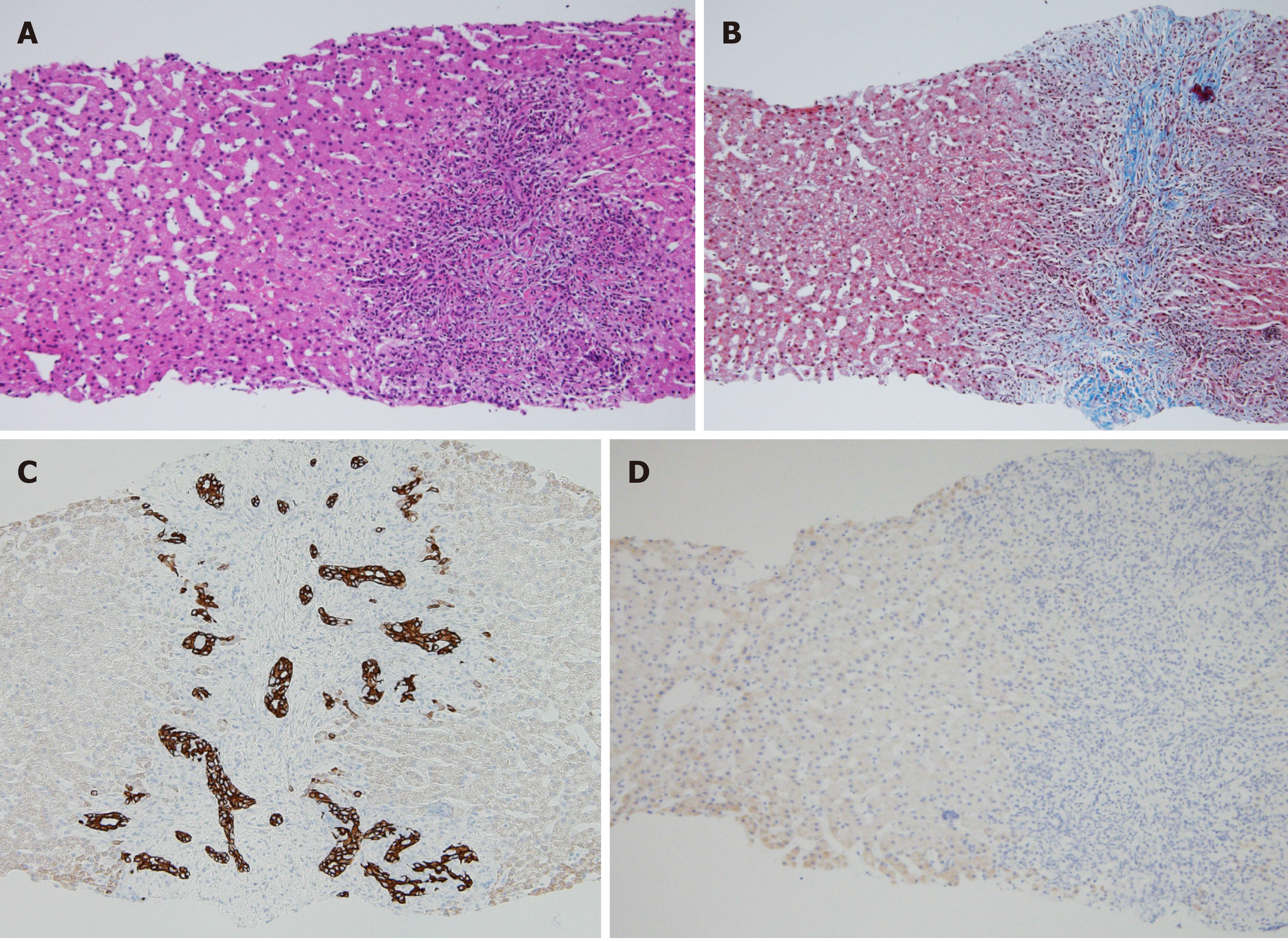
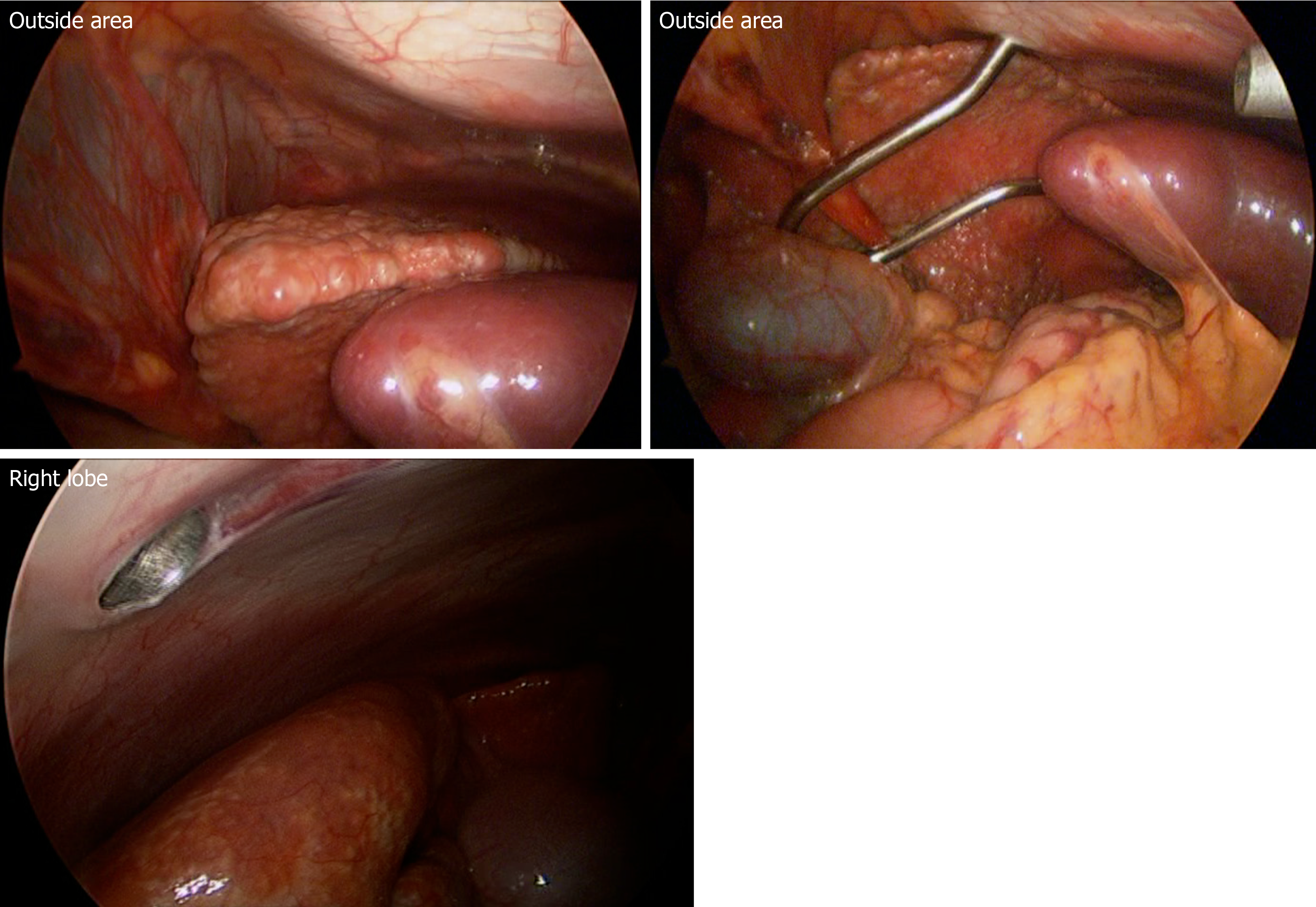
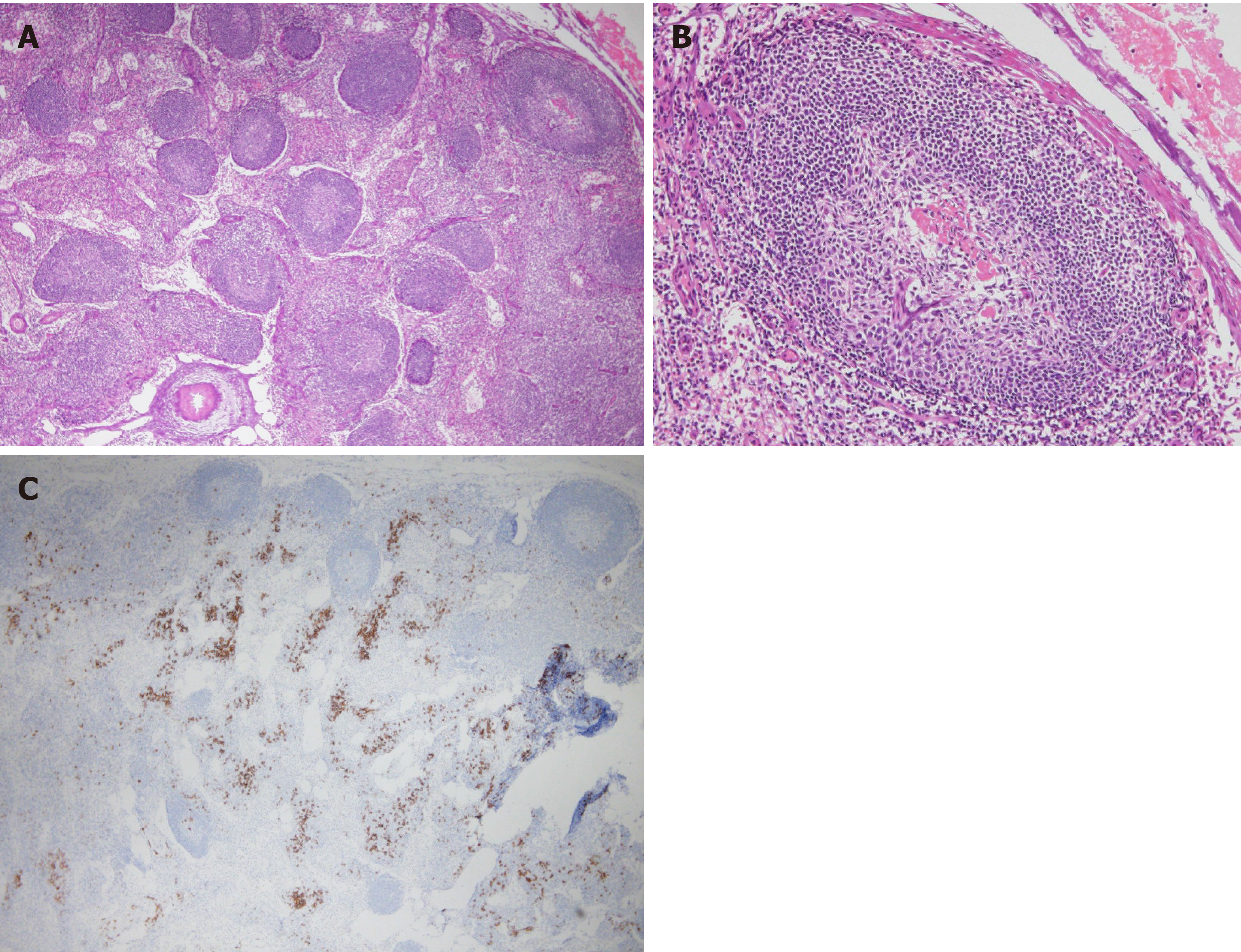
Upon bone marrow examination, no monoclonal cell proliferation was observed. In positron emission tomography with CT, the standardized uptake values for the right hepatic lobe, hepatic portal, and intestinal membrane were about 2.0-3.0. Upper gastrointestinal endoscopy revealed esophageal varices. Liver histology showed sinusoidal dilation in the hepatic lobule, significant inflammatory cell infiltration, bile ductular proliferation, and fibrotic expansion in portal areas (Figure 3). When testing for high serum immunoglobulin G4 (IgG4) levels (191 mg/dL), a special immunohistological staining of the liver tissue was negative for IgG4. Endoscopic retrograde cholangiopancreatography showed no evidence of sclerosing cholangitis, leading to the negative diagnosis of Langerhans cell histiocytosis- or immunodeficiency-related sclerosing cholangitis. Intraoperative findings from laparoscopy revealed roughness of the liver surface, suggesting liver cirrhosis (Figure 4). Laparoscopic lymph node biopsy revealed both hyperplasia and atrophy of lymphoid follicles, lymphoid proliferation with some vascular hyperplasia, and moderate interfollicular plasmacytosis (Figure 5).
Based on the abovementioned findings, the final diagnosis was CD-associated disease.
According to the treatment regimen for CD, 1 mg/kg per day of prednisolone (PSL) was administered for 4 wk. However, there was no improvement in fever and serum CRP. Finally, the patient was switched from PSL to 8 mg/kg per day of tocilizumab, and the fever subsided.
CD is a rare lymphoproliferative disorder first reported by Castleman et al[7] in 1956. To date, there has been no epidemiological study of the disease in children. However, a study conducted in the United States estimated the annual incidence of CD to be 0.24 per million[8]. A study conducted in Japan estimated the prevalence and annual incidence to be approximately 1500 and 1 per million, respectively[9]. Regarding pathological types, the syndrome can be classified into hyaline-vascular type (HV-type), plasma cell type (PC-type), and mixed type. Regarding clinical characteristics, CD can be roughly classified into single (localized) and multiple (systemic). Lymphadenopathy is most frequently observed in the mediastinum, while rare in the hepatic portal region[10]. The close relationship of MCD with HIV and HHV-8 has been emphasized in Western countries[1,2]. However, the relationship is seldom mentioned in studies conducted in Japan[3,4]. Fajgenbaum et al[11] reported HHV-8-negative MCD as idiopathic MCD (iMCD). In Japan, most patients with MCD are classified as having iMCD. In 2010, Takai et al[6] named the TAFRO syndrome based on its key clinical manifestations of thrombocytopenia, anasarca (generalized edema and pleural effusion), fever (pyrexia), reticulin fibrosis (reticulin fibrosis in the bone marrow and the proliferation of megakaryocytes), and organomegaly (e.g., hepatosplenomegaly and multiple lymphadenopathies). The histology of lymph nodes in patients with TAFRO syndrome revealed CD-like (i.e., mixed-type or HV-type) characteristics. Therefore, some researchers classify TAFRO syndrome, which is rare in western regions, as a type of MCD. In most cases, patients with TAFRO syndrome have increased gamma globulin levels, decreased platelet levels, smaller lymph nodes, and significant subacute development of pleural effusion and edema, and follow a progressive (sometimes lethal) course[12,13].
In the present case, the findings from the histopathological examination of the lymph node biopsy, which was performed by a pathologist, Dr. Kojima, a member of the Castleman disease, TAFRO, and related disease research group, indicated a diagnosis of TAFRO syndrome. However, despite the presence of mild ascites, no renal dysfunction, reticulin fibrosis in the bone marrow, general anemia, or thrombocytopenia (100000/μL) were found at the first visit. Thus, the symptomology did not meet the diagnostic criteria for TAFRO syndrome. In addition, the patient had intermittent fever, but he was in good general condition. The patient not presenting with all the symptoms of the TAFRO syndrome might be because he has been receiving treatment early in the disease process due to early diagnosis. Marked hepatosplenomegaly and cirrhosis were particularly dominant in this case. There are 8 case reports of patients with MCD or TAFRO syndrome complicated by hepatobiliary disorders[14-20] (Table 2). Case reports by Baruch et al[14] included the description of cirrhosis in patients with Budd-Chiari syndrome. On the other hand, a study has reported diffuse liver fibrosis, cholangitis, and nodular regenerative hyperplasia (NRH)[17], suggesting that liver disease is associated with MCD and related diseases.
| Year | Age/sex | Diagnosis | Type | Pathological findings of the liver | Outcome | Ref. |
| 1991 | 22/F | CD | Mixed | Liver cirrhosis associated with Budd-Chiari syndrome, an underlying disease | Liver transplantation waiting for Budd-Chiari syndrome | [14] |
| 1995 | 50/M | CD | PC | Diffuse fibrosis | Death due to thrombocytopenia and massive gastrointestinal bleeding | [15] |
| 35/M | CD | PC | Cholestasis and peliosis hepatis | Unknown | ||
| 2003 | 54/M | CD | Mixed | Nodular cirrhosis | Perform liver transplantation and maintain remission | [16] |
| 2005 | 45/M | CD | PC | Nodular regenerative hyperplasia | PSL effective | [17] |
| 2013 | 51/M | CD | - | Liver amyloidosis | Symptoms persist even after lymph node dissection | [18] |
| 2016 | 56/M | TAFRO | Mixed | Expansion of portal area, interface hepatitis, pseudo biliary hyperplasia and cholangitis | Steroid pulse, PSL, tocilizumab, rituximab | [19] |
| 2017 | 26/F | CD | PC | Fibrosis and plasma cell infiltration | PSL effective | [20] |
| This case | 10/M | CD or TAFRO | Mixed | Portal vein area fibrosis, inflammatory cell infiltration, bile duct hyperplasia | PSL ineffective, tocilizumab improves inflammatory response |
The pathogenesis may be explained by the overproduction of IL-6 by the germinal center of the lymph nodes. The detailed mechanisms of MCD and TAFRO syndrome are unknown. However, some patients respond to tocilizumab (humanized anti-human IL-6 receptor monoclonal antibody), suggesting that IL-6 is involved[21,22]. The overproduction of IL-6 promotes differentiation into plasma cells, which in turn produce VEGF and bind to hepatocyte receptors, promoting endothelial permeability and neovascularization. Hypoxia due to blood vessel remodeling, impaired blood flow, and imbalance between demand and supply of oxygen promotes collagen synthesis[23-25] and leads to fibrosis, suggesting that there is a relationship between VEGF and fibrosis.
CD associated disorder should be suspected in patients with fever of unknown origin, hepatosplenomegaly, lymphadenopathy, or polyclonal hypergammaglobulinemia. To date, there is no detailed study of liver pathology in patients with CD-associated disorder. Further understanding of CD-associated disorder may help clarify the mechanism of CD.
Kazuyuki Yoshizaki, Department of Organic Fine Chemicals, The Institute of Scientific and Industrial Research, Osaka University, Osaka, Japan. Masaru Kojima, Department of Diagnostic Pathology, Dokkyo Medical University School of Medicine, Tochigi, Japan.
Manuscript source: Unsolicited Manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Skok P, Tian H S-Editor: Ma YJ L-Editor: A E-Editor: Liu MY
| 1. | Cesarman E, Knowles DM. The role of Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in lymphoproliferative diseases. Semin Cancer Biol. 1999;9:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 148] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Dupin N, Diss TL, Kellam P, Tulliez M, Du MQ, Sicard D, Weiss RA, Isaacson PG, Boshoff C. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood. 2000;95:1406-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Suda T, Katano H, Delsol G, Kakiuchi C, Nakamura T, Shiota M, Sata T, Higashihara M, Mori S. HHV-8 infection status of AIDS-unrelated and AIDS-associated multicentric Castleman's disease. Pathol Int. 2001;51:671-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Kojima M, Nakamura N, Tsukamoto N, Otuski Y, Shimizu K, Itoh H, Kobayashi S, Kobayashi H, Murase T, Masawa N, Kashimura M, Nakamura S. Clinical implications of idiopathic multicentric castleman disease among Japanese: a report of 28 cases. Int J Surg Pathol. 2008;16:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Cronin DM, Warnke RA. Castleman disease: an update on classification and the spectrum of associated lesions. Adv Anat Pathol. 2009;16:236-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 6. | Takai K, Nikkuni K, Shibuya H, Hashidate H. [Thrombocytopenia with mild bone marrow fibrosis accompanied by fever, pleural effusion, ascites and hepatosplenomegaly]. Rinsho Ketsueki. 2010;51:320-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 7. | Castleman B, Iverson L, Menendez VP. Localized mediastinal lymphnode hyperplasia resembling thymoma. Cancer. 1956;9:822-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 8. | Robinson D Jr, Reynolds M, Casper C, Dispenzieri A, Vermeulen J, Payne K, Schramm J, Ristow K, Desrosiers MP, Yeomans K, Teltsch D, Swain R, Habermann TM, Rotella P, Van de Velde H. Clinical epidemiology and treatment patterns of patients with multicentric Castleman disease: results from two US treatment centres. Br J Haematol. 2014;165:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Yoshizaki K. A reference guide for management of Castleman disease. Rinsho Ketsueki. 2017;58:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 10. | Bonekamp D, Horton KM, Hruban RH, Fishman EK. Castleman disease: the great mimic. Radiographics. 2011;31:1793-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 11. | Fajgenbaum DC, van Rhee F, Nabel CS. HHV-8-negative, idiopathic multicentric Castleman disease: novel insights into biology, pathogenesis, and therapy. Blood. 2014;123:2924-2933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 233] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 12. | Ito S, Tsutsumi Y, Kikuchi R, Matsuoka S, Shimoyama N. [Thrombotic microangiopathy developing subsequent to tocilizumab therapy in a patient with TAFRO syndrome]. Rinsho Ketsueki. 2018;59:2432-2437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Hashimoto K, Sano T, Honma Y, Ida M, Tominaga H, Sawada A, Abe T, Takahashi H, Shimada Y, Masaki T, Kamata M, Naito S, Aoyama T, Takeuchi Y, Akiya M, Inukai M, Nakata N. An autopsy case of TAFRO syndrome with membranoproliferative glomerulonephritis-like lesions. CEN Case Rep. 2019;8:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Baruch Y, Ben-Arie Y, Kerner H, Lorber M, Best LA, Gershoni-Baruch R. Giant lymph node hyperplasia (Castleman's disease): a clinical study of eight patients. Postgrad Med J. 1991;67:366-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Molina T, Delmer A, Le Tourneau A, Texier P, Degott C, Audoin J, Zittoun R, Diebold J. Hepatic lesions of vascular origin in multicentric Castleman's disease, plasma cell type: report of one case with peliosis hepatis and another with perisinusoidal fibrosis and nodular regenerative hyperplasia. Pathol Res Pract. 1995;191:1159-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Maloisel F, Andrès E, Chenard MP, Ellero B, Wolf P. Treatment of hepatic relapse of multicentric Castleman's disease with transplantation. Am J Med. 2003;115:160-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Kiyuna A, Sunagawa T, Hokama A, Touyama M, Tomiyama R, Sakugawa H, Kinjo F, Saito A. Nodular regenerative hyperplasia of the liver and Castleman's disease: potential role of interleukin-6. Dig Dis Sci. 2005;50:314-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Gaduputi V, Tariq H, Badipatla K, Ihimoyan A. Systemic Reactive Amyloidosis Associated with Castleman's Disease. Case Rep Gastroenterol. 2013;7:476-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Nagai Y, Ando S, Honda N, Noguchi H, Maemori M, Hayashi T, Sakai H. TAFRO syndrome showing cholangitis on liver biopsy. Rinsho Ketsueki. 2016;57:2490-2495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 20. | Ueki T, Nasuno M, Kaiume H, Hiroshima Y, Sumi M, Watanabe M, Inoue D, Masaki Y, Sato Y, Kojima M, Kobayashi H. Multicentric Castleman's disease with multiple hepatic mass lesions mimicking malignant liver tumors. Rinsho Ketsueki. 2017;58:630-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Nishimoto N, Sasai M, Shima Y, Nakagawa M, Matsumoto T, Shirai T, Kishimoto T, Yoshizaki K. Improvement in Castleman's disease by humanized anti-interleukin-6 receptor antibody therapy. Blood. 2000;95:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 282] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 22. | Sakai K, Maeda T, Kuriyama A, Shimada N, Notohara K, Ueda Y. TAFRO syndrome successfully treated with tocilizumab: A case report and systematic review. Mod Rheumatol. 2018;28:564-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Manotham K, Tanaka T, Matsumoto M, Ohse T, Miyata T, Inagi R, Kurokawa K, Fujita T, Nangaku M. Evidence of tubular hypoxia in the early phase in the remnant kidney model. J Am Soc Nephrol. 2004;15:1277-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 180] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117:557-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 792] [Cited by in RCA: 869] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 25. | Orphanides C, Fine LG, Norman JT. Hypoxia stimulates proximal tubular cell matrix production via a TGF-beta1-independent mechanism. Kidney Int. 1997;52:637-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 151] [Article Influence: 5.4] [Reference Citation Analysis (0)] |