Published online May 6, 2020. doi: 10.12998/wjcc.v8.i9.1632
Peer-review started: December 28, 2019
First decision: January 11, 2020
Revised: January 21, 2020
Accepted: April 4, 2020
Article in press: April 4, 2020
Published online: May 6, 2020
Processing time: 124 Days and 6.3 Hours
Visceral hypersensitivity and psychological performance are the main pathophysiological mechanisms of irritable bowel syndrome (IBS). Previous studies have found that cholecystokinin (CCK) can enhance colon movement and that serotonin transporter (SERT) is a transmembrane transport protein with high affinity for 5-hydroxytryptamine, which can rapidly reuptake 5-hydroxytryptamine and then regulate its action time and intensity. We speculate that SERT and CCK might play a role in the pathogenesis of diarrhea-predominant IBS (IBS-D) by affecting visceral sensitivity and the brain-gut axis.
To determine SERT and CCK levels in IBS-D patients diagnosed using Rome IV criteria and to analyze their associations with abdominal pain, visceral hypersensitivity and psychological performance.
This study collected data from 40 patients with IBS-D at the China-Japan Friendship Hospital from September 2017 to April 2018 and 18 healthy controls. The severity of abdominal pain, visceral sensitivity and psychological performance were evaluated in IBS-D patients and healthy controls, the levels of SERT and CCK in plasma and colonic mucosa were evaluated, and the correlations between them were analyzed.
There were significant differences in the initial sensation threshold (31.00 ± 8.41 mL vs 52.22 ± 8.09 mL, P < 0.001), defecating sensation threshold (51.75 ± 13.57 mL vs 89.44 ± 8.73 mL, P < 0.001) and maximum tolerable threshold (97.25 ± 23.64 mL vs 171.11 ± 20.83 mL, P < 0.001) between the two groups. IBS-D patients had more severe anxiety (7.78 ± 2.62 vs 2.89 ± 1.02, P < 0.001) and depressive (6.38 ± 2.43 vs 2.06 ± 0.73, P < 0.001) symptoms than healthy controls. Significant differences were also found in mucosal CCK (2.29 ± 0.30 vs 1.66 ± 0.17, P < 0.001) and SERT (1.90 ± 0.51 vs 3.03 ± 0.23, P < 0.001) between the two groups. There was a significant positive correlation between pain scores and mucosal CCK (r = 0.96, 0.93, 0.94, P < 0.001). Significant negative correlations between anxiety (r = -0.98; P < 0.001), depression (r = -0.99; P < 0.001), pain evaluation (r = -0.96, -0.93, -0.95, P < 0.001) and mucosal SERT were observed.
IBS-D patients had psychosomatic disorders and visceral hypersensitivity. SERT and CCK might be involved in the pathogenesis of IBS-D by regulating the brain-gut axis and affecting visceral sensitivity. This provides a new potential method for identifying a more specific and effective therapeutic target.
Core tip: This study comprehensively evaluated the symptoms, mental state and visceral sensitivity of patients with diarrhea-predominant irritable bowel syndrome (IBS-D), and further determined serotonin transporter (SERT) and cholecystokinin (CCK) levels in IBS-D patients diagnosed using Rome IV criteria and analyzed their associations with abdominal pain, visceral hypersensitivity and psychological performance. The results showed that the expression of CCK was positively correlated with abdominal pain, while the expression of SERT was negatively correlated with abdominal pain, anxiety and depression, which revealed that SERT and CCK might be involved in the pathogenesis of IBS-D and may provide a new potential method for identifying a more specific and effective therapeutic target.
- Citation: Qin G, Zhang Y, Yao SK. Serotonin transporter and cholecystokinin in diarrhea-predominant irritable bowel syndrome: Associations with abdominal pain, visceral hypersensitivity and psychological performance. World J Clin Cases 2020; 8(9): 1632-1641
- URL: https://www.wjgnet.com/2307-8960/full/v8/i9/1632.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i9.1632
Irritable bowel syndrome (IBS) is the most common gastrointestinal condition[1] and mainly manifests as bloating, chronic or recurrent abdominal pain and a change in frequency or appearance of stool[2,3]. The prevalence of IBS varies from 1.1% to 45.0% among studies in different countries, and a meta-analysis showed that the pooled worldwide prevalence was 11.2%[4]. It is a multifaceted abnormality in neuroga-stroenterology and regulation of the gut-brain axis[5,6].
The newest diagnostic criterion is Rome IV, which was promulgated in 2016[7]. It defines IBS as recurrent abdominal pain on average at least 1 d/wk in the last 3 mo, associated with two or more of the following criteria: (1) Related to defecation; (2) Associated with a change in frequency of stool; and (3) Associated with a change in form (appearance) of stool. IBS was divided into four subtypes based on the predominant stool form, i.e., constipation-predominant IBS (IBS-C), diarrhea-predominant (IBS-D), mixed stool pattern (IBS-M) and (IBS-U)[4], of which IBS-D refers to > 25% of bowel movements with Bristol stool types 6 or 7 and < 25% of bowel movements with Bristol stool types 1 or 2.
Studies focused on IBS and related health problems have been extensively reported. There was no consistency in the etiology and pathogenesis of IBS, and the various factors involved, such as gastrointestinal dysmotility, visceral hypersensitivity, changes in gut barrier function, intestinal microbial dysbiosis, and brain-gut axis dysfunction[8-10]. Depression and anxiety are two of the possible reasons for functional gastrointestinal disorders[11]. A meta-analysis found that patients with IBS had more severe and frequent depressive symptoms than healthy controls[12], which suggests that there were interactions between IBS and psychological factors.
Enterochromaffin cells (ECs) were the first endocrine cells of the gastrointestinal tract to be chemically distinguished[13]. Previous animal studies have shown that 5-hydroxytryptamine (5-HT) and cholecystokinin (CCK) co-expressing EC cells can be seen in the small intestine and large intestine[14,15]. Previous studies have found that 5-HT can inhibit gastric emptying, trigger and enhance the propulsion of the small and large intestine, promote secretion, aggravate intestinal inflammation and regulate appetite[13,16,17]. The function of the serotonin transporter (SERT) is to rapidly re-uptake 5-HT in the effective site, and to regulate the action time and intensity of 5-HT. 5-HT is also found in the limbic system. The study found that patients with lower SERT expression were more likely to have negative emotions (such as somatization, anxiety, depression). CCK can contract smooth muscle in the gallbladder and gastrointestinal tract, and stimulate gland secretion in the pancreas, liver, small intestine and other organs. A previous study showed that exogenous CCK injection did not affect rectal movement or sensation. However, it can increase abdominal pain in IBS patients during rapid intermittent expansion[18]. It has been found that CCK-8 peptide can enhance colonic motility and induce abdominal pain in IBS patients, but the mechanism is unclear.
Previous studies reported that mucosal 5-HT, SERT immunoreactivity and SERT messenger RNA (SERT-mRNA) were all significantly reduced in IBS-C and IBS-D patients[19,20].
This study focused on the pathogenesis of IBS-D from the aspects of abnormal regulation of the gut-brain axis and mental disorders. We aimed to compare IBS-D patients, who met the diagnostic criteria of Rome IV, to healthy controls with regard to clinical characteristics, SERT and CCK levels in plasma and mucous membrane and then to examine the correlations between SERT, CCK and abdominal pain, visceral hypersensitivity and psychological performance.
A total of 40 IBS-D patients, who were treated at the Department of Gastroenterology of the China-Japan Friendship Hospital from September 2017 to April 2018, and 18 age- and sex-matched healthy controls were recruited for the study. All IBS-D patients were diagnosed based on the Rome IV criteria. Healthy controls consisted of patient spouses, medical examiners and volunteers. The patients and healthy controls were included if they did not take the following drugs within 2 wk of the study: Antispasmodics, analgesics, microecological preparations, antidepressants, anti-inflammatory drugs, antibiotics, other drugs and antacids that can affect gastrointestinal motility. The exclusion criteria were as follows: (1) Gastrointestinal organic diseases, endocrine system diseases, metabolic diseases, connective tissue diseases and other organ diseases; (2) A history of gastrointestinal or abdominal surgery; and (3) A previous or current diagnosis of mental illness.
All subjects were informed of the study details and provided written informed consent. This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of China-Japan Friendship Hospital.
Clinical symptoms and psychosocial assessment: The severity of clinical symptoms was evaluated using the IBS symptom severity scale[3], the VAS pain scale and the frequency course scale. The visceral sensitivity index was used to evaluate visceral sensitivity and gastrointestinal-specific anxiety. Psychiatric symptoms were assessed using the validated Hospital Anxiety and Depression Scale[21]. The IBS-specific quality of life was used to evaluate the quality of life of IBS patients in this disease state.
Visceral sensitivity test: The visceral sensitivity test was performed in the Gastroenterology Kinetic Laboratory by the same investigator. Before the examination, the subjects were given glycerin to eliminate feces. The subjects were placed in the left lateral decubitus position and were asked to relax. After the mass was eliminated by digital rectal examination, the lubricating pressure measuring catheter was slowly inserted into the rectum, with an insertion depth of 15 cm and a balloon placed above the anal margin of 8 cm. After the subject had adapted for 3 min, the examination began. Air was slowly injected (at a rate of 10 mL/5 s) into the balloon by a 100 mL syringe. The subject's feeling after every 10 mL gas injected was recorded. When the subject felt the initial swelling, continuous defecation sensation, pain discomfort or intolerance, the amount of gas injected (in mL) was recorded, which reflected the initial feeling threshold, continuous defecation threshold and maximum tolerance threshold, respectively.
Specimen collection: Participants were not allowed to take gastrointestinal motility drugs, laxatives, antidiarrheal drugs, microecological agents, antidepressants or other drugs that affect gastrointestinal motility one week prior to the examination. The participants underwent colonoscopy after standard bowel preparation with polyethylene glycol electrolyte powder (Fortrans, BEAUFOUR IPSEN Industrie, Dreux, France), and four mucosal pinch biopsies were taken from the rectosigmoid junction. Two specimens were immediately fixed in 10% formalin for at least 72 h, embedded in paraffin and sectioned (4 μm) for routine hematoxylin and eosin staining and immunohistochemistry. The other specimens were immediately immersed in storage reagent (RNA-Be-Locker A; Sangon, Shanghai, China) and stored at -80 °C for quantitative real-time polymerase chain reaction (qRT-PCR) analysis. Venous blood was centrifuged at 2000 r/min for 10 min within 30 min after collection, and the supernatant was taken for analysis.
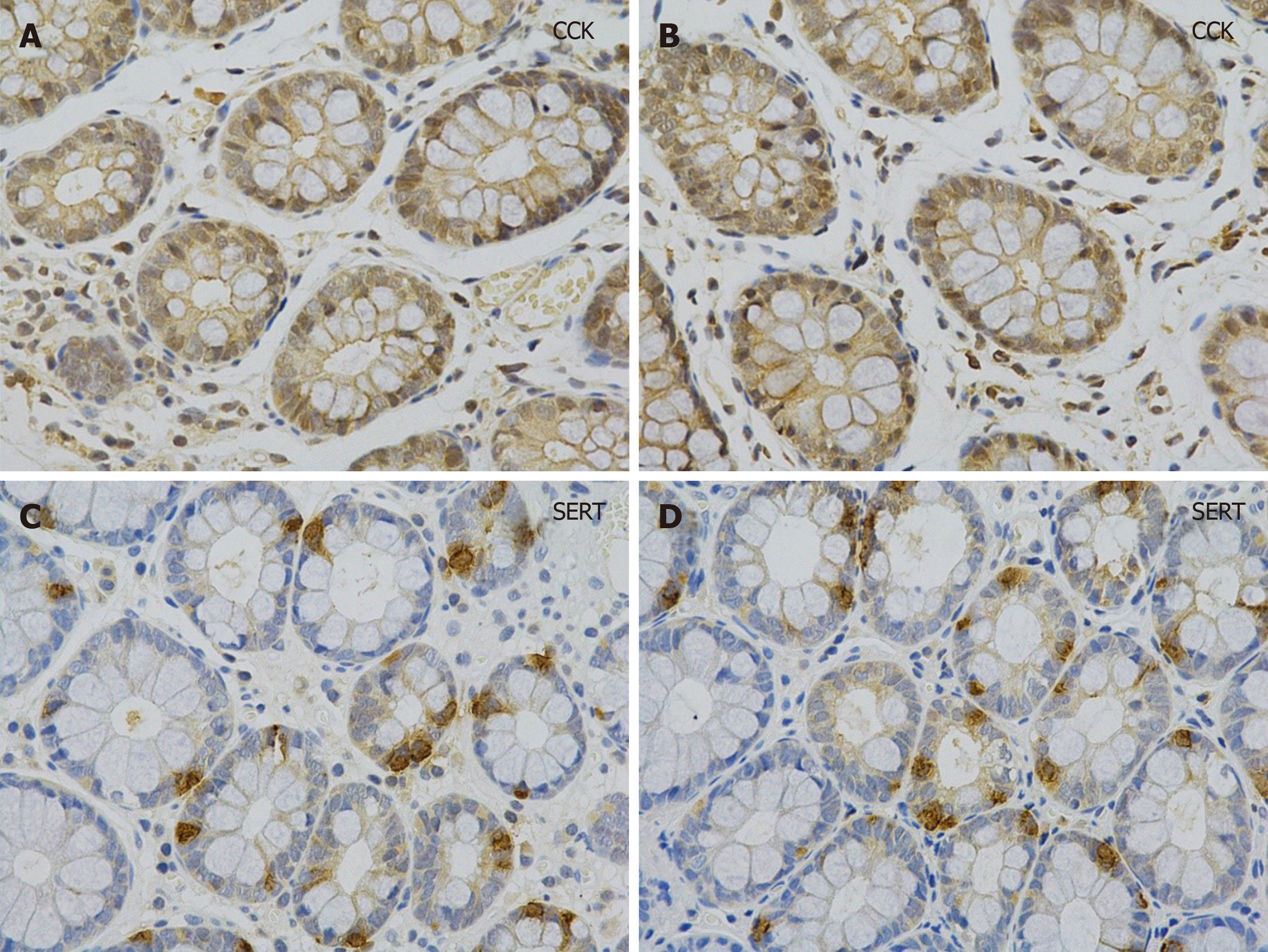
Histology and immunohistochemistry: Paraffin sections were stained with hematoxylin eosin and processed for immunohistochemistry. The latter section was incubated with primary antibody (rabbit polyclonal anti-SERT antibody, 1:400, Affinity Biosciences, United States; rat monoclonal anti-serotonin antibody, 1:100, Santa, United States; rabbit polyclonal anti-CCK antibody, 1:100, Abcam, United Kingdom) overnight at 4 °C after dewaxing, antigen retrieval, endogenous peroxidase inhibition, and nonspecific antigen blocking. After washing with phosphate buffered saline, the sections were incubated with anti-rat and anti-rabbit secondary antibodies (1/200, Zhongshan Golden Bridge, Beijing, China) combined with horseradish peroxidase at room temperature for 1 h and then visualized using diaminobenzidine. Finally, the sections were counterstained with hematoxylin and observed under a light microscope to examine the average optical density (OD value) of positive expression of the stain.
Quantitative real-time PCR detection: The expression of CCK and SERT was analyzed by real-time quantitative PCR. Total RNA in the tissue was extracted by TRIzol Reagent (Invitrogen Life Technologies, Waltham, MA, United States) and reverse transcribed into cDNA. Real-time quantitative PCR was then carried out in a StepOnePlus Real-Time PCR system (Applied Biosystems, Waltham, MA, United States) using the FastStart Universal SYBR Green Master Rox Kit (Roche, Shanghai, China). Finally, the mRNA of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal reference. The primers were designed and synthesized by Beijing Aoke Biotechnology Co., Ltd. The upstream region of SERT was 5'-CAGCGTGTGAAGATGGAGAAG-3', and the downstream region of SERT was 5'-TGGGATAGAGTGCCGTGTGT-3'. The upstream region of CCK was 5'-CAGAGGAGGCAGAATAAGAA-3', and the downstream region of CCK was 5'-CAGGAGTCACAGATGAAGAA-3'. The upstream housekeeping gene GAPDH (internal reference) was 5'-GGAAGCTTGTCATCAATGGAAATC-3', and the downstream housekeeping gene GAPDH was 5'-TGATGACCCTTTTGGCTCCC-3'.
The above were used for quantitative real-time PCR. The total reaction volume was 10 μL, including 5 μL of SYBR master mix (Bio-Rad, United States), 0.2 μL of upstream and downstream primers, 1 μL of template cDNA and 3.6 μL of ddH2O. The reaction conditions consisted of 5 min at 95 °C, 10 s at 94 °C, 10 s at 59 °C, 10 s at 72 °C for 40 cycles, and the temperature change rate was 20 °C/s, followed by 10 s at 95 °C, 15 s at 65 °C and then increased to 95 °C/10 s at a rate of 0.1 °C/s.
The fluorescence signal was continuously monitored for dissolution curve analysis, and GAPDH was used as an internal reference for relative quantitative analysis. The 2-ΔCt method was used to determine the expression level of the SERT gene relative to the internal reference (ΔCt = Ct target gene - Ct internal reference).
Plasma SERT and CCK levels: The levels of SERT and CCK in plasma were determined by double antibody sandwich ELISA (SERT/CCK kit).
Data were analyzed using SPSS version 24.0 (SPSS Inc, Chicago, IL, United States), and the significance level was set at P < 0.05 (two-tailed). The Shapiro-Wilk test was used to test the normal distribution of continuous variables. Comparisons between IBS patients and healthy controls regarding sociodemographic and clinical characteristics were examined using the independent sample t-test, Mann-Whitney U test, and χ2 test as appropriate. The correlations between continuous variables were examined using Pearson correlation analyses if normally distributed; otherwise, Spearman correlation analyses were conducted.
The 40 IBS-D patients had a mean age of 44.50 ± 9.27 years, and 75% (n = 30) of these patients were male. The mean age of the 18 healthy controls was 42.33 ± 12.81 years, and 72.2% (n = 13) were male. The comparisons between IBS-D patients and healthy controls with regard to clinical characteristics are shown in Table 1. There were significant differences in the visceral sensitivity index (75.78 ± 9.31 vs 53.39 ± 4.49, P < 0.001), initial sensation threshold (31.00 ± 8.41 mL vs 52.22 ± 8.09 mL, P < 0.001), defecating sensation threshold (51.75 ± 13.57 mL vs 89.44 ± 8.73 mL, P < 0.001) and maximum tolerable threshold (97.25 ± 23.64 mL vs 171.11 ± 20.83 mL, P < 0.001) between patients and healthy controls. Anxiety (7.78 ± 2.62 vs 2.89 ± 1.02, P < 0.001) and depressive (6.38 ± 2.43 vs 2.06 ± 0.73, P < 0.001) symptoms were also significantly different between IBS-D patients and healthy controls.
| IBS-D patients (n = 40) | HC (n = 18) | P value | |
| Age (yr) | 44.50 ± 9.27 | 42.33 ± 12.81 | 0.47 |
| Male | 30 (75%) | 13 (72.2%) | 0.83 |
| BMI (kg/m2) | 23.95 ± 4.06 | 22.88 ± 2.86 | 0.33 |
| IBS-SSS | 273.25 ± 42.27 | NA | NA |
| VSI | 75.78 ± 9.31 | 53.39 ± 4.49 | < 0.001 |
| IBS-QOL | 48.85 ± 9.79 | NA | NA |
| HADS(A) | 7.78 ± 2.62 | 2.89 ± 1.02 | < 0.001 |
| HADS(D) | 6.38 ± 2.43 | 2.06 ± 0.73 | < 0.001 |
| Visceral sensitivity | |||
| Initial sensation threshold (mL) | 31.00 ± 8.41 | 52.22 ± 8.09 | < 0.001 |
| Defecating sensation threshold (mL) | 51.75 ± 13.57 | 89.44 ± 8.73 | < 0.001 |
| Maximum tolerable threshold (mL) | 97.25 ± 23.64 | 171.11 ± 20.83 | < 0.001 |
| Severity of abdominal pain | |||
| Pain ruler | 2.91 ± 1.63 | NA | NA |
| Pain symptom | 1.35 ± 0.74 | NA | NA |
| Pain frequency | 1.83 ± 1.28 | NA | NA |
The levels of SERT and CCK in plasma were determined by ELISA. CCK and SERT-mRNA expression in colonic mucosa was quantified by qRT-PCR. Immunohisto-chemistry showed that the cytoplasm of cholecystokinin immunoreactive (CCK-IR) cells contained brown granules with a clear background (Figure 1). SERT-positive products were brownish yellow and observed on the membrane (Figure 1). The levels of CCK in the mucosal membrane and plasma were significantly higher in IBS-D patients than in healthy controls, and SERT showed the opposite trend (Table 2).
| IBS-D patients (n = 40) | HC (n = 18) | P value | |
| Plasma | |||
| CCK | 330.88 ± 61.08 | 208.63 ± 30.65 | < 0.001 |
| SERT | 14.26 ± 2.94 | 21.15 ± 2.09 | < 0.001 |
| Mucous membrane (RNA) | |||
| CCK | 2.29 ± 0.30 | 1.66 ± 0.17 | < 0.001 |
| SERT | 1.90 ± 0.51 | 3.03 ± 0.23 | < 0.001 |
| Immunohistochemical OA | |||
| CCK | 0.044 ± 0.007 | 0.028 ± 0.003 | < 0.001 |
| SERT | 0.005 ± 0.001 | 0.010 ± 0.001 | < 0.001 |
Spearman correlations between key continuous variables in IBS-D patients are shown in Table 3, Table 4 and Table 5. In IBS-D patients, CCK expression was positively correlated with the severity of abdominal pain. Furthermore, the level of SERT was negatively correlated with the severity of abdominal pain and visceral sensitivity (Table 3, Table 4 and Table 5).
| SSS | VSI | |
| Initial sensation threshold | -0.64 | -0.47 |
| Defecating sensation threshold | -0.64 | -0.47 |
| Maximum tolerable threshold | -0.64 | -0.45 |
| Pain ruler | Pain symptom | Pain frequency | |
| Plasma CCK | 0.97 | 0.94 | 0.95 |
| Mucous membrane CCK | 0.96 | 0.93 | 0.94 |
| OA CCK | 0.97 | 0.93 | 0.95 |
| HADS(A) | HADS(D) | HAMA | HAMD | Pain ruler | Pain symptom | Pain frequency | |
| Plasma SERT | -0.97 | -0.97 | -0.95 | -0.92 | -0.94 | -0.91 | -0.92 |
| Mucous membrane SERT | -0.98 | -0.99 | -0.97 | -0.94 | -0.96 | -0.93 | -0.95 |
| OA SERT | -0.97 | -0.97 | -0.93 | -0.91 | -0.93 | -0.91 | -0.90 |
In this case-control study, we aimed to evaluate psychological and clinical characteristics and visceral sensitivity in IBS-D patients and determine the roles of SERT and CCK in IBS-D patients.
Depressive and anxiety symptoms were more likely to be experienced by IBS-D patients compared to healthy controls, which agreed with previous studies[22]. Mental disorders, such as depression, anxiety and sleep disorders, are commonly reported in gastrointestinal disorders[23,24]. For example, one cohort study showed that anxiety increased the hazard for postinfectious IBS, and the study also indicated that the mechanism was partly due to the enhancement of susceptibility to develop infectious gastroenteritis[25]. Several studies have demonstrated the effectiveness of psychological treatments on lessening and relieving bowel symptoms in IBS patients, such as cognitive behavioral therapy, mindfulness-based therapies and hypnosis[26-29]. Therefore, psychotherapy should be simultaneously included in clinically IBS-specific treatment to improve IBS patients’ conditions in a timely manner.
Higher visceral sensitivity index, lower initial sensation threshold, lower defecating sensation threshold and lower maximum tolerable threshold were found in IBS-D patients compared with healthy controls. There have been inconsistent reports on the association between visceral sensitivity and functional gastrointestinal (GI) disorders[30]. Some previous studies showed that there were no relationships between hypersensitivity and GI disorder symptoms[31,32], which likely explain our results. In contrast, a large number of studies have identified that IBS patients have higher visceral sensitivity than controls[33] and visceral hypersensitivity is associated with the severity of GI symptoms[30]. The existing discrepancies could be partly due to the various patient-related or research-related factors, such as selection bias in patients or healthy controls and different assessment tools[30].
Mucosa and plasma CCK were significantly higher in IBS patients than in controls. Abdominal pain was reported as a dominant symptom in IBS patients[30], and we identified positive correlations between pain rulers, pain symptoms, pain frequency and mucosa and plasma CCK. Simultaneously, we observed that mucosa and plasma SERT were significantly lower in the IBS-D patients. There were negative correlations between abdominal pain and mucosa and plasma SERT. Negative correlations between psychological performance and mucosa and plasma SERT were also observed.
This study compared the clinical characteristics and biomarker levels between IBS-D patients and healthy controls and the correlation between patients’ GI severity level and biomarker levels. There are some limitations in our study. First, only IBS-D patients were included, and the selection bias could limit the generalization to other types of IBS patients. Second, only patients from one hospital were recruited. Third, the sample size was small. In addition, the disadvantages of a cross-sectional study design lead to the impossibility of identifying causality.
In conclusion, IBS patients had poorer mental health status and visceral hypersensitivity than healthy controls but higher mucous membrane CCK and lower SERT levels. Psychotherapy should be simultaneously included in clinically IBS-specific treatment to improve IBS patients’ conditions in a timely manner, and further large-scale perspective studies are needed to explore the correlation between patients’ GI severity level and biomarker levels.
Diarrhea-predominant irritable bowel syndrome (IBS-D) patients have psychosomatic disorders and visceral hypersensitivity. Preclinical studies have shown that the expression of serotonin transporter (SERT) in the gut can affect the mental and psychological symptoms of IBS patients. Patients with lower expression of SERT are more likely to have negative emotions (somatization, anxiety, depression, hostility). An injection of cholecystokinin (CCK) can enhance and induce colonic motility, resulting in abdominal pain. Few studies have reported the relationship between mucous SERT, CCK levels and symptom severity, anxiety, depression and visceral hypersensitivity in patients. It has been suggested that SERT and CCK may be involved in the pathogenesis of IBS-D.
The present research includes three aspects: (1) General demographic characteristics, symptoms, psychological factors and visceral sensitivity; (2) The expression of SERT and CCK in colonic mucosa of IBS-D patients; and (3) Correlations between CCK, SERT and other parameters. Similar to preclinical studies in IBS-D patients, SERT and CCK may be involved in the pathogenesis of IBS-D, which provides a new potential method for identifying a more specific and effective therapeutic target.
The purpose of this study was to determine the expression of SERT and CCK in colonic mucosa of IBS-D patients, and analyze the relationship between SERT, CCK and general demographic characteristics, symptoms, psychological factors and visceral hypersensitivity.
The participants were evaluated by questionnaires (IBS symptom severity scale, visceral sensitivity index, hospital anxiety and Depression Scale) to obtain clinical and psychological characteristics, and underwent colonoscopy and mucosal biopsies. Visceral sensitivity was detected by a high-resolution manometric system, and the expression of SERT and CCK were detected by immunohistochemistry. Mucosal SERT and CCK mRNA levels were measured by real-time quantitative PCR. These parameters were statistically analyzed using SPSS version 24.0.
The results showed that the anxiety and depression symptoms and visceral sensitivity in IBS-D patients were significantly increased. The levels of CCK in the mucosal membrane and plasma were significantly higher in IBS-D patients than in healthy controls, and SERT showed the opposite trend. The expression of CCK was positively correlated with the severity of abdominal pain, and the level of SERT was negatively correlated with the severity of abdominal pain and visceral sensitivity.
IBS-D patients had psychosomatic disorders and visceral hypersensitivity. SERT and CCK might be involved in the pathogenesis of IBS-D by regulating the brain-gut axis and affecting visceral sensitivity. This provides a new potential method for identifying a more specific and effective therapeutic target.
Our study determined the role of SERT and CCK in the pathogenesis of IBS-D. There are some limitations in our study. First, only IBS-D patients were included, and selection bias could limit the generalization to other types of IBS patients. Second, only patients from one hospital were recruited. Third, the sample size was small. According to the existing research results, no causal inferences can be made. Therefore, more carefully designed clinical studies are needed.
We thank Dr. Du S and Dr. Zhang M for enrollment of participants, and Dr. Gao C, Dr. Liu J and Dr. Liu F for assistance with the colonoscopy tests.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Birk JW, El Amrousy D, Rodrigo L S-Editor: Wang JL L-Editor: Webster JR E-Editor: Qi LL
| 1. | Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313:949-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 740] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 2. | Gralnek IM, Hays RD, Kilbourne A, Naliboff B, Mayer EA. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119:654-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 532] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 3. | Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 1221] [Article Influence: 43.6] [Reference Citation Analysis (1)] |
| 4. | Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712-721.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1415] [Article Influence: 108.8] [Reference Citation Analysis (2)] |
| 5. | Stanghellini V. Functional Dyspepsia and Irritable Bowel Syndrome: Beyond Rome IV. Dig Dis. 2017;35 Suppl 1:14-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Stanghellini V, Chan FK, Hasler WL, Malagelada JR, Suzuki H, Tack J, Talley NJ. Gastroduodenal Disorders. Gastroenterology. 2016;150:1380-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 973] [Article Influence: 108.1] [Reference Citation Analysis (0)] |
| 7. | Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology. 2016;150:1393–1407.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1781] [Cited by in RCA: 1890] [Article Influence: 210.0] [Reference Citation Analysis (3)] |
| 8. | Ohman L, Simrén M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol. 2010;7:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 443] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 9. | Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G775-G785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 284] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 10. | Fukudo S, Kanazawa M. Gene, environment, and brain-gut interactions in irritable bowel syndrome. J Gastroenterol Hepatol. 2011;26 Suppl 3:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Levy RL, Olden KW, Naliboff BD, Bradley LA, Francisconi C, Drossman DA, Creed F. Psychosocial aspects of the functional gastrointestinal disorders. Gastroenterology. 2006;130:1447-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 342] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 12. | Zhang QE, Wang F, Qin G, Zheng W, Ng CH, Ungvari GS, Yuan Z, Mei S, Wang G, Xiang YT. Depressive symptoms in patients with irritable bowel syndrome: a meta-analysis of comparative studies. Int J Biol Sci. 2018;14:1504-1512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Diwakarla S, Fothergill LJ, Fakhry J, Callaghan B, Furness JB. Heterogeneity of enterochromaffin cells within the gastrointestinal tract. Neurogastroenterol Motil. 2017;29:e13101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Cho HJ, Callaghan B, Bron R, Bravo DM, Furness JB. Identification of enteroendocrine cells that express TRPA1 channels in the mouse intestine. Cell Tissue Res. 2014;356:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 15. | Fakhry J, Wang J, Martins P, Fothergill LJ, Hunne B, Prieur P, Shulkes A, Rehfeld JF, Callaghan B, Furness JB. Distribution and characterisation of CCK containing enteroendocrine cells of the mouse small and large intestine. Cell Tissue Res. 2017;369:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. 2013;20:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 453] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 17. | Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 799] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 18. | van der Schaar PJ, van Hoboken E, Ludidi S, Masclee AA. Effect of cholecystokinin on rectal motor and sensory function in patients with irritable bowel syndrome and healthy controls. Colorectal Dis. 2013;15:e29-e34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, Moses PL. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 570] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 20. | Foley S, Garsed K, Singh G, Duroudier NP, Swan C, Hall IP, Zaitoun A, Bennett A, Marsden C, Holmes G, Walls A, Spiller RC. Impaired uptake of serotonin by platelets from patients with irritable bowel syndrome correlates with duodenal immune activation. Gastroenterology. 2011;140:1434-43.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6329] [Cited by in RCA: 7224] [Article Influence: 314.1] [Reference Citation Analysis (0)] |
| 22. | Posserud I, Syrous A, Lindström L, Tack J, Abrahamsson H, Simrén M. Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology. 2007;133:1113-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 23. | Jones MP, Tack J, Van Oudenhove L, Walker MM, Holtmann G, Koloski NA, Talley NJ. Mood and Anxiety Disorders Precede Development of Functional Gastrointestinal Disorders in Patients but Not in the Population. Clin Gastroenterol Hepatol. 2017;15:1014-1020.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 24. | Bouchoucha M, Mary F, Bon C, Bejou B, Airinei G, Benamouzig R. Sleep quality and functional gastrointestinal disorders. A psychological issue. J Dig Dis. 2018;19:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Wouters MM, Van Wanrooy S, Nguyen A, Dooley J, Aguilera-Lizarraga J, Van Brabant W, Garcia-Perez JE, Van Oudenhove L, Van Ranst M, Verhaegen J, Liston A, Boeckxstaens G. Psychological comorbidity increases the risk for postinfectious IBS partly by enhanced susceptibility to develop infectious gastroenteritis. Gut. 2016;65:1279-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Surdea-Blaga T, Baban A, Nedelcu L, Dumitrascu DL. Psychological Interventions for Irritable Bowel Syndrome. J Gastrointestin Liver Dis. 2016;25:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Hanlon I, Hewitt C, Bell K, Phillips A, Mikocka-Walus A. Systematic review with meta-analysis: online psychological interventions for mental and physical health outcomes in gastrointestinal disorders including irritable bowel syndrome and inflammatory bowel disease. Aliment Pharmacol Ther. 2018;48:244-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 28. | Ballou S, Keefer L. Psychological Interventions for Irritable Bowel Syndrome and Inflammatory Bowel Diseases. Clin Transl Gastroenterol. 2017;8:e214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 29. | Kenwright M, McDonald J, Talbot J, Janjua K. Do Symptoms of Irritable Bowel Syndrome Improve when Patients Receive Cognitive Behavioural Therapy for Co-morbid Anxiety Disorders in a Primary Care Psychological Therapy (IAPT) Service? Behav Cogn Psychother. 2017;45:433-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Simrén M, Törnblom H, Palsson OS, van Tilburg MAL, Van Oudenhove L, Tack J, Whitehead WE. Visceral hypersensitivity is associated with GI symptom severity in functional GI disorders: consistent findings from five different patient cohorts. Gut. 2018;67:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 172] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 31. | Sabate JM, Veyrac M, Mion F, Siproudhis L, Ducrotte P, Zerbib F, Grimaud JC, Dapoigny M, Dyard F, Coffin B. Relationship between rectal sensitivity, symptoms intensity and quality of life in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:484-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Boeckxstaens GE, Hirsch DP, Kuiken SD, Heisterkamp SH, Tytgat GN. The proximal stomach and postprandial symptoms in functional dyspeptics. Am J Gastroenterol. 2002;97:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Jarrett ME, Han CJ, Cain KC, Burr RL, Shulman RJ, Barney PG, Naliboff BD, Zia J, Heitkemper MM. Relationships of abdominal pain, reports to visceral and temperature pain sensitivity, conditioned pain modulation, and heart rate variability in irritable bowel syndrome. Neurogastroenterol Motil. 2016;28:1094-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |