Published online May 6, 2020. doi: 10.12998/wjcc.v8.i9.1600
Peer-review started: January 15, 2020
First decision: February 26, 2020
Revised: April 1, 2020
Accepted: April 21, 2020
Article in press: April 21, 2020
Published online: May 6, 2020
Processing time: 105 Days and 23.7 Hours
Acute cardiorenal syndrome type 1 (CRS-1) is defined by a rapid cardiac dysfunction leading to acute kidney injury (AKI). Neutrophil gelatinase-associated lipocalin (NGAL) is expressed on the surface of human neutrophils and epithelial cells, such as renal tubule cells, and its serum (sNGAL) and urinary have been used to predict AKI in different clinical settings.
To characterize CRS-1 in a cohort of patients with acute heart diseases, evaluating the potentiality of sNGAL as an early marker of CRS-1.
We performed a retrospective cohort, multi-centre study. From January 2010 to December 2011, we recruited 202 adult patients admitted to the coronary intensive care unit (CICU) with a diagnosis of acute heart failure or acute coronary syndrome. We monitored the renal function to evaluate CRS-1 development and measured sNGAL levels within 24 h and after 72 h of CICU admission.
Overall, enrolled patients were hemodynamically stable with a mean arterial pressure of 92 (82-107) mmHg, 55/202 (27.2%) of the patients developed CRS-1, but none of them required dialysis. Neither the NGAL delta value (AUC 0.40, 95%CI: 0.25-0.55) nor the NGAL peak (AUC 0.45, 95%CI: 0.36-0.54) or NGAL cut-off (≥ 140 ng/mL) values were statistically significant between the two groups (CRS-1 vs no-CRS1 patients). The area under the ROC curve for the prediction of CRS-1 was 0.40 (95%CI: 0.25-0.55) for the delta NGAL value and 0.45 (95%CI: 0.36-0.54) for the NGAL peak value. Finally, in multivariate analysis, the risk of developing CRS-1 was correlated with age > 60 years, urea nitrogen at admission and 24 h-urine output (AUC 0.83, SE = 60.5% SP = 93%), while sNGAL was not significantly correlated.
In our population, sNGAL does not predict CRS-1, probably as a consequence of the mild renal injury and the low severity of heart disease. So, these data might suggest that patient selection should be taken into account when considering the utility of NGAL measurement as a biomarker of kidney damage.
Core tip: Renal and cardiac injuries are often interrelated, leading to high-risk clinical conditions. In this retrospective study, we evaluated the onset of acute kidney injury in 202 patients affected by acute heart disease [classified as cardiorenal syndrome-1 (CRS-1)], testing the potentiality of serum neutrophil gelatinase-associated lipocalin as a predictive marker of renal damage. Although the prevalence of CRS-1 in our population (27.2%) was consistent with that found in the literature, serum neutrophil gelatinase-associated lipocalin was not associated with CRS-1 development, probably as a consequence of the mild renal and cardiac injuries present in our study population. These data suggest that patient selection is crucial when considering the use of biomarkers.
- Citation: Ferrari F, Scalzotto E, Esposito P, Samoni S, Mistrorigo F, Rizo Topete LM, De Cal M, Virzì GM, Corradi V, Torregrossa R, Valle R, Bianzina S, Aspromonte N, Floris M, Fontanelli A, Brendolan A, Ronco C. Neutrophil gelatinase-associated lipocalin does not predict acute kidney injury in heart failure. World J Clin Cases 2020; 8(9): 1600-1607
- URL: https://www.wjgnet.com/2307-8960/full/v8/i9/1600.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i9.1600
Cardiac and renal diseases are common and frequently coexist in a complex interplay, leading to significantly increased mortality, morbidity and increased care cost[1]. Syndromes describing the interaction between heart and kidney have been defined and classified. The term "cardiorenal syndrome" (CRS) includes disorders of the heart and kidney, whereby acute or chronic dysfunction in one organ may induce acute or chronic dysfunction in the other[2]. The relevance of the kidneys in heart failure is their regulation of fluid and sodium balance: If fluid volume is retained, the possibility of heart failure increases[3]. The acute dialysis quality initiative working group has proposed a consensus definition for CRS, describing five CRS subtypes. Among them, CRS type 1 (CRS-1) encompasses an acute failure of cardiac function leading to acute kidney injury (AKI)[4].
Patients admitted to the coronary intensive care unit (CICU) typically exhibit complex syndromes with numerous pathways that may affect renal function[5]. The spectrum of acute cardiac events that may contribute to AKI includes acute heart failure (AHF), acute coronary syndrome (ACS) cardiogenic shock and contrast medium injection for invasive procedures[6].
Several biomarkers of kidney injury have shown diagnostic and prognostic value[7]. Neutrophil gelatinase-associated lipocalin (NGAL) was introduced as a biomarker for early detection of AKI[8]. NGAL is a ubiquitous 25 kDa protein covalently bound to gelatinase that is expressed on the surface of human neutrophils and epithelial cells, such as renal tubule cells, and which may be over-expressed in ischemic and toxic kidney injuries. Available data indicate that NGAL could be a useful tool for early diagnosis and prognosis of AKI [in terms of prediction of need of renal replacement therapy (RRT) and in-hospital mortality][9].
Based on this evidence, in this study, we characterized CRS-1 in a population of CICU patients, testing the potential of serum NGAL (sNGAL) as an early biomarker of renal damage.
Our study is a retrospective cohort, multi-centre study conducted in three Italian hospitals.
From January 2010 to December 2011, we recruited 202 adult patients (≥ 18 years) admitted to CICU with a diagnosis of AHF or ACS. The following exclusion criteria were adopted: (1) A CICU stay of less than 24 h; (2) Chronic kidney disease (CKD) patients requiring dialysis treatment (CKD 5D); and (3) Informed consent not obtained. We collected demographic and anthropometric data, previous cardiac and renal history, chronic home therapy, co-morbidity and admission diagnosis in the hospital and the CICU. The following biochemical parameters, routinely assessed in our CICU, were measured: Cell blood count, urea, serum creatinine (sCr), electrolytes, albumin, NT-pro-brain natriuretic peptide (NTpro-BNP) and heart injury enzymes, such as troponin. In addition, we assessed and recorded body hydration (cumulative fluid balance on the first day), cardio-circulatory and echocardiographic parameters [systolic/diastolic blood pressure and heart rate, ejection fraction (EF), ventricular volume and presence of valvulopathy], medications used and presence of any mechanical support organ [e.g., intra-aortic balloon pump (IABP), non-invasive mechanical ventilation (NIMV), invasive mechanical ventilation (IMV) or RRT]. Patients were monitored daily until discharge from the CICU. As it is likely, in our cohort of patients, that AKI occurred before hospital admission, we decided to consider pre-admission sCr as a baseline sCr (bsCr). According to published data, pre-admission sCr was defined as the sCr value measured 90-180 d prior to hospital admission[10]. Diagnoses were determined based on sCr and urinary output according to Kidney Disease: Improving Global Outcomes (KDIGO) criteria[11]. CRS-1 was diagnosed when sCr increased by at least 26.5 μmol/L from bsCr during the CICU stay.
The primary aim of this study was to characterize the CRS-1 in our population, and the secondary aim was to evaluate the accuracy of sNGAL in predicting CRS-1. The investigation conforms to the principles outlined in the Declaration of Helsinki. The protocol was approved by the locally appointed Ethics Committee. Informed consent was obtained according to Italian laws.
Serum NGAL was measured within 24 h of CICU admission and after 72 h. We used a Fluorescence Immunoassay, Triage Meter Analyzer (Biosite Incorporated, San Diego, CA, United States) for NGAL analysis. Serum creatinine was measured using the enzymatic method (IL testTM, Instrumentation® Laboratory SpA, Milano, Italy) on an ILab650 analyser (Instrumentation Laboratory, Werfen Group, Barcelona, Spain). We used sNGAL as a continuous variable as well as a dichotomous variable. For the latter, we utilised several cut-off values, derived from our population using a method reported by Liu et al[12]. All laboratory data were analysed by technicians who were blinded to the clinical data.
We first performed a descriptive analysis: mean and standard deviation, median and 25th and 75th quantiles. As the Shapiro-Wilk test did not demonstrate the normal distribution of continuous variables, we adopted non-parametric analysis. We used the Mann Whitney test or the Kruskal Wallis test to compare the collected data of two groups (CRS-1 group vs no-CRS-1 group) or the Spearman test to analyze the correlation between continuous variables. Categorical variables were tested by the chi-squared test or the Fisher exact test, when appropriate. To identify the predictors of CRS-1, we performed a multivariable logistic regression. The final model was determined using both clinical and statistical criteria to obtain the best model in terms of goodness of fit. In the case of collinearity, the variable with a "stronger" association (based on a forward selection process) was used in multivariable analysis. To calculate the discriminatory power of each model, we estimated the areas under the curve (AUC, 95%CI). We also assessed the calibration of the models by applying the Hosmer-Lemeshow test. A P value of less than 0.05 was considered significant. Analyses were conducted using the statistical software STATA version 12.1 (StataCorp, 2012) and the statistical review of the study was performed by a biomedical statistician (Fiorenza Ferrari, MD, MSC, Consultant Intensive Care Unit, I.R.C.C.S Fondazione Policlinico San Matteo- Pavia, Italy).
We enrolled 202 patients. Table 1 shows the characteristics of the CICU patients at the time of admission. During the CICU stay overall 6 patients (3%) died.
| Patient charateristics | All patients (n = 202) | No-CRS-1 patients (n = 147) (72.8%) | CRS-1 patients (n = 55) (27.2%) | P value |
| Age (yr) | 67 (57-77) | 64 (65-72) | 77 (67-83) | < 0.001 |
| Gender: Male/female | 142/60 | 106/40 | 36/20 | 0.30 |
| Creatinine admission (μmol/L) | 78.3 (66.9-97.7) | 72.2 (65.1-82.7) | 124.1 (102-174.2) | < 0.001 |
| Creatinine peak (μmol/L) | 87.1 (72.2-117) | 77.4 (70.4-89.8) | 142.6 (121.4-1.98) | < 0.001 |
| BUN at admission (μmol/L) | 13.2 (10.3-17.8) | 12.1 (10-14.2) | 21.4 (16-25.7) | < 0.001 |
| Urine output by 24 h (mL) | 1585 (900-2625) | 1700 (1100-2650) | 1200 (700-2300) | 0.0034 |
| Furosemide use ≥ 120 mg/24 h | 79 (39.70) | 63 (43.45) | 16 (29.63) | 0.20 |
| ΔNGAL (ng/dL) | -8 [(-29)-17] | -6 [(-24)-17] | -13 [(-112)-20] | 0.14 |
| NGAL peak (ng/dL) | 147 (81-245.5) | 153.5 (80-256) | 121 (86-188) | 0.31 |
| NGAL cut-off (≥ 140 ng/mL) | 102 (50.50) | 79 (48.30) | 23 (41.82) | 0.20 |
| Troponin I peak (μg/L) | 4.72 (0.36-34.51) | 2.21 (0.25-25.26) | 10.67 (1.52-47.76) | 0.02 |
| NTproBNP peak (pg/mL) | 200 (93.60-559.0) | 203.5 (104.5-570.5) | 180.5 (89.0-475.0) | 0.68 |
| K (mmol/L) | 3.9 (3.7-4.2) | 3.9 (3.7-4.1) | 4.2 (3.8-4.5) | < 0.001 |
| Hb (g/L) | 132 (121-143) | 134 (126-145) | 120 (105-133) | < 0.001 |
| Systolic arterial pressure (mmHg) | 135 (120-151) | 139 (120-151) | 135 (110-150) | 0.18 |
| Dyastolic arterial pressure (mmHg) | 70 (60-80) | 70 (65-80) | 65 (55-75) | 0.01 |
| Mean arterial pressure (mmHg) | 92 (82-107) | 94 (86-105) | 89 (75-100) | 0.04 |
| EF ≤ 40% | 44 (32.35) | 34 (34.69) | 10 (26.32) | 0.35 |
| Severe dilation left ventriculus1, n (%) | 22 (11.06) | 15 (10.34) | 7 (12.96) | 0.82 |
| Catecolamine use, n (%) | 10 (4.95) | 7 (4.76) | 3 (5.45) | 0.8 |
| IABP use, n (%) | 12 (5.94) | 6 (4.08) | 6 (12.76) | 0.07 |
| CICU mortality, n (%) | 6 (3) | 3 (2) | 3 (5) | 0.3 |
According to the above-mentioned criteria, 55/202 (27.2%) patients developed CRS-1. CRS-1 patients were older than no-CRS-1 patients [77 (67-83) years vs 64 (65-72) years, P < 0.001] and male gender was prevalent in both groups, although the sex did not affect the risk of CRS-1 (P = 0.22). Overall, mean arterial pressure (MAP) was 92 (82-107) mmHg, while CRS-1 patients had diastolic and mean arterial pressure lower than no-CRS-1 patients (P = 0.01 and P = 0.04, respectively). The proportion of use of catecholamines or levosimendan does not differ between the two groups, while the use of IABP was more frequent in CRS-1 patients (11.1% vs 4.1%, P = 0.003). No differences were found in patients with EF ≤ 40% or left ventriculus severe dilation between groups[13]. CRS-1 patients achieved higher Troponin I values (P = 0.02) than the no-CRS-1 group, but NT-pro BNP did not differ between the two groups (P = 0.68).
During CICU stay 3 CRS-1 patients (5%) and 3 no-CRS-1 patients (2%, P = 0.3 vs CRS-1) died.
On admission to the CICU, sCr and blood urea nitrogen (BUN) of the CSR-1 group were higher than the no-CRS-1 group [142.6 (121.4-1.98) μmol/L vs 77.4 (70.4-89.8) μmol/L, P < 0.001; and 21.4 (16-25.7) μmol/L vs 12.1 (10-14.2) μmol/L, P < 0.001, respectively]. During the CICU stay, CRS-1 group patients reached a higher sCr peak than the no-CRS-1 patients [142.6 (121.4-174.2) μmol/L vs 77.4 (77-89.8) μmol/L, P < 0.001]. Moreover, the two groups differ in the urine output by 24 h (P = 0.0034), but not in the dose of furosemide (P = 0.20).
However, none of our patients required RRT.
Among our population, 65.85% of the patients underwent coronarography (73% out of them underwent additional interventional procedures). Although it is well-known that the volume of contrast media administered in diagnostic coronarography is lower than in interventional procedures, in our cohort, no volume difference was found between patients who developed CRS-1 [100 (80-190) mL] and no-CRS-1 patients [100 (90-130) mL; P = 0.3, Table 1].
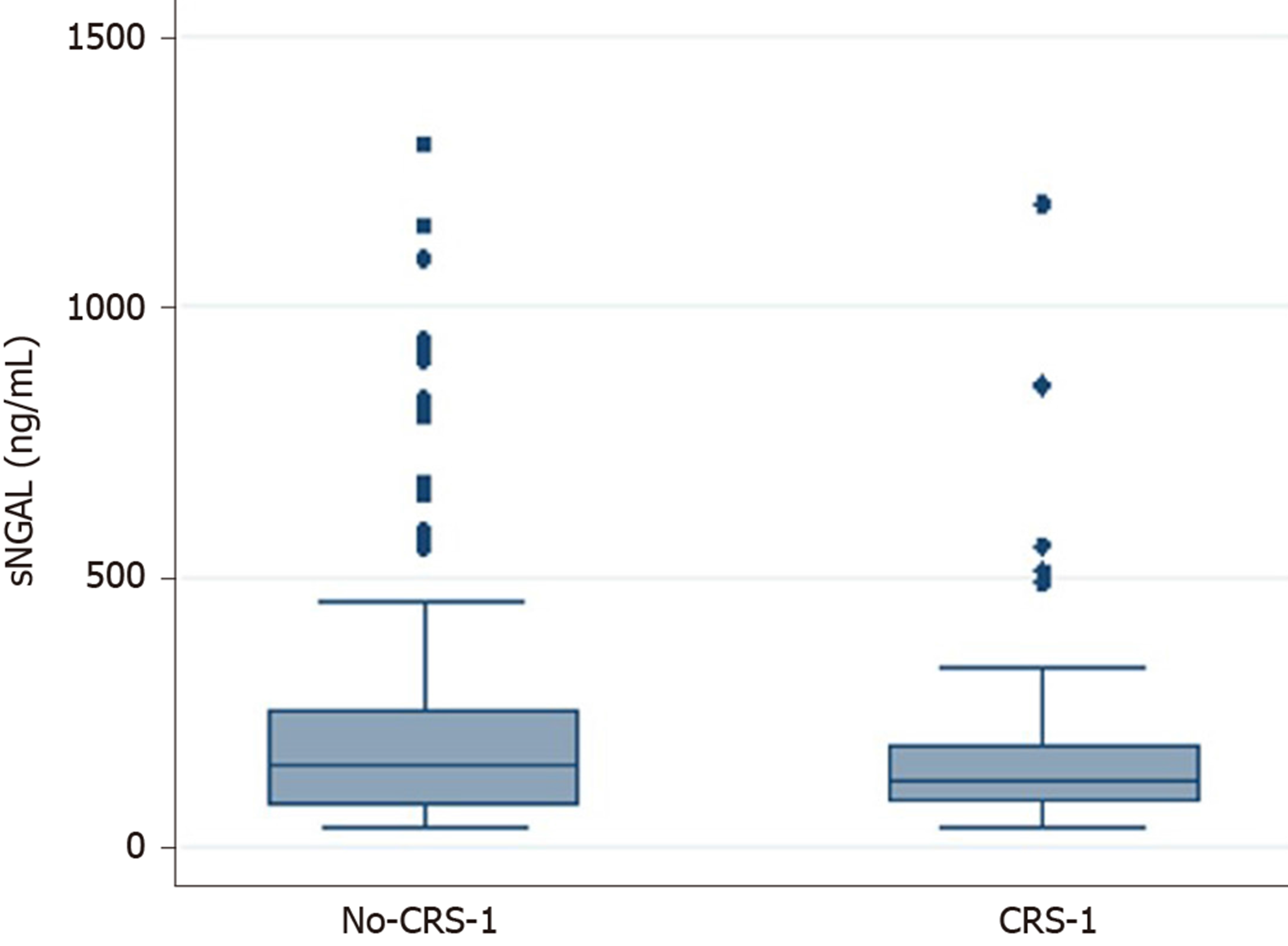
Neither the sNGAL delta value nor the sNGAL peak or sNGAL cut-off (≥ 140 ng/mL)[14] values were statistically significant in the two groups (Table 1, Figure 1). The area under the ROC curve (AUC) for the prediction of CRS-1 was 0.40 (95%CI: 0.25-0.55) for the delta sNGAL value and 0.45 (95%CI: 0.36-0.54) for the NGAL peak. Our population had a cut-off value of 102.2 pg/mL (SE = 69%, SP = 34%, AUC: 0.51).
We found a weak correlation between NT-pro BNP values at the time of admission (r = 0.28, P < 0.001), Troponin I at the time of admission (r = -0.19, P = 0.01), and peak NGAL.
In logistic regression analysis, the best model for predicting the risk of developing CRS-1 includes age > 60 years, BUN at admission, and 24 h-urine output (AUC: 0.83, SE = 60.5% SP = 93%, Akaike's information criterion-AIC-120.9) (Table 2). sNGAL (as a continuous variable, peak value, cut-off or delta value) did not improve the goodness of fit of our model.
| OR | P value | 95%CI | |
| Age > 60 yr | 5.21 | 0.003 | 1.74-15.54 |
| BUN at admission | 1.06 | 0.000 | 1.03-1.08 |
| Diuresis by 24 h | 1.01 | 0.027 | 1.00-1.03 |
In this study, we evaluated the clinical presentation of CRS-1 in a population of CICU patients, focusing on the role of sNGAL as a predictive biomarker. Firstly, CRS-1 prevalence in our population was similar to published data in which CRS-1 occurred in approximately 25% of patients hospitalized for acute decompensated heart failure[5]. We based identification of CRS-1 on the pre-admission bsCR[10] instead of sCr at admission, since, at the time of CICU entry, sCr levels might already be affected by impaired heart performance. Moreover, we excluded CKD patients; therefore, in our population, CRS-1 defined an AKI event as one exclusively related to acute heart failure. Notably, when studying AKI prevalence it should be considered that mortality can represent a competing risk that masks the actual AKI prevalence, i.e., patients may die before the occurrence of AKI[15]. This is not the case of our patient population that presented a low mortality rate, which did not significantly impact on AKI prevalence estimation.
Recent trials have demonstrated that approximately 40% of patients admitted with heart failure diagnoses showed an increased level of sCr, in a setting where the significance of AKI in CKD remains to be established[14]. However, sCr cannot identify the exact mechanism of CRS-1 development, which is reflected by a possibly temporary and benign transient reduction in renal filtration (perhaps due to an impaired renal flow). On the other hand, acute renal tubular damage might lead to a loss of functional nephrons, in which case, the use of NGAL would be justified[16].
NGAL is an acute phase molecule that is released from the immune cells and reaches high serum levels under inflammatory conditions[17]. Several studies have demonstrated that serum NGAL is associated with AKI in different ways, even if its predictive value is influenced by several factors, including baseline renal function, the severity of AKI and age[18,19]. In animal models, recombinant NGAL acts as a trigger of an acute inflammatory response or as a cardiorenal biomarker, modifying cardiac functional parameters[20]. These findings agree with those reported in human myocarditis, whereby NGAL was strongly induced in affected cardiomyocytes, vascular wall cells, fibroblasts and neutrophils. Therefore, NGAL also appears, with some limitations, to be a putative candidate as a predictive marker of renal dysfunction in the context of CRS-1.
Nevertheless, we found that in our population, sNGAL levels (even when considering peak value, delta and cut-off ≥ 140 ng/dL) were not associated with a decline in renal function defined by creatinine changes. Multivariate analysis confirmed the lack of association between sNGAL levels and CRS-1 onset, finding that the best model to describe the risk of developing CRS-1 included only age, BUN and urine output within 24 h. In contrast, we found a correlation between NT-proBNP, troponin I and sNGAL values, which may underline the correlation between cellular damage in the heart and the kidney, likely suggesting that in acute cardiac patients, NGAL levels may not be appropriate as a stand-alone test[13,21,22]. It should be noted that our population appeared to be affected by only mild forms of cardiac and renal damage. Indeed, the mortality rate was low and no patients required non-invasive or invasive ventilation, while few of them needed inotropic drugs (8/202 dopamine, 2/202 levosimendan and 2/202 epinephrine) and in only 5.94 % of the cases, IABP was used (12.76% in CRS-1 vs 4.08% in the no-CRS-1 group, P = 0.07). Furthermore, no patients reached KDIGO stage 3 AKI, requiring RRT.
In patients presenting with CRS-1 we found that EF, left ventricular dilation or the extent of coronaropathy (in terms of the number of damaged coronaries) were not statistically different than the no-CRS-1 group. Therefore, it is possible that the low severity of heart and renal diseases in our population reduced the potential of sNGAL as a biomarker, while the low sNGAL levels might reflect a minor inflammatory response that explains the low prevalence of CRS-1.
The limitations of our study include a lack of consideration of the cytokine pattern or the oxidative stress mechanism, which might have triggered the acute kidney injury as well as heart failure. Moreover, we did not measure urinary NGAL that could be less impacted by extra-renal factors and more accurate in predicting heart failure AKI, even though a recent meta-analysis including patients from 19 studies showed comparable performance for serum and urinary NGAL[23-25]. Finally, two recent papers have demonstrated that an evaluation of the temporal trend of AKI is essential[26,27], while in our study, renal function was not monitored after hospital discharge. In conclusion, we found that in our population, sNGAL (using either ≥ 140 pg/mL, admission value, sNGAL peak levels after three days of CICU stay or sNGAL difference between peak and nadir) did not predict CRS-1. These data underline that further studies are warranted to define the profile of biomarkers for predicting AKI, considering that patient selection and characterization could significantly influence the performance of the different biomolecules.
Cardiac and renal diseases frequently coexist, leading to significantly increased mortality, morbidity and increased care cost. Syndromes describing the interaction between heart and kidney have been defined as "cardiorenal syndrome" (CRS). In particular, acute cardiorenal syndrome type 1 (CRS-1) is defined by a rapid cardiac dysfunction leading to acute kidney injury (AKI). Several biomarkers of kidney injury have shown diagnostic and prognostic value. Among them, neutrophil gelatinase-associated lipocalin (NGAL) has shown promising perspectives.
Due to the high potential clinical impact, the definition and characterization of validated biomarkers may be of help in the prevention and treatment of AKI and CRS-1.
The primary aim of this study was to characterize CRS-1 in a cohort of patients with acute heart diseases, evaluating the potentiality of sNGAL as an early marker of CRS-1.
We performed a retrospective cohort, multicenter study. From January 2010 to December 2011, we evaluated patients admitted to the coronary intensive care unit (CICU) with a diagnosis of acute heart failure or acute coronary syndrome. We monitored the renal function to evaluate CRS-1 development and measured sNGAL levels within 24 h and after 72 h of CICU admission.
202 patients affected by acute heart disease were enrolled. Out of them, 55 patients (27.2%) developed CRS-1, but none required dialysis. Neither the NGAL delta value (AUC 0.40, 95%CI 0.25-0.55) nor the NGAL peak (AUC 0.45, 95%CI 0.36-0.54) or NGAL cut-off (≥ 140 ng/mL) values were statistically significant between two groups of patients (CRS-1 vs no-CRS1). The area under the ROC curve for the prediction of CRS-1 was 0.40 (95%CI 0.25-0.55) for the delta NGAL value and 0.45 (95%CI: 0.36-0.54) for the NGAL peak value. In multivariate analysis, the risk of developing CRS-1 was correlated with age > 60 years, urea nitrogen at admission and 24 h-urine output (AUC: 0.83, SE = 60.5% SP = 93%), while sNGAL was not significantly correlated.
We found that in our population, although the prevalence of CRS-1 (27.2%) was consistent with that found in the literature, sNGAL levels were not associated with a decline in renal function defined by creatinine changes. This finding could be, at least in part, a consequence of the mild renal and cardiac injuries present in our study population, which might have reduced the potential of sNGAL as a biomarker.
Our data underline that further studies are warranted to define the profile of biomarkers for predicting AKI, considering that patient selection and characterization could significantly influence the performance of the different biomolecules.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang ZH S-Editor: Gong ZM L-Editor: A E-Editor: Liu JH
| 1. | Heywood JT. The cardiorenal syndrome: lessons from the ADHERE database and treatment options. Heart Fail Rev. 2004;9:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bellomo R, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, Hillege H, House AA, Katz N, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P; Acute Dialysis Quality Initiative (ADQI) consensus group. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010;31:703-711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 592] [Cited by in RCA: 657] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 3. | Brisco MA, Testani JM. Novel renal biomarkers to assess cardiorenal syndrome. Curr Heart Fail Rep. 2014;11:485-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Ronco C, Ronco F. Cardio-renal syndromes: a systematic approach for consensus definition and classification. Heart Fail Rev. 2012;17:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Bagshaw SM, Cruz DN, Aspromonte N, Daliento L, Ronco F, Sheinfeld G, Anker SD, Anand I, Bellomo R, Berl T, Bobek I, Davenport A, Haapio M, Hillege H, House A, Katz N, Maisel A, Mankad S, McCullough P, Mebazaa A, Palazzuoli A, Ponikowski P, Shaw A, Soni S, Vescovo G, Zamperetti N, Zanco P, Ronco C; Acute Dialysis Quality Initiative Consensus Group. Epidemiology of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant. 2010;25:1406-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 6. | McCullough PA, Stacul F, Becker CR, Adam A, Lameire N, Tumlin JA, Davidson CJ; CIN Consensus Working Panel. Contrast-Induced Nephropathy (CIN) Consensus Working Panel: executive summary. Rev Cardiovasc Med. 2006;7:177-197. [PubMed] |
| 7. | Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231-1238. [PubMed] |
| 8. | Chen TH, Chang CH, Lin CY, Jenq CC, Chang MY, Tian YC, Hung CC, Fang JT, Yang CW, Wen MS, Lin FC, Chen YC. Acute kidney injury biomarkers for patients in a coronary care unit: a prospective cohort study. PLoS One. 2012;7:e32328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Ronco C. NGAL: an emerging biomarker of acute kidney injury. Int J Artif Organs. 2008;31:199-200. [PubMed] |
| 10. | Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, Bittleman D, Cruz D, Endre Z, Fitzgerald RL, Forni L, Kane-Gill SL, Hoste E, Koyner J, Liu KD, Macedo E, Mehta R, Murray P, Nadim M, Ostermann M, Palevsky PM, Pannu N, Rosner M, Wald R, Zarbock A, Ronco C, Kellum JA, Acute Disease Quality Initiative Workgroup 16. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13:241-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 1058] [Article Influence: 132.3] [Reference Citation Analysis (0)] |
| 11. | KDIGO. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1-138. [DOI] [Full Text] |
| 12. | Liu KD, Yang W, Go AS, Anderson AH, Feldman HI, Fischer MJ, He J, Kallem RR, Kusek JW, Master SR, Miller ER, Rosas SE, Steigerwalt S, Tao K, Weir MR, Hsu CY; CRIC Study Investigators. Urine neutrophil gelatinase-associated lipocalin and risk of cardiovascular disease and death in CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2015;65:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Forman DE, Butler J, Wang Y, Abraham WT, O'Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB, Krumholz HM. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61-67. [PubMed] |
| 14. | Maisel AS, Mueller C, Fitzgerald R, Brikhan R, Hiestand BC, Iqbal N, Clopton P, van Veldhuisen DJ. Prognostic utility of plasma neutrophil gelatinase-associated lipocalin in patients with acute heart failure: the NGAL EvaLuation Along with B-type NaTriuretic Peptide in acutely decompensated heart failure (GALLANT) trial. Eur J Heart Fail. 2011;13:846-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 201] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 15. | Zhang Z. Survival analysis in the presence of competing risks. Ann Transl Med. 2017;5:47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 16. | Palazzuoli A, McCullough PA, Ronco C, Nuti R. Kidney disease in heart failure: the importance of novel biomarkers for type 1 cardio-renal syndrome detection. Intern Emerg Med. 2015;10:543-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Xu G, Ahn J, Chang S, Eguchi M, Ogier A, Han S, Park Y, Shim C, Jang Y, Yang B, Xu A, Wang Y, Sweeney G. Lipocalin-2 induces cardiomyocyte apoptosis by increasing intracellular iron accumulation. J Biol Chem. 2012;287:4808-4817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 18. | Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, van Veldhuisen DJ, Hillege HL. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail. 2007;13:599-608. [PubMed] |
| 19. | McIlroy DR, Wagener G, Lee HT. Neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery: the effect of baseline renal function on diagnostic performance. Clin J Am Soc Nephrol. 2010;5:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 20. | Latouche C, El Moghrabi S, Messaoudi S, Nguyen Dinh Cat A, Hernandez-Diaz I, Alvarez de la Rosa D, Perret C, López Andrés N, Rossignol P, Zannad F, Farman N, Jaisser F. Neutrophil gelatinase-associated lipocalin is a novel mineralocorticoid target in the cardiovascular system. Hypertension. 2012;59:966-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Lameire NH, Vanholder RC, Van Biesen WA. How to use biomarkers efficiently in acute kidney injury. Kidney Int. 2011;79:1047-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Cheng H, Chen YP. Clinical prediction scores for type 1 cardiorenal syndrome derived and validated in chinese cohorts. Cardiorenal Med. 2015;5:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Devarajan P. Neutrophil gelatinase-associated lipocalin: a promising biomarker for human acute kidney injury. Biomark Med. 2010;4:265-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 228] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 24. | Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A; NGAL Meta-analysis Investigator Group. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 860] [Cited by in RCA: 912] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 25. | Bruetto RG, Rodrigues FB, Torres US, Otaviano AP, Zanetta DM, Burdmann EA. Renal function at hospital admission and mortality due to acute kidney injury after myocardial infarction. PLoS One. 2012;7:e35496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 546] [Reference Citation Analysis (0)] |
| 26. | Hsu RK, McCulloch CE, Heung M, Saran R, Shahinian VB, Pavkov ME, Burrows NR, Powe NR, Hsu CY; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team. Exploring Potential Reasons for the Temporal Trend in Dialysis-Requiring AKI in the United States. Clin J Am Soc Nephrol. 2016;11:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2013;24:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 388] [Article Influence: 29.8] [Reference Citation Analysis (0)] |