Published online Apr 6, 2020. doi: 10.12998/wjcc.v8.i7.1337
Peer-review started: November 24, 2019
First decision: December 30, 2019
Revised: February 11, 2020
Accepted: March 5, 2020
Article in press: March 5, 2020
Published online: April 6, 2020
Processing time: 134 Days and 4.2 Hours
The aim of this study was to report a case of a patient who suffered from severe concentrated sulfuric acid burns while working at high altitude. This patient recovered after systemic treatment. We also provide a literature review for a better understanding of the disease.
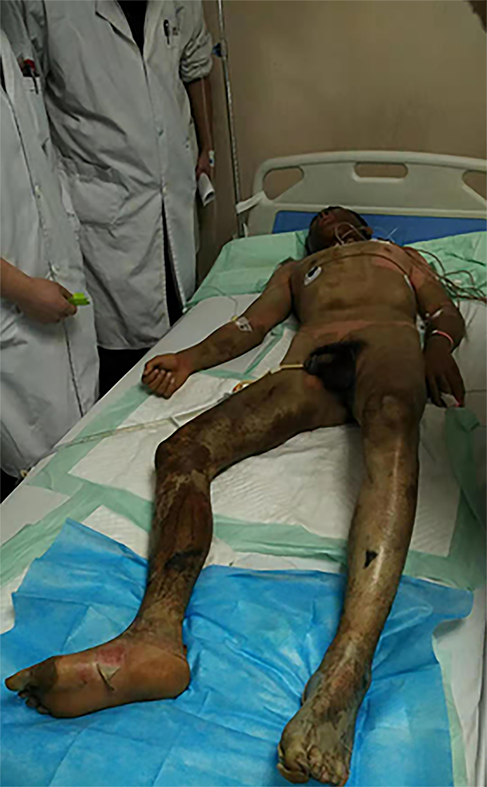
A 30-year-old male, who was working in a local chemical plant in Xining (the Qinghai provincial capital, China) at an altitude of 2261 m, suffered from burns 85% TBSA (III° 70%TBSA, deep II° 15%TBSA) after a tank containing 80% concentration of sulfuric acid exploded. The patient immediately received a series of first aid treatments, as well as rigorous wound managements after admission, which included protection for the whole body and organs, prevention and treatment of eye burns, and the appropriate oxygen therapy. After 65 d of treatment, the burn wounds had completely healed, and the patient was transferred to another specialized hospital for further eye treatment. The first aid before admission and the emergency treatment of wounds following admission were appropriate. No severe complications of sepsis, severe renal insufficiency, septic costal chondritis, corneal perforation or other burns occurred during the treatment.
The main causes of concentrated sulfuric acid burns consisted of accidental burns at work, accidents in the outside, factitious injuries and improper laboratory operations. The clinical manifestations were mostly deep II° and III° burns, with a formation of brown-black, leather-like eschar on the wound surface and locally embolized dendrite-like vessels. The clear cause of the injury and typical clinical manifestations in this case made it easy to diagnosis. However, adult cases with severe concentrated sulfuric acid burns in high altitude areas are rare, so the successful treatment of this case is of great significance.
Core tip: Severe concentrated sulfuric acid burns are rare in the world. We herein present a rare case of extensive concentrated sulfuric acid burns caused by a tank in a factory containing an 80% concentration of sulfuric acid. The worker received a series of treatments that included first aid immediately following the burn, as well as rigorous wound managements after admission, which included protection for the whole body and organs, prevention and treatment of eye burns, and the appropriate oxygen therapy. After the 65-d of treatments, the burn wounds completely healed, and the patient was transferred to another specialized hospital for further eye treatment.
- Citation: Zhao RM, Li Y, Chao SW, Wang HJ. Systemic treatment for severe concentrated sulfuric acid burns in an adult male at high altitude: A case report. World J Clin Cases 2020; 8(7): 1337-1342
- URL: https://www.wjgnet.com/2307-8960/full/v8/i7/1337.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i7.1337
Severe chemical burns rarely occur in adults in high altitude regions. However, we report such an adult case with severe concentrated sulfuric acid burns who was successfully treated. After on-the-spot first aid, the patient was transferred to our hospital for systemic treatment. This study summarizes the clinical manifestations, diagnosis and treatment of this case.
A 30-year-old male,when he was at work,a tank containing 80% sulfuric acid exploded, resulting in burns on his face, neck, limbs, trunk, buttocks, and perineum.
A 30-year-old male presented to the Burns and Plastic Department of Affiliated Hospital of Qinghai University complaining of worsening concentrated sulfuric acid burns. When the patient was at work, a tank containing 80% sulfuric acid exploded, resulting in burns on the face, neck, limbs, trunk, buttocks, and perineum. At 5 min after the injury, the patient was rescued by his colleagues who helped him take off all his clothes and washed him with cold water for about 20 min.accompanied by obvious thirst, dyspnea, dry pharyngeal pain, stomach discomfort, and no urine.7 hours after admission, the patient suddenly vomited. The vomitus was black and brown stomach contents, about 300ml.
The patient has been in good health.
At 1 h after the injury, the patient was sent to the Burns and Plastic Department of Affiliated Hospital of Qinghai University by the emergency ambulance service. Upon admission, 85% of the patient’s body surface area was burned . The clinical manifestations were mostly deep II° and III° burns, with a formation of brown-black, leather-like eschar on the wound surface and locally embolized dendrite-like vessels.The patient showed pharyngeal pain, hoarseness, dyspnea after the accident and his nasal hair is scorched.(Figure 1)
He had a blood pressure of 110/60 mmHg, pulse rate of 135 times/min and respiratory rate of 35 times/min,Hemoglobin 193.0g/L,White blood cell count 21.94×109/L,Urine protein(++),Urine occult blood test(++), Blood urea nitrogen7.3mmol/L,Glucose 10.9mmol/L, Creatinine76umol/L,Uric acid 296umol/L.
The diagnosis by our department was as follows: (1) chemical burns 85% TBSA (III° 70%TBSA, deep II° 15%TBSA)caused by concentrated sulfuric acid; (2) Severe burn shock; (3) Moderate inhalation injury; (4) Severe sulfuric acid burns of conjunctiva at both canthus; and (5) Acute renal insufficiency.
Subclavian vein access was immediately established after admission, and rehydration therapy was implemented. During the first 24 h after admission, we estimated a total liquid volume of 11790 mL, a 2:1 ratio of electrolyte liquid to colloid, and a basic water content of 3380 mL in the patient. The second 24 h showed a total liquid volume of 8940 mL, a 1.68:1 ratio of electrolyte liquid to colloid, and a basic water content of 4000 mL. In the shock stage, the urine volume was 149 mL/h. The vital signs of the patient were stable, with a pulse rate of 84 times/min, SaO2 of 99%, and respiratory rate of 17 times/min. The amounts of electrolyte liquids and colloidal rehydration were moderate, and the urine volume was satisfactory. Thus, the patient was considered to be stable in the shock stage.
The patient showed pharyngeal pain, hoarseness, dyspnea and other symptoms after the injury. Upon admission, with a respiratory rate of 35 times/min, the patient was immediately administered with oxygen inhalation with an oxygen flow of 5 L/min via nasal catheter. Tracheotomy was performed within 3 h after admission, and endotracheal intubation was performed to deliver oxygen with a continuous low flow. Other medications included the injection of 3 mL 0.9% normal saline and 15 mg ambroxol hydrochloride, the administration of 2.5 mL salbutamol sulfate every 6 h, mist aspiration, and diluting sputum to ensure airway patency.
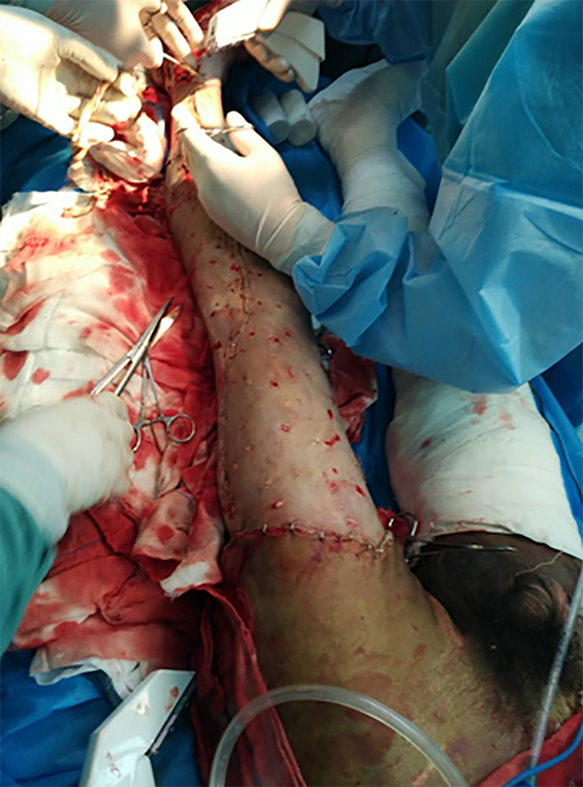
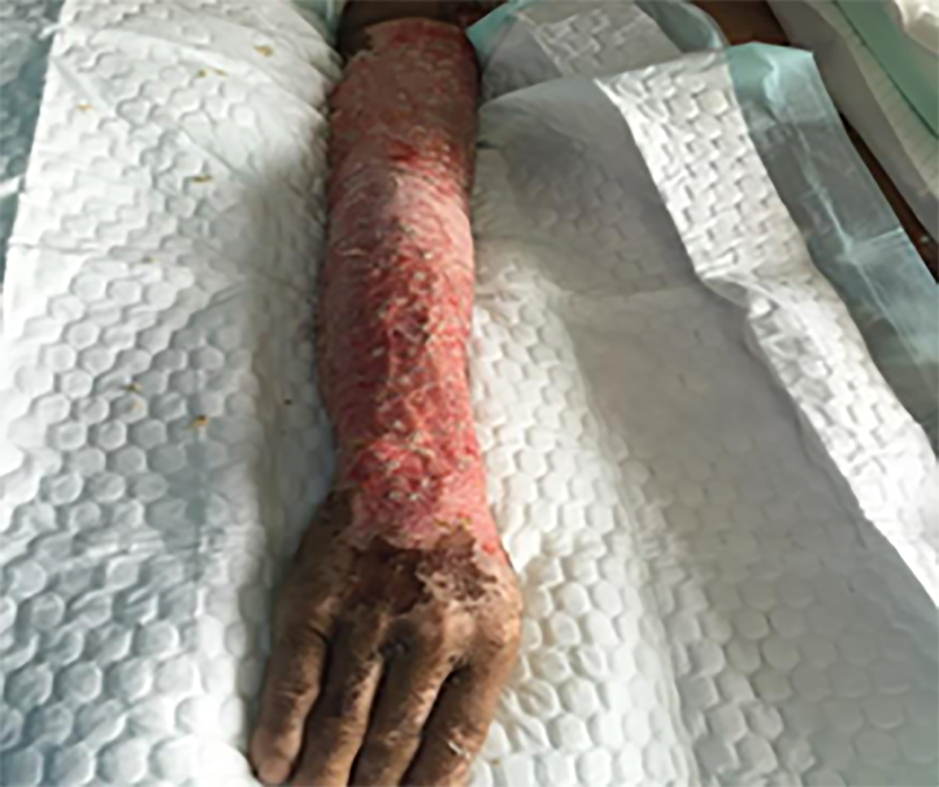
After admission, the wound was exposed and 5% sodium bicarbonate was applied to keep the wound wet. The wetted yarn with sodium bicarbonate was wrapped in the first 3 d. The pH test paper was used to determine the pH value of the wounds during the dressing changes. Dressings were changed daily. The depth of the wound was re-evaluated 48 h after injury.After the shock period, the eschar on the deeper wound is coated with iodine, and the shallow wound healed with the administration of a growth factor. On the 5th d after admission, all vital signs of the patient were determined as reasonable. A 2%TBSA blade thick skin slice of the left thigh was prepared for the particulate skin paste. The eschar of the rest of the lower limb wound was excised and a "microparticle allograft skin transplantation" was performed, with an excised eschar area of about 35% (Figure 2). On the 15th, 35th and 49th d after admission, the scalp was prepared for eschar cutting and skin grafting. On the 65th d after admission, all wounds were closed (Figure 3).
Since the patient's admission, cefmenoxime hydrochloride had been intravenously administered for an anti-infective treatment. On the 3rd d after admission, biapenem was administered as a replacement for antibiotic therapy (0.3 g for every 8 h). At 1 d later, the eschar cutting microparticle allograft transplantation of both lower limbs was performed. On the 9th d of antibiotic administration, fluconazole was taken orally, and antibiotics were discontinued when the general situation of the patient had improved. After treatment, bacterial cultures indicated a Staphylococcus aureus and Pseudomonas aeruginosa infection. Based on results from the bacterial cultures and drug sensitivity tests, sensitive antibiotics were selected for further anti-infective treatment to ensure better efficacy.
The patient received an urgent ophthalmic consultation after admission, and the examination results were as follows: Right VOD index/anterior, 15 marks, and left VOD index/anterior, 15 marks. The PE examination revealed bilateral eyelid burns, conjunctiva and severe corneal haze. In addition, the anterior chamber was normal. The pupil was faintly visible, which was round and about 3.5 mm in diameter. We observed delayed pupillary light reflex. Crystalline lens, vitreous and fundus were also examined. The Binocular touch Tn. diagnosis was sulfuric acid burns of conjunctiva at both canthus. The ophthalmic treatments were as follows: (1) Rinsing bilateral conjunctival sac with a 0.9% sodium chloride injection of 800 mL; (2) Subconjunctival injection with 0.5 mL vitamin C; (3) Treatments of Pranoprofen eye drops, Recombinant human epidermal growth factor eye drops, ofloxacin eye drops(3 to 5 times a day, one drop each time) and Compound Tropicamide eye liquid (1 to 2 times a day, one drop each time) with an interval of at least 30 min between each eye drop treatment; and (4) Corneal nutritional therapy. The patient was regularly followed up by the department of ophthalmology and the medications were adjusted.
On the 65th d after admission, the burn wounds of the patient had completely healed, and the patient was transferred to a specialized hospital in Beijing for further eye treatment. No follow-up was conducted after discharge.
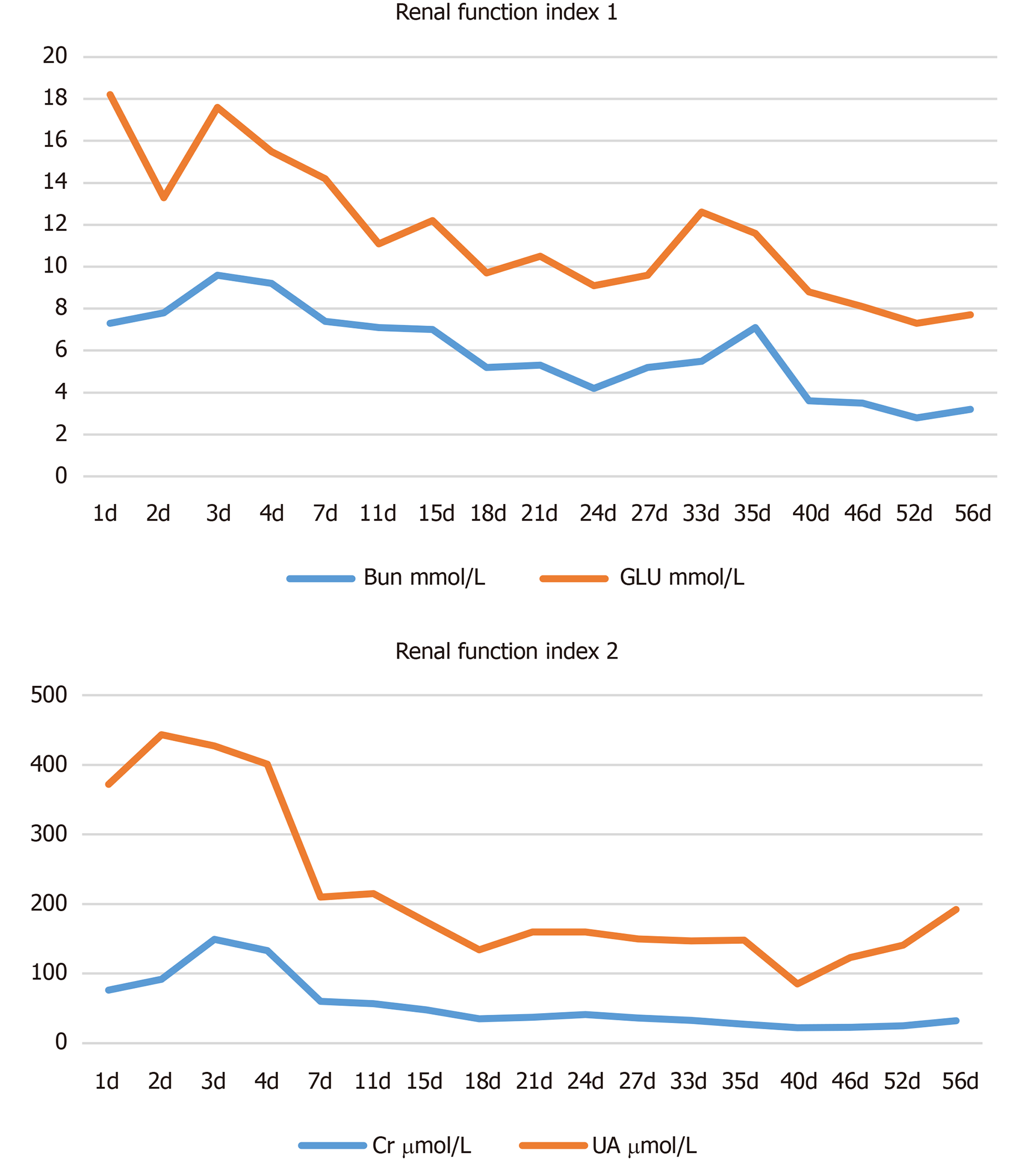
Though chemical burns, such as acid burns and alkali burns, account for a small proportion of all burns, they may result in relatively deep wounds and bring about high mortality and disability rates[1]. This is why successful treatment is key. In high altitude and cold areas, this case of severe extensive concentrated sulfuric acid burns is extremely rare. In clinical practices for this case, we gained the following experience. For emergency treatment, the patient received a continuous flow of cold water flushing for 20 min prior to admission and a wet application of 5% sodium bicarbonate solution on the wounds. At 5 d after admission, we performed the cutting eschar and skin graft surgery to completely remove the effective residues, especially sulfuric acid residue, on the skin so as to stop further damage to the body[2]. According to the depth and distribution of the wounds, the eschar and skin grafts were cut into batches, and the wound surface was covered with prepared scalp skin. During the treatment, appropriate surgical arrangements and microparticle allogeneic skin coverage ultimately brought favorable efficacy for the burn wounds. In one case, there was a secondary injury during the rescue of the chemical burn[3], but the patient's colleagues conducted the operation in the process of first aid treatment, so no secondary injury occurred during the process. This is largely due to the standardization of operations and routine safety training of rescue personnel. We summarize the features of the injured wound surface of concentrated sulfuric acid burns. Firstly, sustained thermal burns appear due to the interaction of sulfuric acid with the skin. When concentrated sulfuric acid contacts the skin, the resulting chemical reaction releases heat that brings sustained thermal damage to the skin[4]. The sustained heat dehydrates local tissues and cells, and the consequential skin coagulation and necrosis may produce eschars and damage the microvascular system[5]. Thus, we found that the wounds of this patient were brown-black and dry with clear boundaries and some hollows upon admission. Secondly, an inhalation injury usually follows concentrated sulfuric acid burns[6]. The accident scene is a relatively closed working environment. The explosion caused chemical reactions between concentrated sulfuric acid, air and water, which produced a high amount of SO3 in the air[7]. The poisonous gas stimulated and damaged the respiratory tract of the patient. On the 2nd d after admission, a bronchoscopy examination was performed on the patient and revealed edema, congestion and adherent necrotic tissue in the mucosa of the atmospheric tube above the trachea uplift. No obvious bleeding was observed. A large amount of white viscous sputum was observed in the accessory tube below the trachea uplift, which can be removed after treatment. Tracheotomy within 3 h after admission was of great significance for first aid. Thirdly, concentrated sulfuric acid and other chemical burns often affect the eyes, accounting for 11.5% to 22.1% of all eye injuries. Routine treatment for acute chemical burns includes immediate and adequate cleaning to remove pathogenic factors and minimize damage[8]. However, during first aid, the patient kept closing his eyes due to pain, so his colleagues omitted the serious consequences of eye burns, missing the greatest opportunity of irrigating the eyes to minimize damage. After admission, the patient was treated with anti-inflammation medications, epidermal growth-promoting drugs, corneal nutrition agents and other drugs. No complications such as corneal lysis, eyeball extirpation and symblepharon were observed during hospitalization, and therapeutic efficacy was acceptable. The patient was sent to our hospital for the prevention and treatment of shock 1 h after injury. After the first 24 h of fluid replacement, the patient complained of thirst, which was due to excessive crystal fluid supplementation. The late reduction of crystal fluid, in addition to increased water and colloidal fluid perfusion, relieved the symptoms. This reminds us of the ratio of crystal, colloid and water in clinical practices. Xining is located in a plateau with an average altitude of 2261 m, an average atmospheric pressure of 77.3 kPa (or 579 mmHg), and an oxygen partial pressure of 16 kPa (or 122 mmHg). The hypoxic environment is detrimental to the recovery of severe wounds[9]. Thus, high-flow oxygen inhalation in the early stage and continuous low-flow oxygen injection in the later stage are necessary for a patient with severe burns, providing favorable conditions for further wound healing. In addition, timely liquid treatment after admission, urine alkalization, correction of fluids, electrolytes, acid-base balance disorders and other treatments effectively protect the kidney (Figure 4).
The main causes of concentrated sulfuric acid burns consist of accidental burns at work, accidents outside, factitious injuries and improper laboratory operations. The clinical manifestations were mostly deep II° and III° burns with a formation of brown-black, leather-like eschar on the wound surface and locally embolized dendrite-like vessels. With a clear cause of injury in this case and typical clinical manifestations, it was easy to make a diagnosis. However, adult cases with severe concentrated sulfuric acid burns in high altitude areas are rare, so the successful treatment of this case is of great significance.
The author thanks and has gratitude for all the medical staff of burn and plastic surgery in Affiliated hospital of Qinghai University , as well as for the patient and his family members. Thanks to the increasingly powerful China for providing the good working environment for the author and team. I love you, China.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Coban YK, Vaudo G S-Editor: Zhang L L-Editor: Filipodia E-Editor: Xing YX
| 1. | Moore K. Hot Topics: Chemical Burns in the Emergency Department. J Emerg Nurs. 2015;41:364-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Palao R, Monge I, Ruiz M, Barret JP. Chemical burns: pathophysiology and treatment. Burns. 2010;36:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Coban YK, Ozkan KU. Danger of being second victim when resuscitating chemically burned patient. Burns. 2005;31:668-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Yin S. Chemical and Common Burns in Children. Clin Pediatr (Phila). 2017;56:8S-12S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 6. | Rong XZ, Xu YH. One case with severe inhalation injury was cured successfully. J First Mil Med Univ. 2001;21:652. [DOI] [Full Text] |
| 7. | Fang ZY, Gao XS, Wu ZL, Xu FX. Burn Theory and Practice. Shenyang: Liaoning Science and Technology Publisher 1989; 457-464 [ISBN 7-5381-0311-2/R· 53]. |
| 8. | Sharma N, Kaur M, Agarwal T, Sangwan VS, Vajpayee RB. Treatment of acute ocular chemical burns. Surv Ophthalmol. 2018;63:214-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 137] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 9. | de Smet GHJ, Kroese LF, Menon AG, Jeekel J, van Pelt AWJ, Kleinrensink GJ, Lange JF. Oxygen therapies and their effects on wound healing. Wound Repair Regen. 2017;25:591-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |