Published online Apr 6, 2020. doi: 10.12998/wjcc.v8.i7.1326
Peer-review started: December 17, 2019
First decision: February 20, 2020
Revised: March 5, 2020
Accepted: March 10, 2020
Article in press: March 10, 2020
Published online: April 6, 2020
Processing time: 110 Days and 17 Hours
Apatinib is a small-molecule multitargeted tyrosine kinase inhibitor. Apatinib has demonstrated encouraging antitumor activities. This study aimed to observe the efficacy and safety of apatinib for the treatment of multiple brain micrometastases.
We report two patients with multiple brain micrometastases after failure of second-line treatment. Both patients had extracerebral metastases. When the patients took 250 mg/d apatinib orally, the intracerebral lesions disappeared. The extracerebral lesions were partially alleviated. Both patients had a progression-free survival of more than 12 mo and were still stable. The safety was good. The main adverse events (AEs) were mild hypertension and proteinuria, which could be controlled.
Apatinib has clear efficacy and good tolerance in patients with multiple brain micrometastases after failure of second-line treatment.
Core tip: Brain metastases are the most common intracranial tumor, and the average survival time of patients with brain metastases after symptomatic treatment with chemotherapy, radiotherapy, and hormonal drugs is less than 1 year. We report two patients with multiple brain micrometastases after failure of second-line treatment; both had extracerebral metastases. When the patients were treated with apatinib, the intracerebral lesions disappeared, and the extracerebral lesions were partially alleviated. Both patients had a progression-free survival of more than 12 mo and were still stable. Apatinib, a small-molecule oral inhibitor with anti-angiogenic function, may be an option for treating brain metastases.
- Citation: Guo JH, Wang YY, Zhang JW, Liu PM, Hao YJ, Duan HR. Clinical effects of apatinib mesylate for treatment of multiple brain micrometastases: Two case reports. World J Clin Cases 2020; 8(7): 1326-1336
- URL: https://www.wjgnet.com/2307-8960/full/v8/i7/1326.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i7.1326
Brain metastases (BMs) are the most common intracranial tumors in adults, occurring in 20%-40% of cancer patients[1-3]. Once brain metastasis is found, the natural course of disease is 4-8 wk, and the average survival time is less than 2 mo[4-6]. The average survival time of BM patients after symptomatic treatment with chemotherapy, radiotherapy, and hormonal drugs is less than 1 year[7]. It is generally believed that systemic chemotherapy is not effective in the treatment of brain metastases due to the presence of the blood-brain barrier. The main treatments include stereotactic radiosurgery, surgical resection, whole brain radiation therapy, or a combination of these methods[8]. With the advent of molecularly targeted drugs, there are more treatment options for patients with brain metastases[9]. However, it was found that brain metastases have molecular changes that are different from those in primary tumors[10]. The proliferation and migration of malignant tumor cells are closely related to abnormal angiogenesis. Anti-angiogenic drugs alone or in combination with chemotherapy have become the treatment for a variety of malignant tumors[11]. Apatinib is a small-molecule multitargeted tyrosine kinase inhibitor (TKI) that highly selectively binds and inhibits vascular endothelial growth factor receptor 2 (VEGFR-2) activity, blocks the binding of VEGFR-2 to vascular endothelial growth factor (VEGF), and blocks VEGF/VEGFR-2 mediated signal transduction by suppressing several signaling pathways, including the Raf/MEK/Erk pathway, the p38-MAPK pathway, and the PI3K/AKT/mTOR pathway[12], thereby inhibiting vascular endothelial cell proliferation and migration and reducing tumor neovascularization[13]. Apatinib has demonstrated encouraging antitumor activities and tolerable toxicities in several solid tumors, including lung carcinomas, breast cancer, hepatocellular carcinoma, and osteosarcoma[14]. Apatinib inhibits glycolysis by suppressing the VEGFR2/ AKT1/SOX5/GLUT4 signaling pathway in ovarian cancer cells[15]. Furthermore, apatinib inhibits the function of ATP-binding cassette subfamily B member 1 (ABCB1) in certain cancers and reverses ABCG2 (BCRP/MXR/ABCP)- and P-glycoprotein (ABCB1/MDR1)-mediated multidrug resistance[16,17]. The efficacy of apatinib in advanced gastric cancer has been verified in phases II and III clinical studies[18,19]. In addition, the efficacy of apatinib in the treatment of other malignant tumors, including lung cancer[20], esophageal cancer[21,22], liver cancer[23], and colorectal cancer[24], has also been confirmed by a series of clinical studies. Apatinib has also been reported for the treatment of cerebral edema[25] and glioma[26], but there are few reports on the treatment of brain metastases. We observed the use of single-agent apatinib for the treatment of two patients with multiple micrometastases who failed second-line therapy, one with esophageal squamous cell carcinoma and the other with cervical adenocarcinoma. Both patients with intracranial lesions achieved complete remission.
Case 1: An 82-year-old man presented with a chief complaint of lower back pain for 4 wk.
Case 2: A 40-year-old woman presented with chief complaints of left neck pain and lower back pain for 4 wk.
Case 1: The patient had lower back pain and slight nausea after eating solid food over the last 4 wk. The patient’s daily bowel habits had been changed considerably for 3 mo.
Case 2: The patient had frequent irregular vaginal bleeding over the last 5 wk that began from June 2017. She suffered left neck pain and lower back since March 2018. Her symptoms worsened over the last month.
Case 1: He had coronary heart disease for 5 years and hypertension for 2 years.
Case 2: She underwent a cesarean operation 1 year prior.
Case 1: The patient did not have a history of smoking or drinking.
Case 2: The patient did not have a history of smoking or drinking.
Case 1: The patient’s body was lean with a navicular abdomen.
Case 2: Multiple metastases on the left neck lymph nodes were collectively approximately 4 cm × 4 cm and rock-hard. In addition, the mass, with poor movement, may have adhered to the tissue structure of the muscle. The patient felt left neck pain and lower back pain.
Case 1: On April 2, 2017, the pathological results (esophageal biopsy) showed esophageal squamous cell carcinoma. The results of immunohistochemistry indicated LCA (-), P53 (+), CK5/6 (+), and P63 (+).
Case 2: On July 6, 2017, cervical smear confirmed cervical adenocarcinoma. On September 6, 2017, postoperative pathology showed cervical adenocarcinoma stage IIA2, with moderate differentiation, infiltration of the whole cervix (neck junction and endometrium), and lymph node metastasis (sentinel 2/4). On March 9, 2018, the patient underwent left supraclavicular lymph node biopsy, and postoperative pathology showed metastatic adenocarcinoma. Carbohydrate antigen 125, a blood tumor marker of ovarian cancer, was high at 127.9 µ/mL (reference range: <35 U/mL). On January 8, 2019, the patient developed proteinuria 2+ with a white blood cell count of 12274.7/μL and a bacterial count of 25044.6/μL.
Case 1: On March 23, 2017, computed tomography (CT) showed upper esophageal wall thickening and stenosis. On March 25, 2017, gastroscopy showed suspected esophageal cancer, superficial gastritis, duodenitis, and duodenal bulb polyps. Pain relief was achieved after treatment with traditional Chinese medicine. On April 4, 2017, the patient developed hoarseness, and CT showed a space-occupying lesion in the upper part of the esophagus, which was considered esophageal cancer, and multiple nodules in both lungs and mediastinal lymph nodes, which were considered metastases.
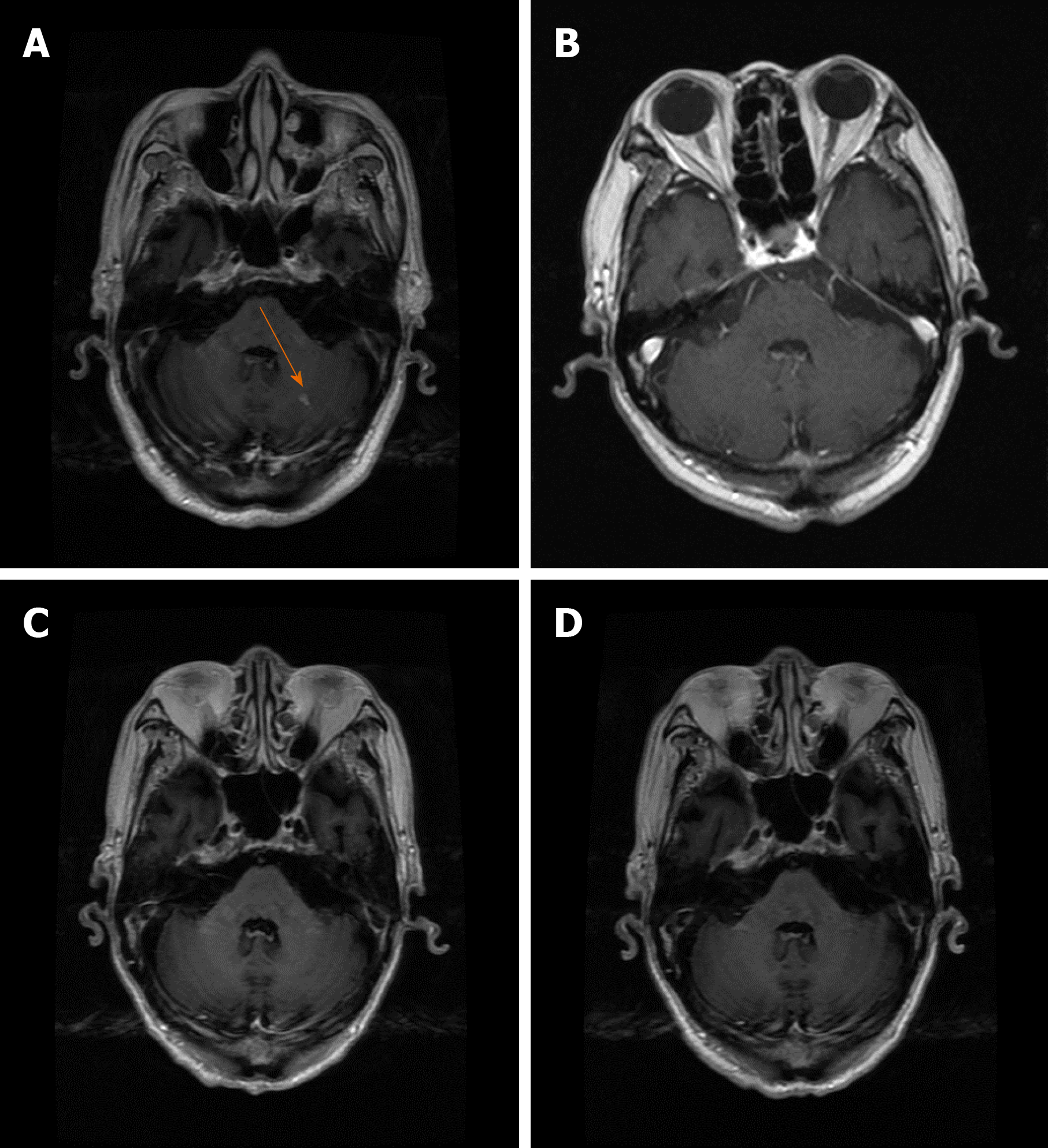
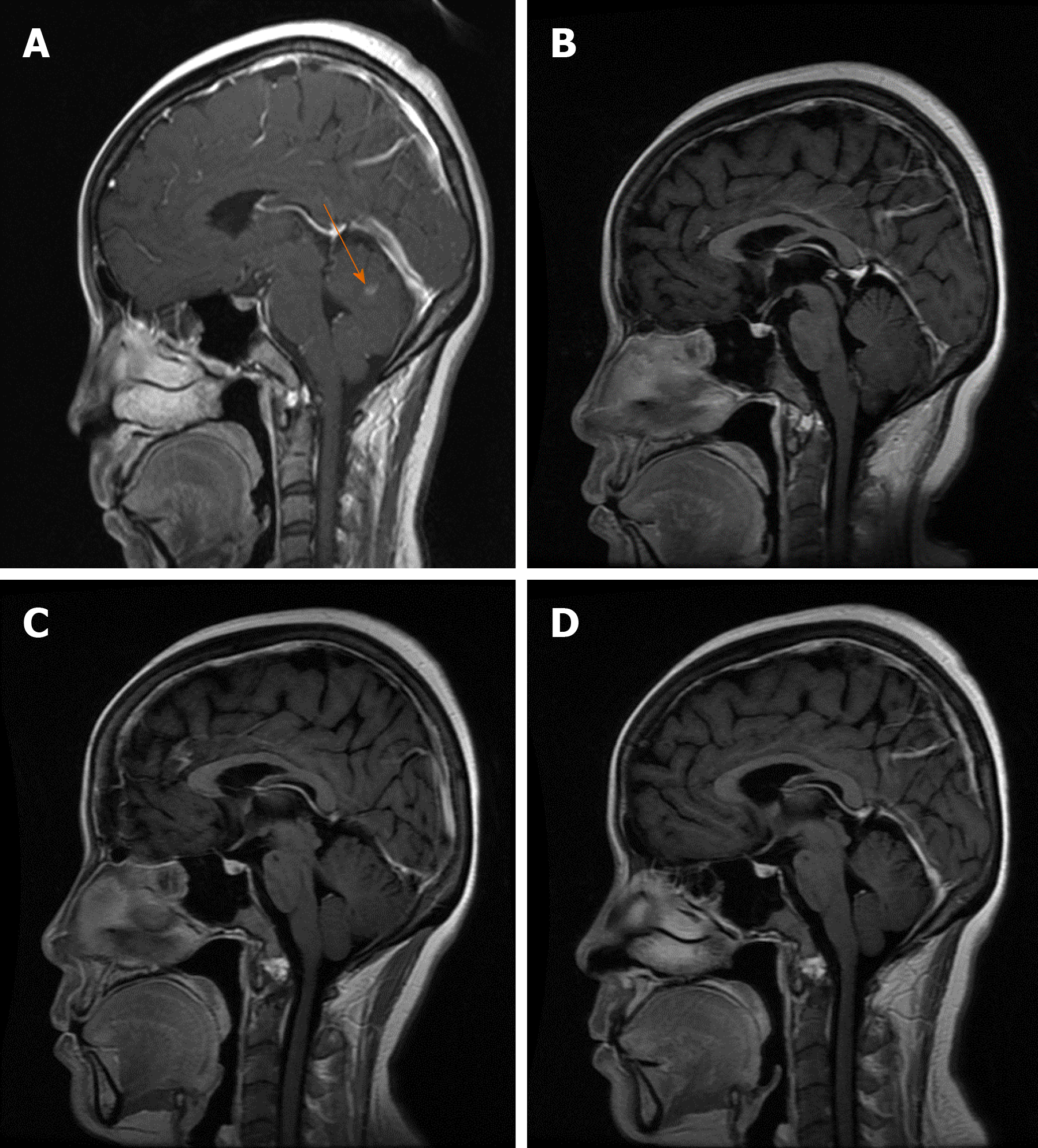
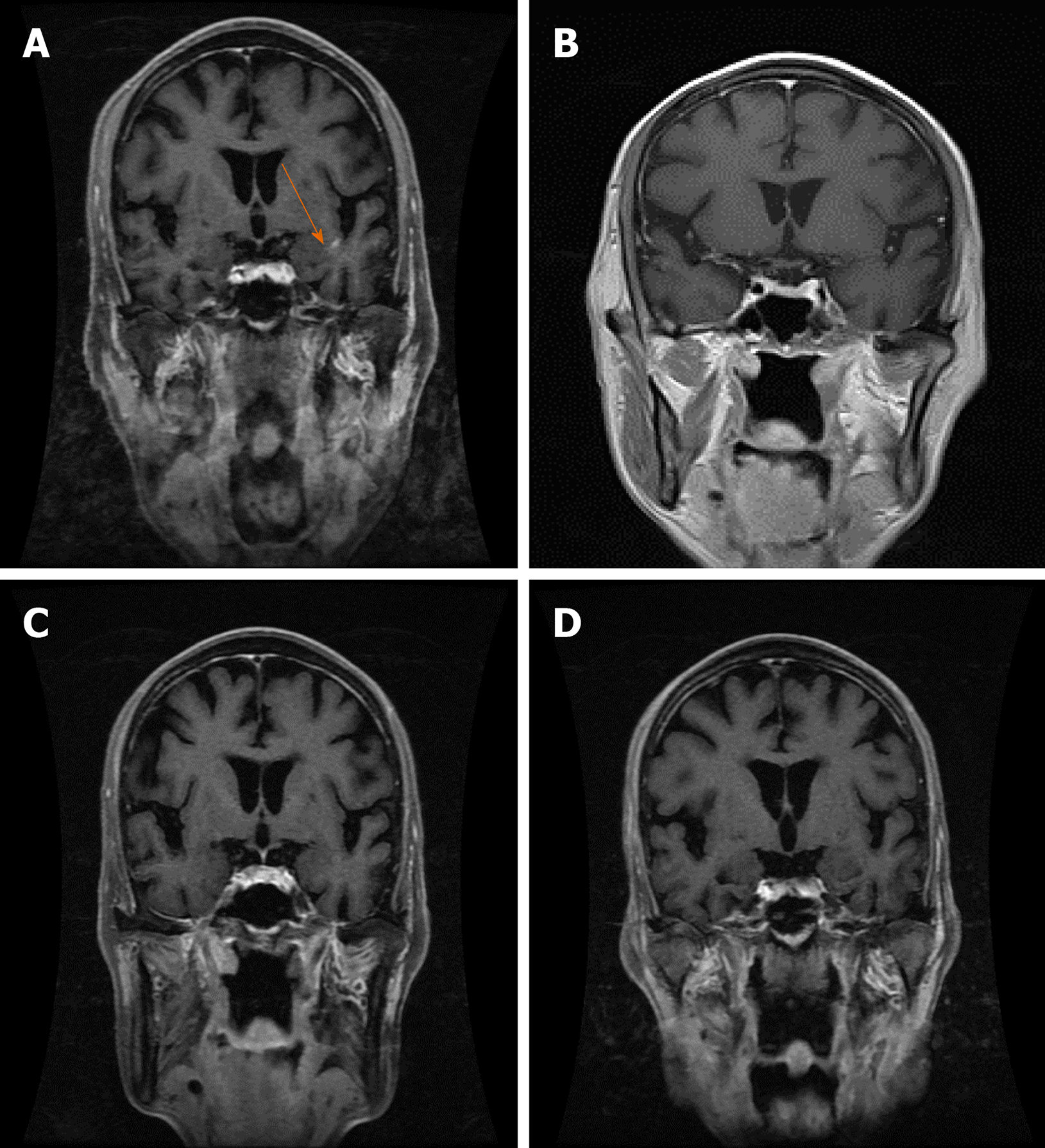
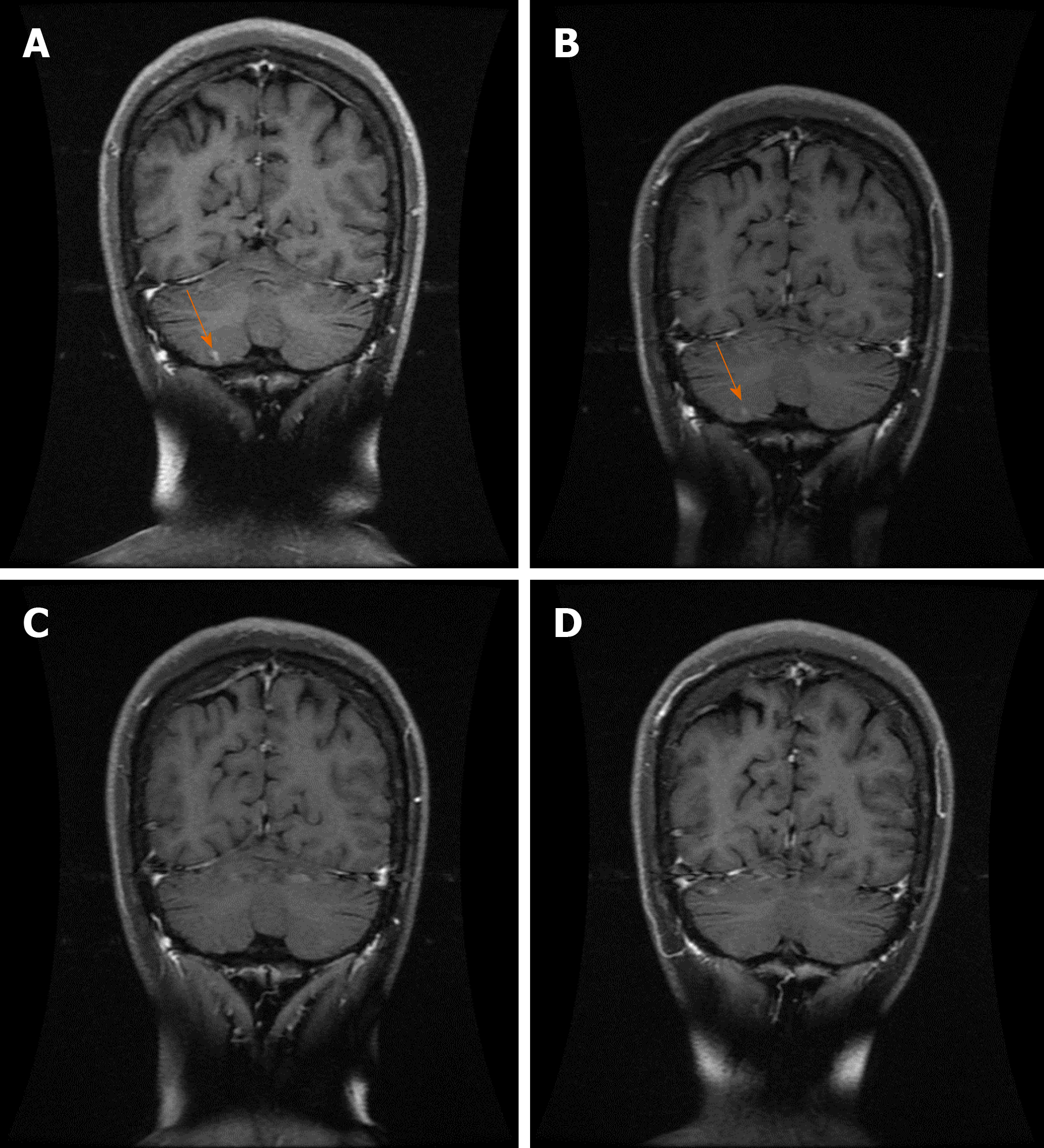
On June 7, 2018, the patient reappeared with hoarseness, accompanied by difficulty swallowing and cough. CT showed that the esophageal wall was unevenly thickened; the upper esophageal nodule showed a soft tissue shadow, the tracheal boundary was unclear, and there was left pleural effusion. On July 14, 2018, the patient developed headache and nausea. Magnetic resonance imaging (MRI) showed abnormally enhanced lesions in the left cerebellar hemisphere (Figure 1A and 2A), temporal lobe (Figure 3A), and basal ganglia (Figure 4A), which were considered metastases. On August 10, 2018, CT showed that the esophageal wall was unevenly thickened. The upper part of the esophageal nodule showed a soft tissue shadow. The nodular soft tissue was slightly smaller than the previously observed nodule. The left pleural effusion decreased. On August 29, 2018, MRI showed that the temporal lobe and basal ganglia lesions were unclear and that the left cerebellar lesions shrank. On September 14, 2018, CT showed that the esophageal wall was unevenly thickened and the upper esophagus protruded to the left front and was smaller than the old lesion. On October 18, 2018, MRI showed pons, bilateral thalamic-basal ganglia, bilateral ventricular cleft cerebral infarction, and bilateral ventricular and bilateral frontal cortex demyelination; contrast-enhanced cranial MRI showed no clear abnormal enhancement (Figures 1B-4B).
On February 18, 2019, contrast-enhanced brain MRI showed no obvious abnormal enhancement (Figures 1C-4C). On May 14, 2019, CT showed that the upper part of the esophageal wall was unevenly thickened, the upper esophagus protruded to the left front, and the upper part of the esophagus was paralyzed. On July 16, 2019, CT showed that the upper wall of the esophagus was unevenly thickened, the upper part of the upper esophagus protruded from the left side, and the upper part of the esophagus was paralyzed.
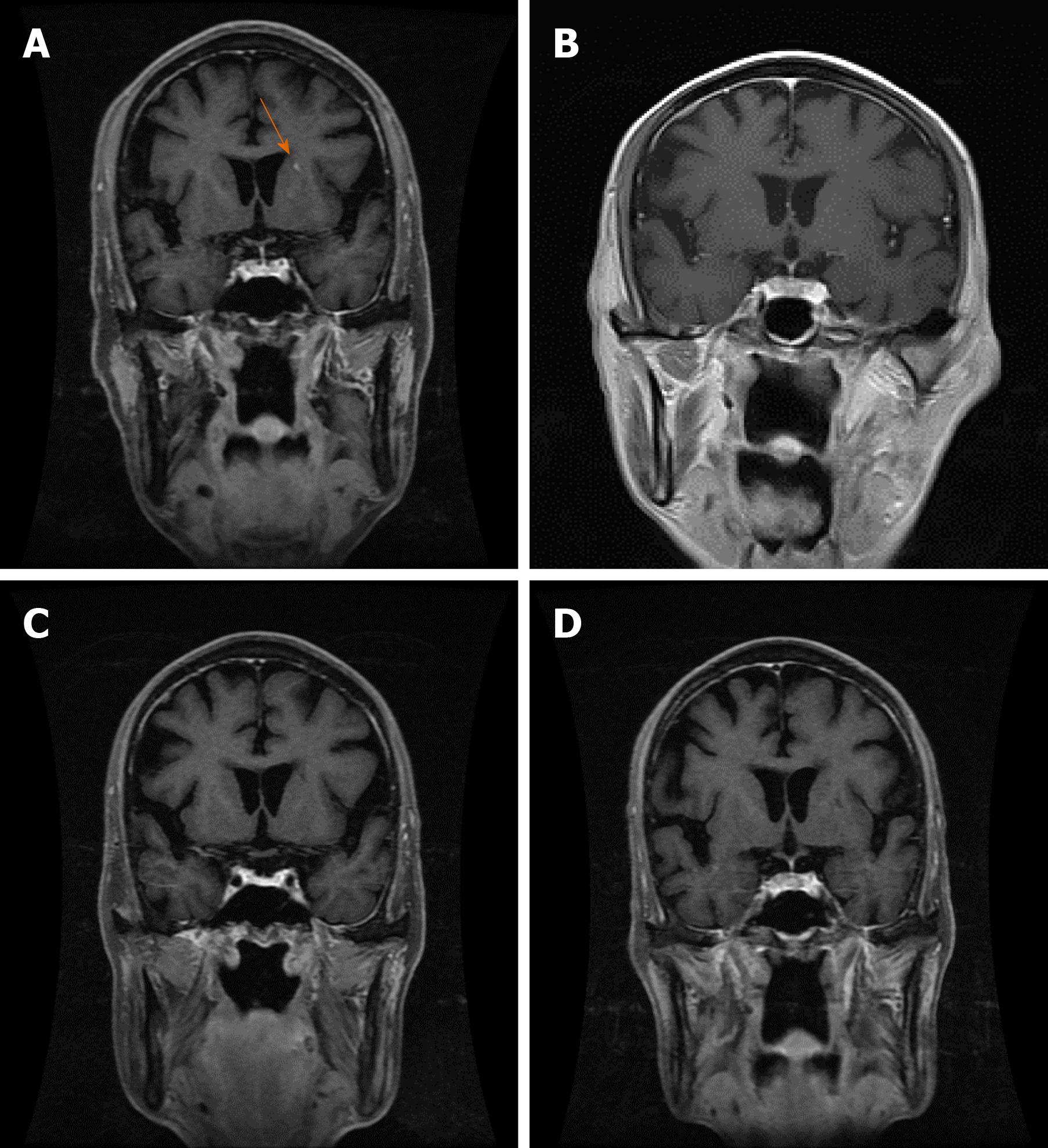
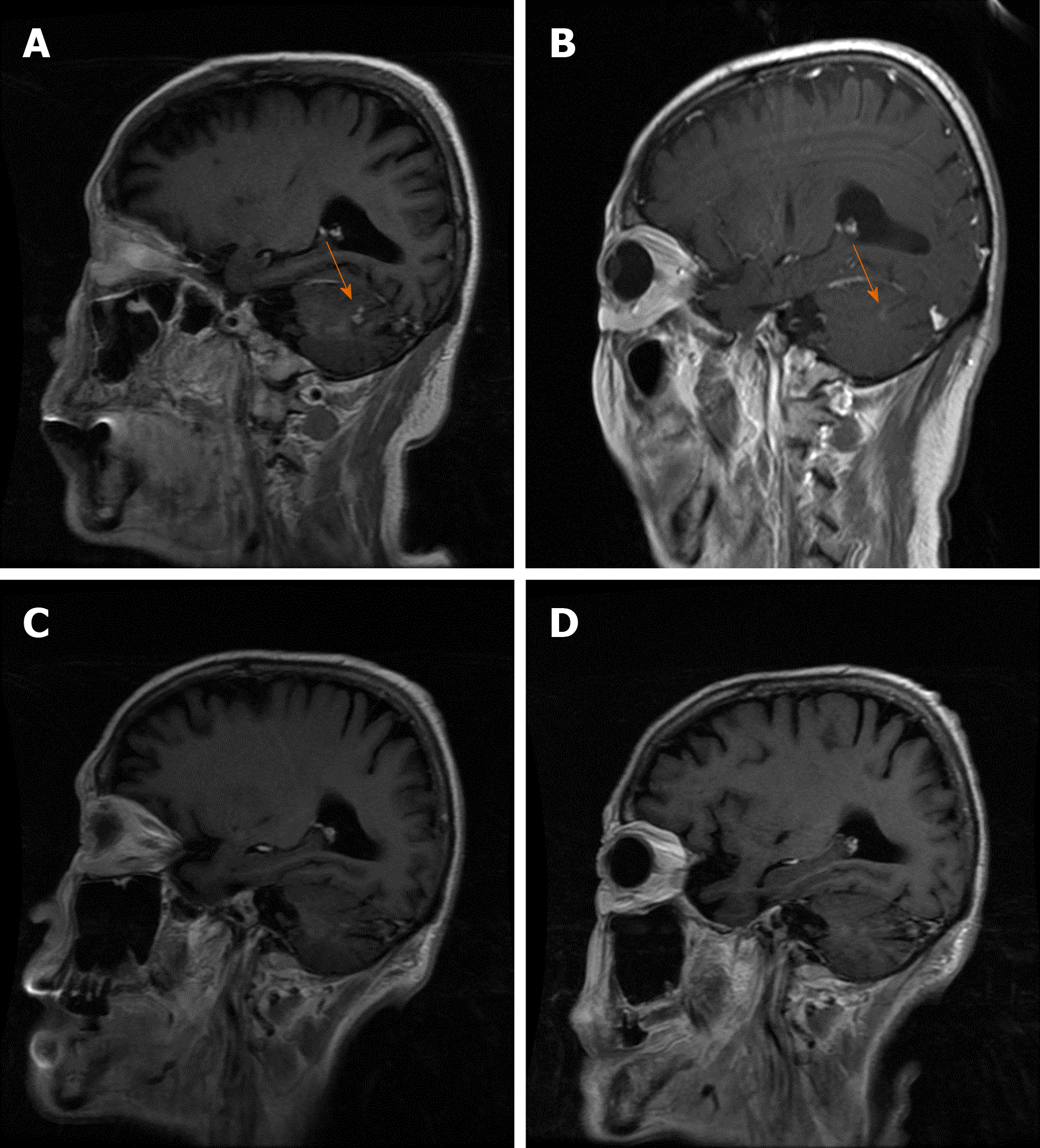
Case 2: On March 5, 2018, positron emission tomography-CT showed that the left lymph nodes in the left axillary fossa and supraorbital sac and multiple retroperitoneal lymph nodes had active metabolism, which suggested metastases. On June 2, 2018, MRI showed left lateral ventricle paraventricular white matter and an abnormally enhanced lesion in the lower right cerebellar hemisphere (Figures 5A and 6A), which were considered metastases. On July 10, 2018, CT showed slightly enlarged lymph nodes of the left axilla. On October 7, 2018, contrast-enhanced MRI showed that there was no obvious abnormality in the brain and that the intracranial lesion completely disappeared (Figures 5B and 6B). On January 9, 2019, MRI showed spotty abnormal enhancement of the left thalamus and temporal lobe, suggesting dynamic follow-up; the original diffuse lesion in the left frontal lobe showed no clear enhancement (Figures 5C and 6C). On January 11, 2019, CT showed slightly larger lymph nodes of the left axilla, enlarged retroperitoneal lymph nodes, and a small amount of fluid in the pelvic cavity. On March 13, 2019, CT showed that the left axillary and retroperitoneal lymph nodes were stable; there was some pelvic fluid, and the abdominal wall structure was blurred. On May 10, 2019, an enhanced brain MRI scan showed no clear abnormal enhancement (Figures 5D and 6D). On July 25, 2019, an enhanced brain MRI showed no clear abnormal enhancement. On July 24, 2019, CT showed a few small lymph nodes on both sides of the neck, slightly larger lymph nodes of the left axilla, and a small amount of pelvic cavity effusion.
Final pathology confirmed esophageal squamous cell carcinoma in the upper part of the esophagus; multiple metastases in both lungs and mediastinal lymph nodes; and multiple micrometastases in the left cerebellar hemisphere, temporal lobe, and basal ganglia.
Final pathology confirmed cervical adenocarcinoma with moderate differentiation, infiltration of the whole cervix (neck junction and endometrium), visible cancer involvement, and lymph node metastasis (sentinel 2/4); multiple lymph node metastases in the left axillary fossa and supraorbital sac, and retroperitoneal lymph nodes; and multiple micrometastases in the left lateral ventricle paraventricular white matter and the lower right cerebellar hemisphere.
On April 4, 2017, the patient presented with hoarseness, and after receiving traditional Chinese medicine treatment for 2 mo, the patient’s hoarseness was not relieved. Subsequently, the TP regimen treatment was given for 1 cycle, which was stopped because of serious bone marrow suppression and secondary pulmonary infection in July 2017. Paclitaxel was given for 3 cycles from July 31 to September 25, 2017. The therapeutic evaluation indicated a partial response (PR). Thereafter, local radiation using gamma knife radiotherapy at 60 Gy was administered in 30 fractions from October 23 to November 23, 2017. The therapeutic evaluation indicated a complete response. In December 2017, the patient developed radiation pneumonitis and repeated infections. Chemotherapy was stopped, and intermittent treatment with traditional Chinese medicine and regular review of the condition were implemented.
On June 7, 2018, the patient reappeared with hoarseness accompanied by difficulty swallowing and cough. The therapeutic evaluation indicated progressive disease (PD), and the patient received single-agent gemcitabine chemotherapy for 1 cycle from June 15, 2018. On July 21, 2018, the patient began oral apatinib 250 mg daily. On September 14, 2018, gastroscopy showed esophageal cancer and fistula formation (approximately 20-23 cm from the incisors to see deep ulcer formation). On September 7, 2018, DSA-guided jejunal nutrition tube implantation was performed. The intracranial and extracranial lesions continued to shrink, and treatment continued.
From July 11 to August 10, 2017, the patient underwent chemotherapy with a platinum-based regimen for 2 cycles. On September 6, 2017, the patient underwent surgery (general hysterectomy + pelvic lymph node dissection + bilateral accessory resection + intestinal adhesion lysis). After the operation, the patient continued the platinum-based regimen for 4 cycles, which ended on January 25, 2018. In October 2017, a total of 46 Gy of pelvic radiotherapy was performed.
From April 14 to July 4, 2018, the patient underwent 4 cycles of gemcitabine plus cisplatin regimen chemotherapy and developed grade IV leukocyte and platelet decrease due to myelosuppression, which improved after supportive symptomatic treatment. On June 2, 2018, the patient developed headache, nausea, and vomiting. The therapeutic evaluation indicated a PD. On July 12, 2018, the patient began to take apatinib monotherapy 250 mg daily, which was adjusted to 500 mg daily for oral administration after 1 wk because the patient could not tolerate the dose and was later readjusted to 250 mg. On August 21, 2018, MRI showed that the right lobes and right cerebellar hemisphere were enhanced, revealing a new lesion. The patient had no obvious symptoms, and the extracranial lesions continued to shrink. The patient continued to take 250 mg apatinib daily. The therapeutic evaluation indicated a PR.
On January 08, 2019, the patient developed proteinuria 2+ with a white blood cell count of 12274.7 μL and a bacterial count of 25044.6 μL, for which urinary tract infection was considered. The patient's urine protein disappeared after anti-infective treatment. On January 11, 2019, CT showed slightly larger lymph nodes of the left axilla, enlarged retroperitoneal lymph nodes, and a small amount of fluid in the pelvic cavity. The therapeutic evaluation was stable disease. On May 8, 2019, lymph node enlargement in the left neck was obvious after the patient stopped taking apatinib for 1 wk. Color ultrasound showed that there were several hypoechoic lesions on the left neck, and the largest lymph node was approximately 17 mm × 7.6 mm. We regarded lymph node enlargement as local PD, and the patient was treated with radiofrequency ablation for metastatic left neck lymph node enlargement. Thereafter, the patient continued to take apatinib monotherapy (250 mg po qd).
On October 18, 2018, after 3 mo of oral apatinib, the brain metastases completely disappeared. On July 16, 2019, the therapeutic evaluation indicated a PR, and the progression-free survival for apatinib monotherapy was 13 mo. The intracranial lesions completely disappeared (Figures 1D-4D), and the extracranial disease was stable. Because of the combined esophageal fistula, the patient’s fluid diet and apatinib were administered through a jejunal nutrient tube. On August 16, 2019, the patient died of severe lung infection.
The patient performed well after taking apatinib monotherapy (250 mg po qd), and the carbohydrate antigen 125 level returned to 25.7 U/mL as normal (reference range: < 35 U/mL). The therapeutic evaluation was stable disease. After 13 mo of oral administration of apatinib, the patient still had complete disappearance of intracranial lesions and partial remission of extracranial lymph node metastasis. In September 2019, the patient still had complete disappearance of intracranial lesions, the lymph node metastasis in the left neck enlarged and increased in number rapidly, and the patient took external beam radiation therapy and continued to take apatinib monotherapy (250 mg po qd). The patient is still alive now.
Two patients developed grade I to II adverse reactions during treatment, namely, one case of hypertension and one case of proteinuria. The adverse reactions were tolerated after treatment with drugs or without special treatment.
Angiogenesis is closely related to the occurrence, development, and metastasis of malignant tumors[27]. Tumor cells produce a variety of substances that lead to angiogenesis, which provides essential nutrients (including oxygen and nutrients) for the further growth of tumors and the excretion of metabolites[28]. In addition, tumor cells are mainly metastasized to other parts of the body through vascular dissemination[29]. Stromal cells in the tumor microenvironment play an important role in promoting tumor invasion and metastasis[30]. Stromal cells can promote angiogenesis and basement membrane destruction through the production of cytokines, growth factors, and chemokines (e.g., VEGF, matrix metalloproteinase, fibroblast growth factor, and transforming growth factor-beta) to enhance the invasion and metastasis of tumors[31]. VEGF-C is a mitogen directed against lymphatic endothelial cells and has a dual role in stimulating blood vessels and lymphan-giogenesis[32]. Studies have shown that high expression of VEGF-C is positively associated with postoperative recurrence and brain metastasis in patients with lung cancer[33]. Therefore, inhibiting tumor angiogenesis can not only inhibit the growth of tumor cells but also reduce the occurrence of tumor metastasis.
Mesylate apatinib is a small-molecule multitargeted TKI that highly selectively binds and inhibits VEGFR-2 activity, blocks VEGFR-2 to bind VEGF, and blocks VEGF/VEGFR-2-mediated signal transduction, thereby inhibiting vascular endothelial cell proliferation and migration and reducing tumor neovascularization[13]. Phases I, II, and III clinical trials of apatinib have shown that patients with advanced gastric cancer after failure to standard chemotherapy have clear objective efficacy and significant survival benefit. Therefore, the State Food and Drug Administration approved apatinib for the treatment of advanced gastric cancer or gastroesophageal junction adenocarcinoma in October 2014. In addition, the efficacy of apatinib in the treatment of other malignant tumors, including lung cancer, breast cancer, liver cancer, and colorectal cancer, has also been confirmed by a series of clinical studies. At present, the clinical trial of apatinib for the treatment of brain metastases from solid malignant tumors is basically in the stage of clinical exploration. Yang et al[34] used apatinib combined with sustained nutrition support to treat gastric cancer patients with brain metastasis and obtained a 2-year survival time. It is suggested that apatinib combined with sustained nutritional support may be an option for the treatment of gastric cancer with brain metastasis. Hu et al[35] reported a 39-year-old Chinese woman diagnosed with stage III triple-negative breast cancer with brain, lung, and bone metastases who chose apatinib + CPT-11 + S1 as a sixth-line treatment after progression through fifth-line treatment. The results showed that the effects of brain, lung metastasis, and cerebral edema were reduced for 7 mo. Zhang et al[26] tried apatinib in two patients with refractory recurrent glioblastomas and achieved significant efficacy, especially in case 1, who responded to apatinib rapidly and had rapidly reduced central nervous symptoms. It has been reported that apatinib effectively suppressed cell proliferation by regulating glycolysis through the VEGFR2/AKT1/GSK3β/SOX5 signaling pathway in ovarian cancer cells[25]. Preclinical findings have indicated that apatinib also appeared to reverse multidrug resistance by inhibiting the transport function of multidrug resistance protein 1 (ABCB1), multidrug resistance-associated protein 1 [ATP-binding cassette (ABC) subfamily C member 1], and breast cancer resistance protein (ATP binding cassette subfamily G member 2)[17,36]. Apatinib has been found to repress the expression of STAT3 and BCL-2 and suppress the growth of osteosarcoma[37]. Apatinib has a strong inhibitory effect on recurrent malignant gliomas, probably because its interaction with intracellular VEGFR-2 is stronger than that of other TKIs (such as sorafenib, pazopanib, and sunitinib)[38]. Cowey et al[39] found that VEGFR-2 has a high specificity for apatinib.
The results of this study showed that apatinib had a definite effect on multiple micrometastatic brain tumors. Apatinib can inhibit the proliferation of intracranial vascular endothelial cells through the blood-brain barrier, reduce tumor neovascularization, and thus inhibit tumor growth. Apatinib mesylate is expected to be an optional targeted drug for patients with multiple micrometastases in the brain. However, it is still in the stage of clinical exploration. There is no clear recommen-dation or guidance for the initial dose and maintenance dose for brain metastases. In addition, its long-term efficacy and adverse reactions need further research. Due to the small number of cases, we could conduct multicenter clinical observations to provide more information on treatments for brain metastases.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Soriano-Ursúa MA S-Editor: Tang JZ L-Editor: Wang TQ E-Editor: Xing YX
| 1. | Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1998. CA Cancer J Clin. 1998;48:6-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1809] [Cited by in RCA: 1687] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 2. | Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 905] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 3. | Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003;29:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 449] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 4. | Hardesty DA, Nakaji P. The Current and Future Treatment of Brain Metastases. Front Surg. 2016;3:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Markesbery WR, Brooks WH, Gupta GD, Young AB. Treatment for patients with cerebral metastases. Arch Neurol. 1978;35:754-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 146] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Nathoo N, Chahlavi A, Barnett GH, Toms SA. Pathobiology of brain metastases. J Clin Pathol. 2005;58:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 136] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Freilich RJ, Seidman AD, DeAngelis LM. Central nervous system progression of metastatic breast cancer in patients treated with paclitaxel. Cancer. 1995;76:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Di Lorenzo R, Ahluwalia MS. Targeted therapy of brain metastases: latest evidence and clinical implications. Ther Adv Med Oncol. 2017;9:781-796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Lin NU. Targeted therapies in brain metastases. Curr Treat Options Neurol. 2014;16:276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Lowery FJ, Yu D. Brain metastasis: Unique challenges and open opportunities. Biochim Biophys Acta Rev Cancer. 2017;1867:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 11. | Abdollahi A, Folkman J. Evading tumor evasion: current concepts and perspectives of anti-angiogenic cancer therapy. Drug Resist Updat. 2010;13:16-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 12. | Zhao D, Hou H, Zhang X. Progress in the treatment of solid tumors with apatinib: a systematic review. Onco Targets Ther. 2018;11:4137-4147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Li X, Xu A, Li H, Zhang B, Cao B, Huang J. Novel role of apatinib as a multi-target RTK inhibitor in the direct suppression of hepatocellular carcinoma cells. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1693-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Scott AJ, Messersmith WA, Jimeno A. Apatinib: a promising oral antiangiogenic agent in the treatment of multiple solid tumors. Drugs Today (Barc). 2015;51:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 15. | Chen L, Cheng X, Tu W, Qi Z, Li H, Liu F, Yang Y, Zhang Z, Wang Z. Apatinib inhibits glycolysis by suppressing the VEGFR2/AKT1/SOX5/GLUT4 signaling pathway in ovarian cancer cells. Cell Oncol (Dordr). 2019;42:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Zhang Q, Song Y, Cheng X, Xu Z, Matthew OA, Wang J, Sun Z, Zhang X. Apatinib Reverses Paclitaxel-resistant Lung Cancer Cells (A549) Through Blocking the Function of ABCB1 Transporter. Anticancer Res. 2019;39:5461-5471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Mi YJ, Liang YJ, Huang HB, Zhao HY, Wu CP, Wang F, Tao LY, Zhang CZ, Dai CL, Tiwari AK, Ma XX, To KK, Ambudkar SV, Chen ZS, Fu LW. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res. 2010;70:7981-7991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 277] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 18. | Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y, Sun G, Yang Y, Wang L, Xu N, Cheng Y, Wang Z, Zheng L, Tao M, Zhu X, Ji D, Liu X, Yu H. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013;31:3219-3225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 415] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 19. | Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R, Wang Z, Wang Q, Ouyang X, Yang Y, Ba Y, Liang J, Lin X, Luo D, Zheng R, Wang X, Sun G, Wang L, Zheng L, Guo H, Wu J, Xu N, Yang J, Zhang H, Cheng Y, Wang N, Chen L, Fan Z, Sun P, Yu H. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol. 2016;34:1448-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 724] [Article Influence: 80.4] [Reference Citation Analysis (1)] |
| 20. | Jiang Q, Zhang NL, Ma DY, Tan BX, Hu X, Fang XD. Efficacy and safety of apatinib plus docetaxel as the second or above line treatment in advanced nonsquamous NSCLC: A multi center prospective study. Medicine (Baltimore). 2019;98:e16065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Wang W, Zhang L, Xie Y, Zhen T, Su G, Zang Q. Fatal hemoptysis in patients with advanced esophageal cancer treated with apatinib. Onco Targets Ther. 2018;11:2565-2570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Li J, Wang L. Efficacy and safety of apatinib treatment for advanced esophageal squamous cell carcinoma. Onco Targets Ther. 2017;10:3965-3969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Kong Y, Sun L, Hou Z, Zhang Y, Chen P, Cui Y, Zhu X, Song T, Li Q, Li H, Zhang T, Qin L. Apatinib is effective for treatment of advanced hepatocellular carcinoma. Oncotarget. 2017;8:105596-105605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Gou M, Si H, Zhang Y, Qian N, Wang Z, Shi W, Dai G. Efficacy and safety of apatinib in patients with previously treated metastatic colorectal cancer: a real-world retrospective study. Sci Rep. 2018;8:4602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Song Y, Liu B, Guan M, Liu M. Successful treatment using apatinib in intractable brain edema: A case report and literatures review. Cancer Biol Ther. 2018;19:1093-1096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Zhang H, Chen F, Wang Z, Wu S. Successful treatment with apatinib for refractory recurrent malignant gliomas: a case series. Onco Targets Ther. 2017;10:837-845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 46672] [Article Influence: 3333.7] [Reference Citation Analysis (4)] |
| 28. | Viallard C, Larrivée B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20:409-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 1003] [Article Influence: 125.4] [Reference Citation Analysis (0)] |
| 29. | Harlozinska A. Progress in molecular mechanisms of tumor metastasis and angiogenesis. Anticancer Res. 2005;25:3327-3333. [PubMed] |
| 30. | Denton AE, Roberts EW, Fearon DT. Stromal Cells in the Tumor Microenvironment. Adv Exp Med Biol. 2018;1060:99-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 192] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 31. | Yan Y, Wang LF, Wang RF. Role of cancer-associated fibroblasts in invasion and metastasis of gastric cancer. World J Gastroenterol. 2015;21:9717-9726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 32. | Yonemura Y, Endo Y, Fujita H, Fushida S, Ninomiya I, Bandou E, Taniguchi K, Miwa K, Ohoyama S, Sugiyama K, Sasaki T. Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin Cancer Res. 1999;5:1823-1829. [PubMed] |
| 33. | Chen G, Liu XY, Wang Z, Liu FY. Vascular endothelial growth factor C: the predicator of early recurrence in patients with N2 non-small-cell lung cancer. Eur J Cardiothorac Surg. 2010;37:546-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Yang Y, Pei X, Yang M. Combination of apatinib and continuous nutritional support for a gastric cancer patient with brain metastasis prolongs survival. J Clin Pharm Ther. 2018;43:726-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Hu T, Liu C, Li Q, Xiong J, Ma Y, Wu G, Zhao Y. Apatinib + CPT-11 + S-1 for treatment of refractory brain metastases in patient with triple-negative breast cancer: Case report and literature review. Medicine (Baltimore). 2018;97:e0349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Tong XZ, Wang F, Liang S, Zhang X, He JH, Chen XG, Liang YJ, Mi YJ, To KK, Fu LW. Apatinib (YN968D1) enhances the efficacy of conventional chemotherapeutical drugs in side population cells and ABCB1-overexpressing leukemia cells. Biochem Pharmacol. 2012;83:586-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 37. | Liu K, Ren T, Huang Y, Sun K, Bao X, Wang S, Zheng B, Guo W. Apatinib promotes autophagy and apoptosis through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death Dis. 2017;8:e3015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 212] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 38. | Li J, Zhao X, Chen L, Guo H, Lv F, Jia K, Yv K, Wang F, Li C, Qian J, Zheng C, Zuo Y. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer. 2010;10:529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 208] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 39. | Cowey CL, Sonpavde G, Hutson TE. New advancements and developments in treatment of renal cell carcinoma: focus on pazopanib. Onco Targets Ther. 2010;3:147-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |