Published online Mar 6, 2020. doi: 10.12998/wjcc.v8.i5.995
Peer-review started: November 26, 2019
First decision: December 30, 2019
Revised: January 8, 2020
Accepted: February 15, 2020
Article in press: February 15, 2020
Published online: March 6, 2020
Processing time: 100 Days and 20.3 Hours
Multiple acyl-CoA dehydrogenase deficiency (MADD) is an uncommon autosomal recessive disorder of mitochondrial fatty acid beta-oxidation. Syncope is a transient loss of consciousness due to acute global cerebral hypoperfusion. Late-onset MADD with syncope has not been reported previously.
We report a 17-year-old girl with exercise intolerance and muscle weakness. She felt palpitation and shortness of breath after short bouts of exercise. She also suffered from a transient loss of consciousness many times. Muscle biopsy showed lipid storage. Genetic mutation analysis indicated a compound heterozygous mutation c.250G > A (p.A84T) and c.872T > G (p.V291G) in the ETFDH gene. The results of Holter electrocardiogram monitoring showed supraventricular tachycardia when the patient experienced a loss of consciousness. After treatment with riboflavin and carnitine, muscle weakness and palpitation symptoms improved rapidly. No loss of consciousness occurred, and the Holter electrocardiogram monitoring was normal.
Late-onset MADD with supraventricular tachycardia can cause cardiac syncope. Carnitine and riboflavin supplement were beneficial for treating the late-onset MADD with cardiac syncope. Attention should be paid to the prevention of cardiac syncope when diagnosing late-onset MADD.
Core tip: Multiple acyl-CoA dehydrogenase deficiency (MADD) is an uncommon autosomal recessive disorder of mitochondrial fatty acid beta-oxidation. Syncope is a transient loss of consciousness due to acute global cerebral hypoperfusion. Late-onset MADD with syncope has not been reported previously. Here, we present a case of late-onset MADD with cardiac syncope, which suggested that we should pay attention to the monitoring of cardiac function and the prevention of cardiac syncope when we diagnose and treat late-onset MADD. Carnitine and riboflavin supplement were beneficial for treating the late-onset MADD with cardiac syncope.
- Citation: Pan XQ, Chang XL, Zhang W, Meng HX, Zhang J, Shi JY, Guo JH. Late-onset multiple acyl-CoA dehydrogenase deficiency with cardiac syncope: A case report. World J Clin Cases 2020; 8(5): 995-1001
- URL: https://www.wjgnet.com/2307-8960/full/v8/i5/995.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i5.995
Multiple acyl-CoA dehydrogenase deficiency (MADD) is an autosomal recessive inherited metabolic disorder that is also known as glutaric aciduria type II. MADD is mainly caused by mutations in the ETFA, ETFB, or ETFDH genes. Among them, ETFDH gene mutations have been reported to be the major cause of MADD. Mutations in the ETFA, ETFB, and ETFDH genes result in the deficient function of the alpha and beta subunits of the electron transfer protein (ETF) or electron transfer flavoprotein ubiquinone oxidoreductase (ETF-QO)[1,2]. Additionally, mutations in the SLC52A, SLC25A32, and FLAD1 genes can also lead to MADD[3,4]. The clinical phenotype is heterogeneous and can be divided into two forms of phenotype: Neonatal-onset and late-onset. Neonatal-onset MADD is severe and predominantly characterized by non-ketotic hypoglycemia, hepatomegaly, metabolic acidosis, hypotonia, severe encephalopathy, and cardiac damage[5]. Late-onset MADD mainly features proximal limb and neck muscle weakness, fatigue, and myalgia[5]. Diagnosis is based on urine organic acid and serum acyl-carnitines. However, the diagnosis of late-onset MADD remains challenging due to the atypical elevation of acyl-carnitine levels, secondary carnitine deficiency, and a narrow detectable period[6,7].
Syncope is a clinical syndrome characterized by a transient loss of consciousness. The main reason for this is temporary and self-terminating global cerebral hypoperfusion. The characteristics of syncope are abrupt onset, short duration, and spontaneous occurrence. The premonitory symptoms of syncope include dizziness, blurred vision, and limb weakness. The transient loss of consciousness is always combined with hypotension, paleness, and a cold sweat, sometimes with limb jerks and the incontinence of urine or feces. The transient loss of consciousness always recovers within several minutes. Syncope is classified into two categories: Reflex syncope and cardiac syncope. Reflex syncope affects up to 40% of the population. A tilt table test is an important diagnostic tool for reflex syncope[8]. Cardiac syncope is the second most common syncope and includes arrhythmic syncope and cardiac organic syncope. Both bradyarrhythmias and tachyarrhythmias may predispose individuals to arrhythmic syncope. Cardiomyopathy, valvular disease, and coronary heart disease can lead to cardiac organic syncope. A 12-lead electrocardiogram (ECG) can be used to diagnose arrhythmic cardiac syncope. Holter ECG monitoring is of more value if syncope is very frequent. Syncope is also needed to distinguish epileptic seizures, functional transient loss of consciousness, and a group of rare causes[9].
This is the first report of late-onset MADD with cardiac syncope. In this report, we described a patient presenting with exercise intolerance, muscle weakness, palpitation, and a transient loss of consciousness. A diagnosis of late-onset MADD with cardiac syncope was finally made.
A 17-year-old girl had experienced exercise intolerance and general fatigue for 4 years. Over those 4 years, she always felt palpitation and shortness of breath after short bouts of exercise. Within the past year, she had started to have progressive proximal limb weakness and myalgia. The symptoms above gradually worsened and then “dropped head syndrome” occurred. She received vitamin therapy for approximately half a year and showed significant improvement. Then, she stopped the vitamin therapy by herself. She presented with proximal limb weakness again after short bouts of exercise, with severe palpitation, but without chest tightness, chest pain, or chest discomfort. Within the past year, she had experienced a transient loss of consciousness several times, presenting with paleness, cold sweats, and blurred vision. This loss of consciousness lasted 1-2 min every time. The patient once had a loss of consciousness on the supine, the blood pressure was 122/76 mmHg, and the loss of consciousness lasted about 1 min.
The patient had no previous medical history.
None.
The heart rate was 120 bpm. The blood pressure was 118/75 mmHg. No abnormalities were observed in the general physical examination. The Medical Research Council muscle strength scores were as follows: Proximal limbs (4/5) and neck flexion (2/5). Deep tendon reflexes were not elicited. Sensory examination was intact to pinprick.
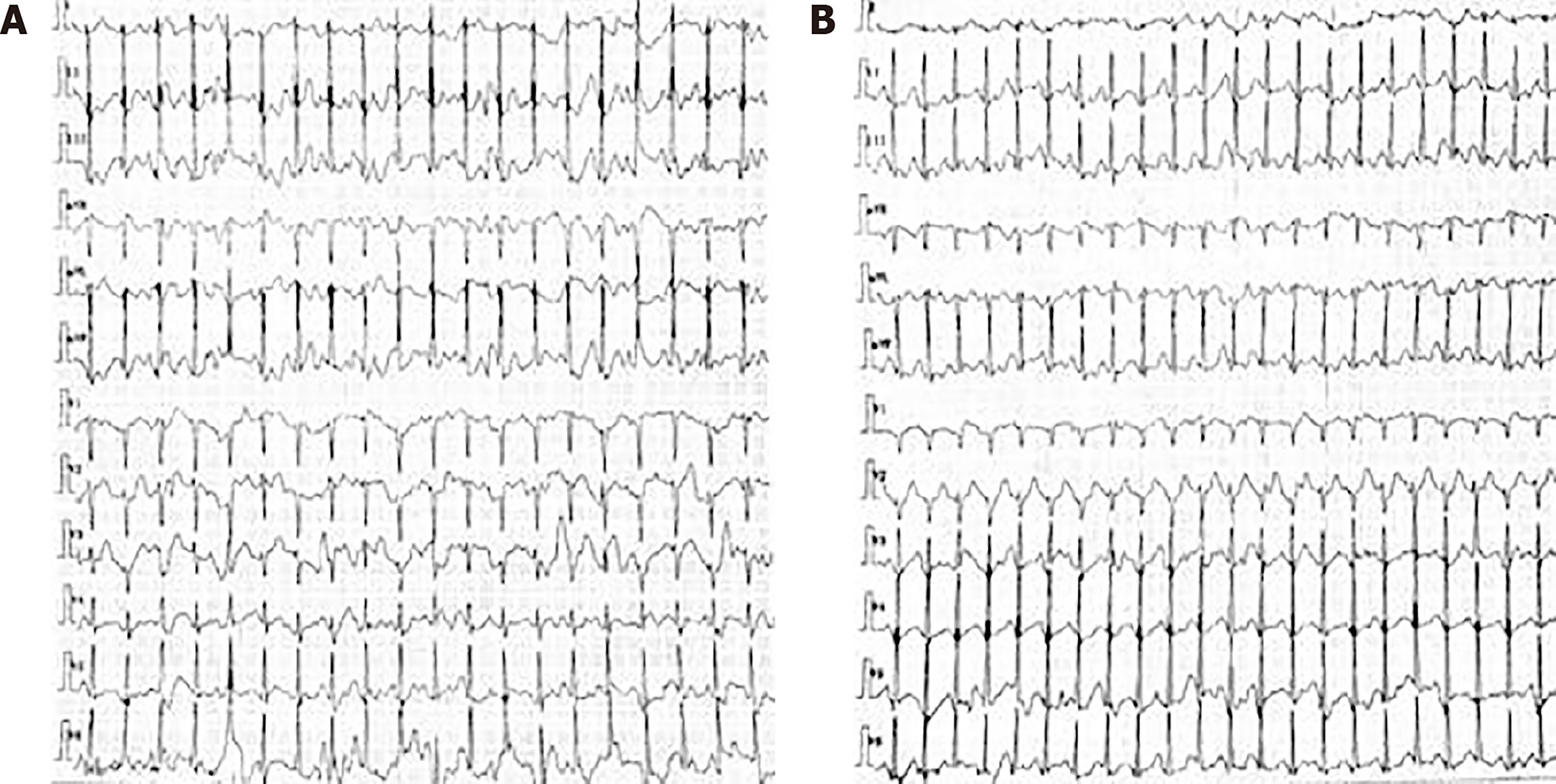
The following serum biochemistry markers were abnormal: ALT was 157 U/L (7-40 U/L) and AST was 114 U/L (13-35 U/L). Serum CK, LDH, electrolytes, total protein, lactic acid, creatinine, and urea nitrogen were within normal limits. The free carnitine level was 4.40 (normal range: 10-60 mmol/L), and the serum acyl-carnitine analysis suggested decreased C2 (4.10 mmol/L; normal range: 6-30 mmol/L), C3 (0.27 mmol/L; 0.5-4 mmol/L), and C4DC (0.17 mmol/L; 0.2-1.2 mmol/L) levels and increased C16 (C16:1: 0.22 mmol/L, 0.02-0.2 mmol/L; C16:2: 0.07 mmol/L, 0-0.05 mmol/L) levels compared to the normal limits. Organic acid analysis was normal. Motor and sensory nerve conduction studies in the four limbs showed normal results. A needle electromyogram showed mildly decreased motor unit action potentials in the left quadriceps femoris, biceps femoris, and tibialis anterior muscle. The 12-lead ECG was normal when she had a physical examination before the limb weakness. The Holter ECG monitoring showed supraventricular tachycardia before treatment, and the heart rate was 158 bpm (Figure 1A). The Holter ECG monitoring showed supraventricular tachycardia when the patient presented with a loss of consciousness, and the heart rate was 184 bpm (Figure 1B). After the treatment, the Holter ECG was normal. Cardiac MRI did not show abnormal lipid deposition in cardiomyocytes. The echocardiography did not show ventricular hypertrophy or dilatation. There was no abnormal discharge in the interictal electroencephalogram. Our patient underwent two tilt table tests before and after treatment. No abnormality was found in the two-tilt table test. Fatty liver was noticed in the abdomen on ultrasound examination.
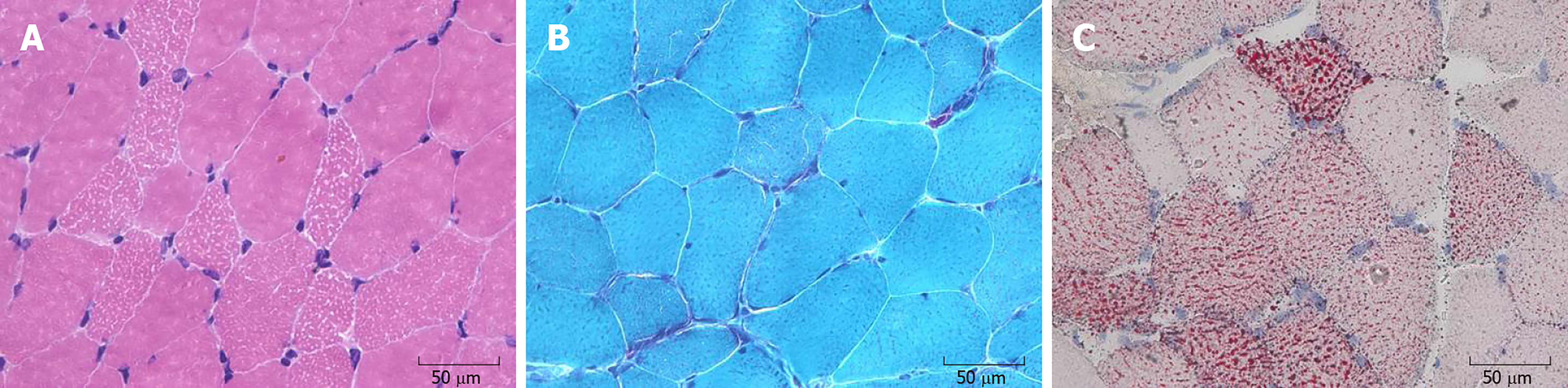
The histochemical staining of all the muscular sections demonstrated similar outcomes. Hematoxylin and eosin staining showed muscle fibers with mild variability in size. Some myofibers were modified by small vacuoles, which were most mainly seen in type I fibers (Figure 2A). On modified Gomori trichrome staining, occasional atypical ragged red fibers were observed (Figure 2B). Oil red O staining showed predominant lipid accumulation in type I fibers (Figure 2C).
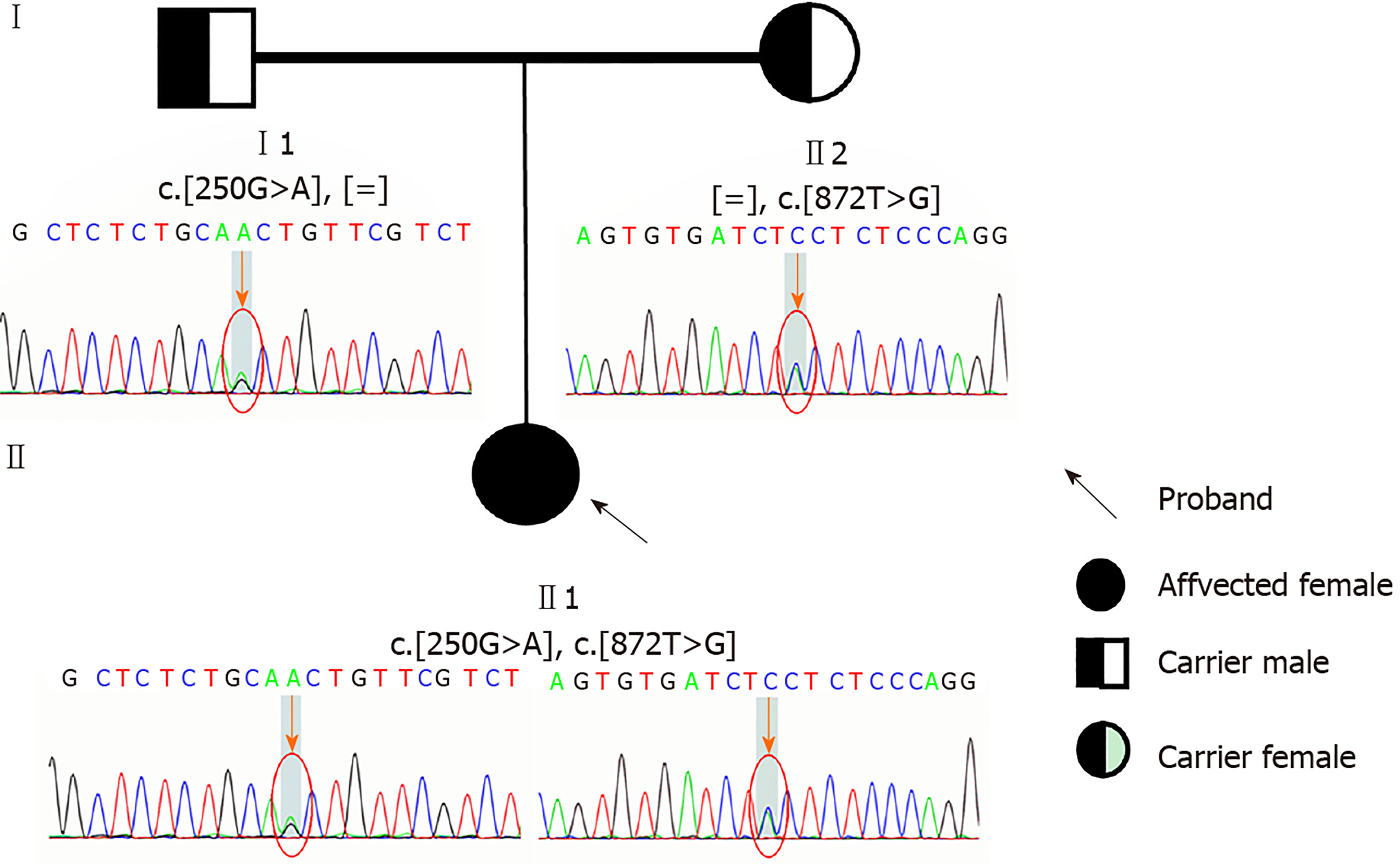
After receiving informed consent, we sequenced ETFDH, ETFA, ETFB, SLC52A, SLC25A32, FLAD1, and mitochondrial genes. Compound heterozygous mutations were identified in the ETFDH gene: c.250G>A (p.A84T) and c.872T>G (p.V291G). The c.872T>G (p.V291G) variant was inherited from her mother and the c.250G>A (p.A84T) variant from her father. The two mutations had been already reported previously. The variants c.250G>A (p.A84T) and c.872T>G (p.V291G) had a very low allele frequency in the 1000 genomes database, ExAC database, and gnomAD database. No other mutations were identified in ETFA, ETFB, SLC52A, SLC25A32, FLAD1, and mitochondrial genes (Figure 3).
Late-onset MADD with cardiac syncope.
The patient was initially treated with riboflavin (30 mg/d), L-carnitine (1 g/d), coenzyme Q10 (50 mg/d), and β-adrenergic blockers.
After 2 wk, she presented with significant improvement in exercise intolerance, general fatigue, and muscle weakness, but palpitation and the loss of consciousness still occurred occasionally. After 1 mo, the muscle strength returned to nearly normal, and there were no symptoms of palpitations or the loss of consciousness. The Holter ECG monitoring was normal. The patient continued to take riboflavin (15 mg/d), L-carnitine (500 mg/d), coenzyme Q10 (50 mg/d), and β-adrenergic blockers.
Here, we report a case of late-onset MADD with syncope. The diagnosis of MADD was based on the symptoms of exercise intolerance, muscle weakness, and myalgia. The result of muscle biopsy indicated a typical myopathological pattern of lipid storage myopathy, which was greatly valuable in pinpointing the diagnosis of MADD. Finally, genetic screening provided definitive diagnostic evidence of late-onset MADD caused by mutations in the ETFDH gene[2].
However, the patient presented with significant palpitation and shortness of breath, predominantly after short exercise bouts. The Holter ECG monitoring showed supraventricular tachycardia. Some studies have shown that cardiac damage can be found in late-onset MADD, including cardiomyopathy and arrhythmia, which may lead to cardiomegaly, cardiac diastolic dysfunction, and heart failure[10-12]. In late-onset MADD, the mutation in ETFDH leads to abnormal ETF-QO structure, causing the dysfunction of different acyl-CoA dehydrogenases and defects in the transfer of electrons generated by dehydrogenation reactions. These lead to the accumulation of long-chain fatty acids and acyl-carnitine in different tissues. The accumulation of acyl-carnitine may result in low serum free carnitine levels, which may exacerbate the abnormalities of fatty acid oxidation in the tissues. Excessive accumulation of fatty acid and acyl-carnitine in cardiac myocytes can cause calcium overload, leading to abnormal myocardial electrical activity and arrhythmia. The abnormal deposition of acyl-carnitine in cardiac myocytes disturbs the Na+/K+-ATPase and increases its activity, leading to the excitation of the α-1-adrenergic receptor of cardiac myocytes and the destruction of the polarization, hyperpolarization, and depolarization of the cardiac myocardium. These can cause arrhythmia[13-15]. In our patient, the serum free carnitine level was decreased before treatment. After treatment, serum free carnitine returned to normal levels, and palpitation symptoms improved significantly. We thought that carnitine deficiency was a part of the essential cause of arrhythmia. Another study found that ETF-QO is an important mitochondrial enzyme of the respiratory chain responsible for passing electrons coming from β-oxidation to the complex of the respiratory chain, and the dysfunction of the ETF-QO protein affects the function of the mitochondrial respiratory chain, resulting in a decrease in ATP, the accumulation of a large number of reactive oxygen species, and abnormal cell excitability and electrical activity[16-18]. In this patient, abnormal mitochondria were found in the muscle biopsies, and we considered mitochondrial dysfunction as another potential cause of arrhythmia.
In addition to cardiac damage, the patient also presented with a transient loss of consciousness. The clinical manifestation of our patient was different from that of classic epilepsy[19]. To identify the causes of the transient loss of consciousness, tilt table testing was conducted. The results are inconsistent with the diagnostic criteria for reflex syncope[20]; thus, reflex syncope was ruled out. We noticed that the arrhythmia of our patient occurred at the onset of the disease, and she presented with a transient loss of consciousness accompanied by arrhythmia during the course of the disease. We thought that cardiac syncope may be the cause of the loss of consciousness. The Holter ECG monitoring showed supraventricular tachycardia when the patient presented with a loss of consciousness. Therefore, we considered that the supraventricular tachycardia was a cause of the cardiac syncope. For our patient, she did not present with palpitation, shortness of breath, or loss of consciousness, and the 12-lead ECG was normal before limb weakness. After the treatment with riboflavin, there were no symptoms of palpitations or the loss of consciousness and the Holter ECG monitoring was normal. We consider that the paroxysmal supraventricular tachycardia was less likely to be congenital. Some studies have shown that abnormal lipid storage in skeletal muscle may be associated with cardiac syncope but only in the neonatal-onset form[21]. Cardiac syncope has not been reported in patients with late-onset MADD. Currently, there is no definite conclusion about the mechanism of cardiac syncope caused by supraventricular tachycardia. Inadequate autonomic vascular responsiveness is now recognized as having a crucial role in most cases. In the episodes of supraventricular tachycardia, a shortened diastolic time leads to a decrease in left ventricular filling and an increase in sympathetic nerve tension, resulting in a decrease in ventricular volume. The excessive contraction of stimulated ventricular C fibers in the inferior and posterior walls of the left ventricle leads to the increased activity of the vagus nerve. Therefore, the increased activity of the vagus nerve is the main cause of syncope in supraventricular tachycardia[22]. Therefore, for our patient, the main cause of supraventricular tachycardia was the abnormal accumulation of long-chain fatty acids and mitochondrial dysfunction in cardiomyocytes, which affected the cardiac conduction system and resulted in cardiac syncope.
It was notable that the muscle strength and palpitation symptoms improved after treatment with riboflavin and carnitine, and the Holter ECG monitoring was normal. We inferred that riboflavin and carnitine were beneficial for MADD with arrhythmia. Carnitine can promote long-chain fatty acid oxidation in mitochondria[23]. This could reduce the production and toxicity of acyl-carnitine. In our patient, supraventricular tachycardia did not occur after carnitine supplementation, which showed that carnitine supplementation was significant in improving arrhythmia.
In conclusion, we report a late-onset MADD patient with cardiac syncope caused by a mutation in the ETFDH gene. The main reason for arrhythmia in our patient was a lack of free carnitine and mitochondrial dysfunction. Riboflavin and carnitine supplementation play an important role in MADD patients with arrhythmia. Therefore, attention should be paid to the monitoring of cardiac function and the prevention of syncope in the diagnosis and treatment of MADD.
We thank the patient and her family for kindly giving us permission to publish this data. We thank Pathology Lab of our Department for supporting the pathological data.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barik R, Teragawa H S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Liu JH
| 1. | Angelini C, Tavian D, Missaglia S. Heterogeneous Phenotypes in Lipid Storage Myopathy Due to ETFDH Gene Mutations. JIMD Rep. 2018;38:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Olsen RK, Olpin SE, Andresen BS, Miedzybrodzka ZH, Pourfarzam M, Merinero B, Frerman FE, Beresford MW, Dean JC, Cornelius N, Andersen O, Oldfors A, Holme E, Gregersen N, Turnbull DM, Morris AA. ETFDH mutations as a major cause of riboflavin-responsive multiple acyl-CoA dehydrogenation deficiency. Brain. 2007;130:2045-2054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 228] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 3. | Mosegaard S, Bruun GH, Flyvbjerg KF, Bliksrud YT, Gregersen N, Dembic M, Annexstad E, Tangeraas T, Olsen RKJ, Andresen BS. An intronic variation in SLC52A1 causes exon skipping and transient riboflavin-responsive multiple acyl-CoA dehydrogenation deficiency. Mol Genet Metab. 2017;122:182-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Olsen RKJ, Koňaříková E, Giancaspero TA, Mosegaard S, Boczonadi V, Mataković L, Veauville-Merllié A, Terrile C, Schwarzmayr T, Haack TB, Auranen M, Leone P, Galluccio M, Imbard A, Gutierrez-Rios P, Palmfeldt J, Graf E, Vianey-Saban C, Oppenheim M, Schiff M, Pichard S, Rigal O, Pyle A, Chinnery PF, Konstantopoulou V, Möslinger D, Feichtinger RG, Talim B, Topaloglu H, Coskun T, Gucer S, Botta A, Pegoraro E, Malena A, Vergani L, Mazzà D, Zollino M, Ghezzi D, Acquaviva C, Tyni T, Boneh A, Meitinger T, Strom TM, Gregersen N, Mayr JA, Horvath R, Barile M, Prokisch H. Riboflavin-Responsive and -Non-responsive Mutations in FAD Synthase Cause Multiple Acyl-CoA Dehydrogenase and Combined Respiratory-Chain Deficiency. Am J Hum Genet. 2016;98:1130-1145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 5. | Olsen RK, Andresen BS, Christensen E, Bross P, Skovby F, Gregersen N. Clear relationship between ETF/ETFDH genotype and phenotype in patients with multiple acyl-CoA dehydrogenation deficiency. Hum Mutat. 2003;22:12-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 173] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Wen B, Li D, Li W, Zhao Y, Yan C. Multiple acyl-CoA dehydrogenation deficiency as decreased acyl-carnitine profile in serum. Neurol Sci. 2015;36:853-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 7. | Pollard LM, Williams NR, Espinoza L, Wood TC, Spector EB, Schroer RJ, Holden KR. Diagnosis, treatment, and long-term outcomes of late-onset (type III) multiple acyl-CoA dehydrogenase deficiency. J Child Neurol. 2010;25:954-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Kapoor WN. Syncope. N Engl J Med. 2000;343:1856-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 221] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 9. | Task Force for the Diagnosis and Management of Syncope. European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS), Moya A, Sutton R, Ammirati F, Blanc JJ, Brignole M, Dahm JB, Deharo JC, Gajek J, Gjesdal K, Krahn A, Massin M, Pepi M, Pezawas T, Ruiz Granell R, Sarasin F, Ungar A, van Dijk JG, Walma EP, Wieling W. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30:2631-2671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1194] [Cited by in RCA: 1226] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 10. | Zhu M, Zhu X, Qi X, Weijiang D, Yu Y, Wan H, Hong D. Riboflavin-responsive multiple Acyl-CoA dehydrogenation deficiency in 13 cases, and a literature review in mainland Chinese patients. J Hum Genet. 2014;59:256-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Xi J, Wen B, Lin J, Zhu W, Luo S, Zhao C, Li D, Lin P, Lu J, Yan C. Clinical features and ETFDH mutation spectrum in a cohort of 90 Chinese patients with late-onset multiple acyl-CoA dehydrogenase deficiency. J Inherit Metab Dis. 2014;37:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Lan MY, Fu MH, Liu YF, Huang CC, Chang YY, Liu JS, Peng CH, Chen SS. High frequency of ETFDH c.250G>A mutation in Taiwanese patients with late-onset lipid storage myopathy. Clin Genet. 2010;78:565-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Vasiljevski ER, Summers MA, Little DG, Schindeler A. Lipid storage myopathies: Current treatments and future directions. Prog Lipid Res. 2018;72:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Bers DM, Despa S. Na/K-ATPase--an integral player in the adrenergic fight-or-flight response. Trends Cardiovasc Med. 2009;19:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Bonnet D, Martin D, Pascale De Lonlay, Villain E, Jouvet P, Rabier D, Brivet M, Saudubray JM. Arrhythmias and conduction defects as presenting symptoms of fatty acid oxidation disorders in children. Circulation. 1999;100:2248-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 187] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Wajner M, Amaral AU. Mitochondrial dysfunction in fatty acid oxidation disorders: insights from human and animal studies. Biosci Rep. 2015;36:e00281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 17. | Debashree B, Kumar M, Keshava Prasad TS, Natarajan A, Christopher R, Nalini A, Bindu PS, Gayathri N, Srinivas Bharath MM. Mitochondrial dysfunction in human skeletal muscle biopsies of lipid storage disorder. J Neurochem. 2018;145:323-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Akar FG. Mitochondrial targets for arrhythmia suppression: is there a role for pharmacological intervention? J Interv Card Electrophysiol. 2013;37:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Thijs RD, Surges R, O'Brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019;393:689-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 1237] [Article Influence: 206.2] [Reference Citation Analysis (0)] |
| 20. | Adkisson WO, Benditt DG. Pathophysiology of reflex syncope: A review. J Cardiovasc Electrophysiol. 2017;28:1088-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Dunnigan A, Pierpont ME, Smith SA, Breningstall G, Benditt DG, Benson DW. Cardiac and skeletal myopathy associated with cardiac dysrhythmias. Am J Cardiol. 1984;53:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | van Dijk JG, Thijs RD, Benditt DG, Wieling W. A guide to disorders causing transient loss of consciousness: focus on syncope. Nat Rev Neurol. 2009;5:438-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Wang ZY, Liu YY, Liu GH, Lu HB, Mao CY. l-Carnitine and heart disease. Life Sci. 2018;194:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |