Published online Mar 6, 2020. doi: 10.12998/wjcc.v8.i5.954
Peer-review started: November 3, 2019
First decision: December 4, 2019
Revised: January 14, 2020
Accepted: February 15, 2020
Article in press: February 15, 2020
Published online: March 6, 2020
Processing time: 123 Days and 23 Hours
A 46-year-old male underwent ascending aortic replacement, total arch replacement, and descending aortic stent implantation for Stanford type A aortic dissection in 2016. However, an intraoperative stent-graft was deployed in the false lumen inadvertently. This caused severe iatrogenic thoracic and abdominal aortic dissection, and the dissection involved many visceral arteries.
The patient had pain in the chest and back for 1 mo. A computed tomography scan showed that the patient had secondary thoracic and abdominal aortic dissection. The ascending aortic replacement, total arch replacement, and descending aortic stent implantation for Stanford type A aortic dissection were performed 2 years prior. An intraoperative stent-graft was deployed in the false lumen. Endovascular aneurysm repair was performed to address this intractable situation. An occluder was used to occlude the proximal end of the true lumen, and a covered stent was used to direct blood flow back to the true lumen. A three-dimensional printing technique was used in this operation to guide pre-fenestration. The computed tomography scan at the 1stmo after surgery showed that the thoracic and abdominal aortic dissection was repaired, with all visceral arteries remaining patent. The patient did not develop renal failure or neurological complications after surgery.
The total endovascular repair for false lumen stent-graft implantation was feasible and minimally invasive. Our procedures provided a new solution for stent-graft deployed in the false lumen, and other departments may be inspired by this case when they need to rescue a disastrous stent implantation.
Core tip: In this work, we report an extremely rare case of repair for intraoperative stent-graft deployed in the false lumen of Stanford type A aortic dissection. This is very rare because the patient survived this catastrophic thoracic surgery for 2 years. We performed a well-designed total endovascular repair to draw blood back to the true lumen. Our report presents a new solution for incorrect stent-graft implantation. Other departments may be inspired by this case when they need to rescue an incorrect stent implantation. Moreover, we discuss regular rescue methods and preventive measures used for stent implantation of the false lumen.
- Citation: Li XR, Tong YH, Li XQ, Liu CJ, Liu C, Liu Z. Total endovascular repair of an intraoperative stent-graft deployed in the false lumen of Stanford type A aortic dissection: A case report. World J Clin Cases 2020; 8(5): 954-962
- URL: https://www.wjgnet.com/2307-8960/full/v8/i5/954.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i5.954
The use of elephant trunk stent can prevent distal residual dilation and anastomotic leakage after total arch replacement and shows survival benefits[1,2]. Unfortunately, when the true lumen is too compressed to identify, the stent may enter the false lumen. The prevalence of intraoperative stent-graft deployed in the false lumen is relatively rare. However, malignant stent implantation is also a complication of thoracic endovascular aortic repair. Therefore, every surgeon should pay more attention to its treatment. We report our experience with total endovascular repair of an intraoperative stent-graft deployed in the false lumen.
At 2 years after surgery for aortic dissection, chest tightness lasted for 1 mo.
The patient was admitted to the department of cardiothoracic surgery of our hospital 2 years prior due to repeated chest tightness. He underwent ascending aortic replacement, total arch replacement, and descending aortic stent implantation for Stanford type A aortic dissection on January 2016. The patient recovered well after the operation and the symptom of chest tightness was slightly relieved. However, 1 mo ago, the patient felt chest tightness after a little exercise, accompanied by left limb paresthesia, and it was only slightly relieved after rest. The patient had no chest or back lancinate pain or radiating pain, and no nausea, vomiting or other discomfort.
The patient underwent surgery for aortic dissection 2 years prior. He had hypothyroidism for 2 years and was treated with Euthyrox. In addition, he had a history of coronary heart disease for 1 mo.
The patient’s parents were healthy and his personal history was normal.
On admission, the patient's body temperature was 36.8°C, pulse was 95 times/min, and blood pressure was 159/77 mm/Hg. His breathing was steady, averaging 18 breaths per minute. The patient had an old surgical scar in the middle of chest, no deformity of chest and breath sounds in both lungs were normal. There was no abnormal uplift in his precardiac region, and no obvious expansion of his cardiac dullness boundary. His heart rate was regular at 95 beats/min and there was no pathological murmur. The patient has a weak carotid pulse on the right side. There were no positive signs in abdomen and no swelling in the lower limbs.
There was no significant abnormality in preoperative blood routine and stool routine examination, and the occult blood test was negative. The patient had hypothyroidism, presenting with elevated thyrotrophin(83.41 mIU/L) and multiple positive thyroid antibodies. Biochemical examination showed that the estimated glomerular filtration rate was 77.2 mL/min/1.73 m2, and urine protein was weakly positive in urine analysis, indicating slightly abnormal renal function.
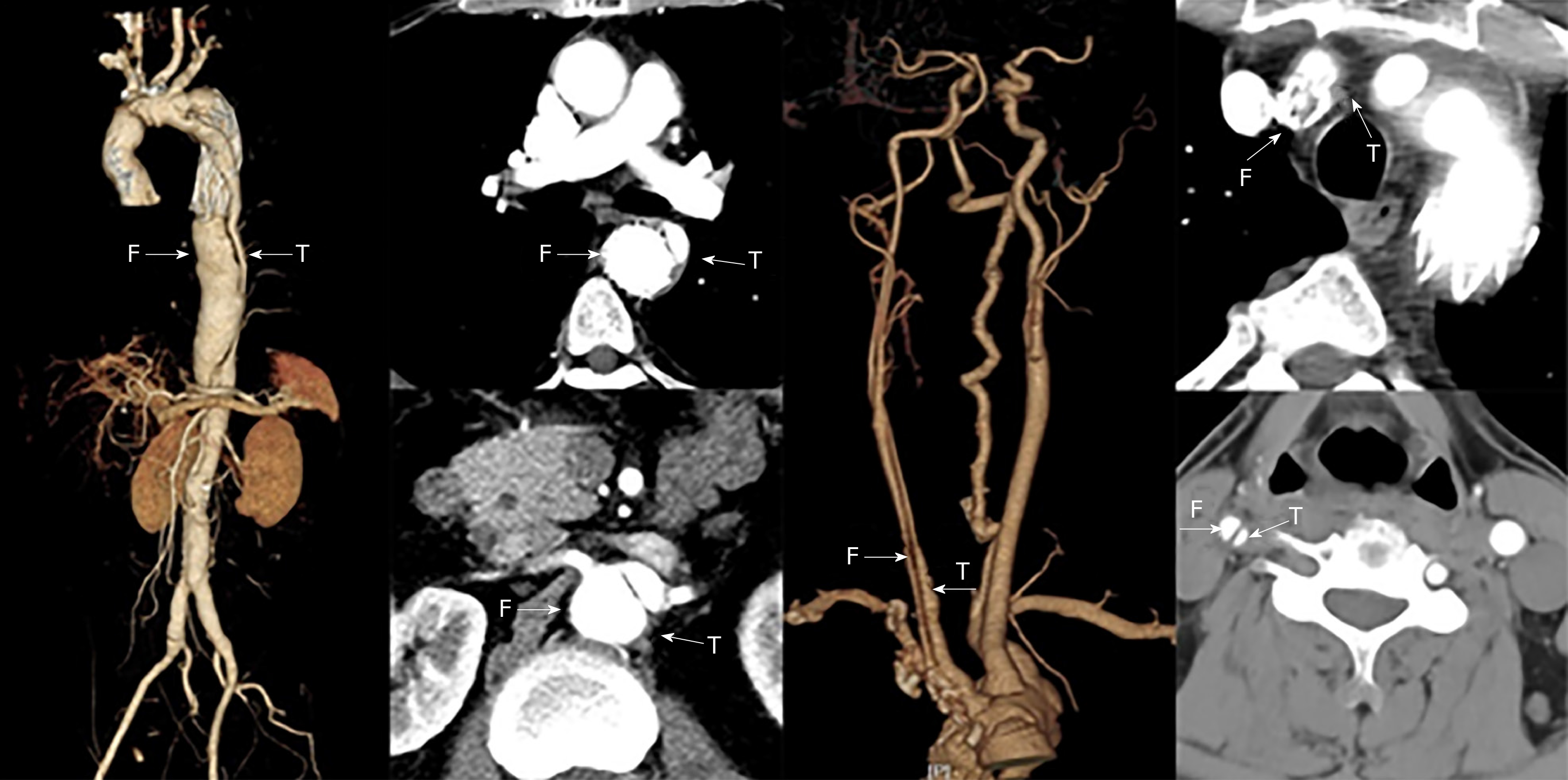
Vascular ultrasound revealed dissection in the right carotid artery and the blood was decreased in the right internal carotid artery. The stenosis of the innominate artery was approximately 50%-69%.There was no obvious abnormality in the right subclavian artery. An intraoperative stent-graft was deployed in the false lumen inadvertently in the surgery 2 years prior. This caused severe iatrogenic thoracic and abdominal aortic dissection, and the dissection involved many visceral arteries. The computed tomography angiography (CTA) showed that the blood supply of celiac artery and superior mesenteric artery came from the true lumen and the blood supply of both renal arteries came from the true and false lumen (Figure 1). CTA examination of the neck revealed that the stent of the innominate artery was located in the false lumen, and the right internal carotid artery also formed dissection. The patient survived after the wrong stent implantation, because there were a large number of intimal tears in the distal aorta which communicated between the true and false lumens. A series of endovascular operations were performed to address the intractable situation (Table 1).
| Date | July 16, 2018 | July 18, 2018 | August 1, 2018 | December 10, 2018 |
| Operation | Aortography | Left subclavian artery-right carotid artery artificial vascular bypass surgery | Thoracic aortic stent implantation and Interventional occlusion operation | Abdominal aortic stent implantation |
Thoracic aortic dissection (after stent implantation), coronary heart disease, hypothyroidism, and cholecystolithiasis.
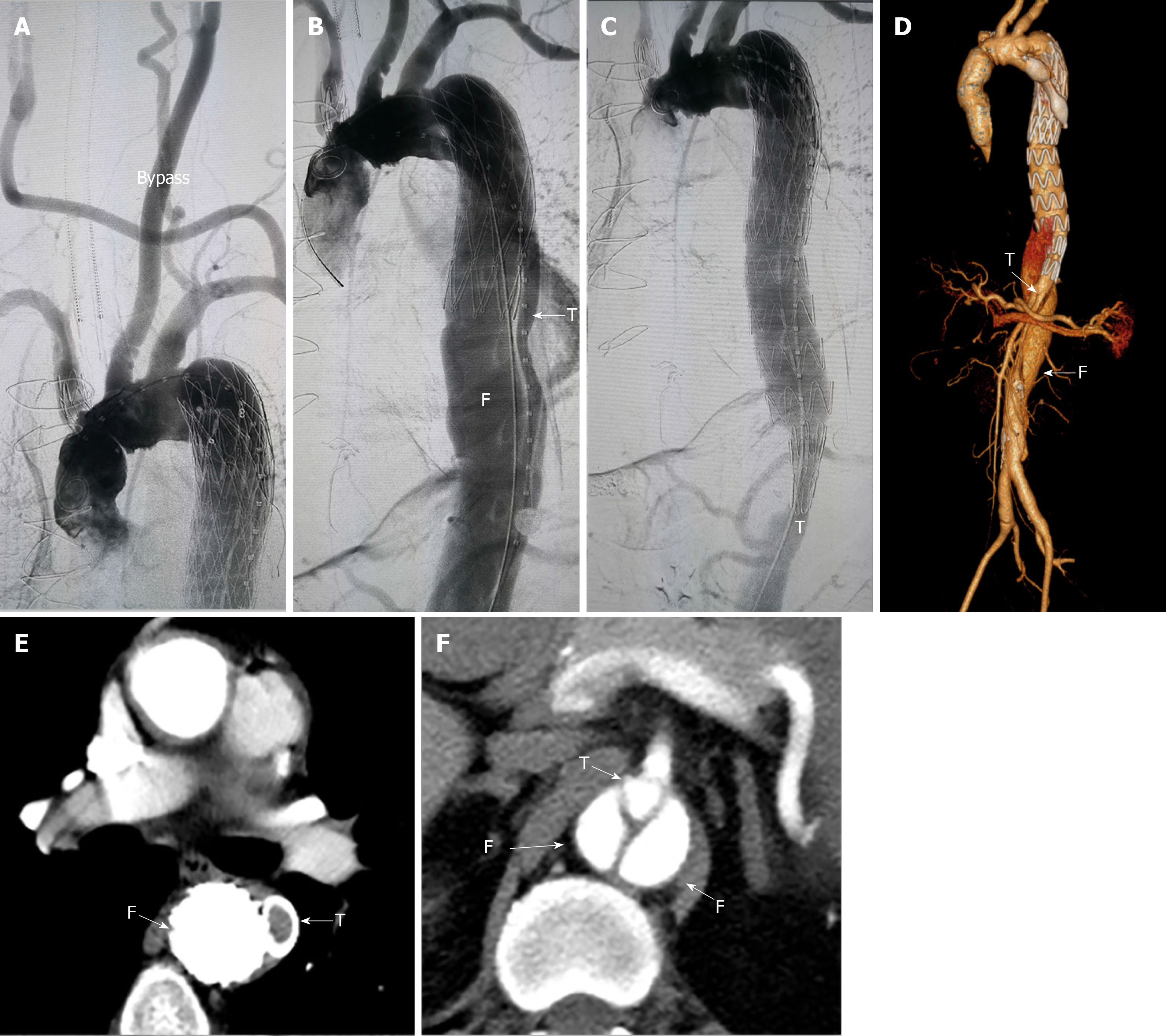
In July 2018, left subclavian artery-right carotid artery artificial vascular bypass surgery was performed to repair the right common carotid artery dissection (Figure 2A). After 1 mo, thoracic endovascular aortic repair was performed. Under general anesthesia, an occluder (28 mm, WBFDQ-I 16, Shanghai memory alloy) was used to occlude the true lumen (Figure 2B). A covered stent (32 mm × 200 mm, Ankura, Ankuracompany, China) was deployed through the intimal tear to overlap with the original stent in the false lumen for approximately 3 cm. The proximal end of the covered stent was in the false lumen and the distal end was in the true lumen. It drew the blood flow back to the true lumen successfully (Figure 2C). A CTA examination 3 mo after surgery showed that the descending aortic dissection was completely repaired (Figure 2D-F).
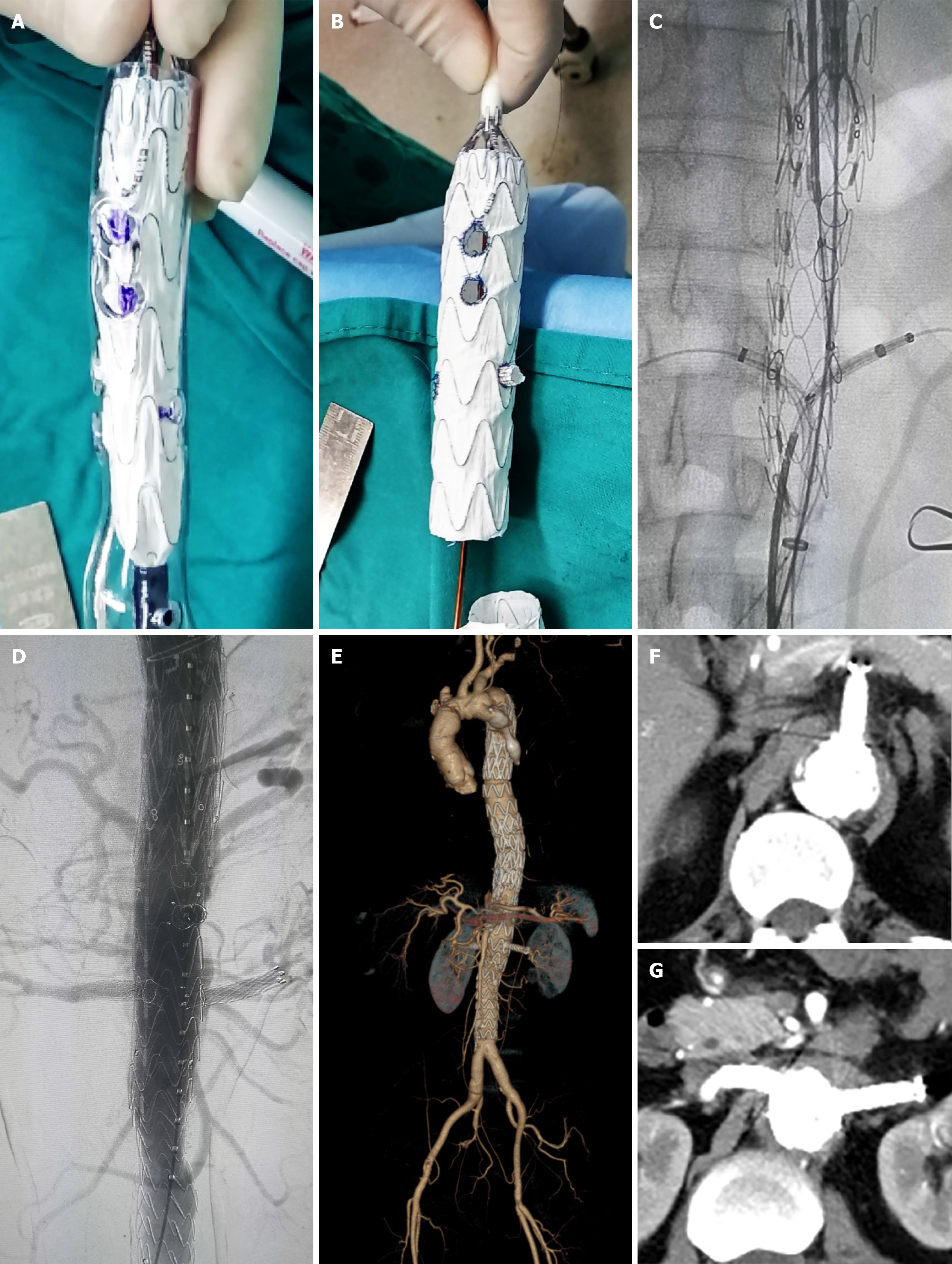
The patient underwent the second stage of surgery in December 2018. Before surgery, the positions of the visceral arteries were located by the three-dimensional (3D) printing technique. The 3D printing guide plate was designed by Geomagic Design Direct 2014 software. During the operation, the covered stent (28 mm × 24 mm × 160 mm, Ankura, Ankura Company, China) was fenestrated at the positions of the celiac trunk artery, superior mesenteric artery, and bilateral renal arteries according to the custom 3D printing model. A very short-branched stent was sutured at the position of left renal artery to avoid endoleak (Figure 3A, B). The customized stent was deployed in the true lumen of abdominal aorta from the right femoral approach. Then two branched stents were deployed in the superior mesenteric artery, and the right renal artery and balloons were used to expand these branched stents. Another two covered stents were deployed at the distal end of the fenestrated stent to deal with endoleak (Figure 3C, D). The thoracic and abdominal aortic dissection was successfully repaired, with all the visceral arteries preserved. The operation lasted approximately 7.5 h and the radiation dose was 1529 mGy. The volume of intraoperative hemorrhage was approximately 500 mL and the blood transfusion volume was approximately 600 mL.
Patients were given oral antihypertensive drugs to control their blood pressure every day after discharge. The patient received long-term antiplatelet management with rivaroxaban (5 mg/d) and aspirin (100 mg/d) to prevent in-stent restenosis. In addition, regular examinations of aortic CTA were necessary.
The patient suffered from acute renal failure after the thoracic aortic stent implantation and interventional occlusion operation. However, he did not develop renal failure or spinal cord injury after the second EVAR. A computed tomography scan was performed at 3 mo after the operation. The results showed that all of the stents were in the right positions and all of the branch arteries were patent (Figure 3E).
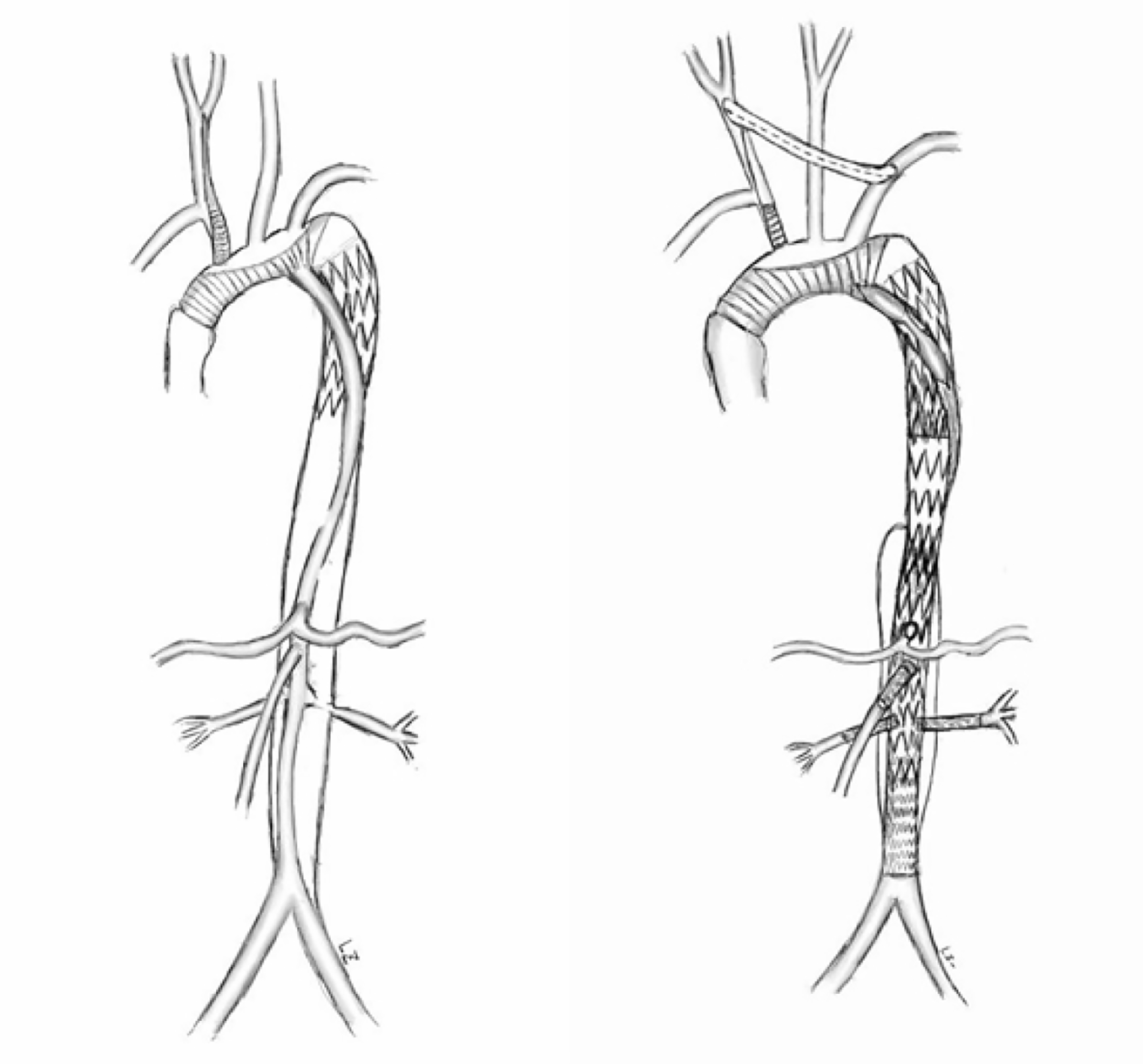
Inappropriate deployment of the stent-graft into the false lumen may lead to hypoperfusion of important viscera and lethal aortic rupture during or after surgery. This patient survived after previous catastrophic stent implantation because there were a large number of intimal tears, which communicated between the true and false lumens but eventually led to severe aortic disease. There are many ways to rescue incorrect stent implantation. Conventional open surgery can be adopted to replace the relevant aortic segments. However, this kind of surgery is very dangerous in high-risk patients and requires extracorporeal circulation[3,4]. EVAR was proved to be minimally invasive and had lower perioperative morbidity and mortality than open surgery[5-7]. However, this less invasive approach still has several complications such as spinal cord injury, renal failure, and neurological complications[8,9]. Thus, operations need to be well designed to reduce these complications (Figure 4).
This operation had several technical challenges. First, we need to divert blood to the true lumen. Ma et al[10] adopted fenestration technique to puncture the septum between lumens, artificially creating a break to connect the true and false lumens. Then, a covered stent was deployed through the break to connect the true and false lumens. In this way, the blood was drawn back into the true lumen. In our case, fenestration was not necessary because there was already a break at the distal end of the aorta. A balloon was used to expand the break, and a covered stent was used to connect the elephant trunk stent in the original false lumen with the true lumen. An occluder was deployed at the proximal end of the true lumen to repair the anastomotic leakage to avoid high-risk fenestration of the aortic arch. Second, personalized stent was the key to retain visceral blood supply. After the first stage of surgery, we repaired the abdominal aortic dissection with a custom stent graft, which was fenestrated with 3D printing guidance. Before the implantation, the stent was released in the 3D-printed vascular model and the positions of fenestration were accurately determined by the model.
With the development of endovascular techniques, there have been many reports about successful rescue after false lumen stent-graft implantation. However, there are serious complications because of incorrect stent implantation including death from intraoperative or postoperative aortic rupture. A large amount of blood flow into the false lumen and less blood is in the true lumen which may eventually lead to multiple organ failure and even death. The pressure in the false lumen was too great which lead to the formation of a dissecting aneurysm at the distal end. Eventually the aneurysm ruptures and patients die. Thus, preventing stents from being deployed in the false lumen is the most crucial step. Some cardiac surgery departments have grasped the concept of modern hybrid aortic surgery. The guide wires can be used to locate the positions of stents and perform angiography at any time. In addition, another stent can be implanted at the distal end of elephant trunk stent to avoid possible complications. Furthermore, the hemodynamic changes in complicated dissections and position of the septum can be assessed using intravascular ultrasound[11,12]. Most importantly, the true and false lumens should be carefully identified by preoperative computed tomography. Usually the true lumen is smaller and the false lumen is larger. The beak sign and a large cross-sectional area indicate a false lumen. Intimal calcification is common in the true lumen and mural thrombus is more common in the false lumen.
To reduce the risk of stent rupture into the false lumen, the length and size of the stents should be carefully selected according to the aortic diameter and the position of the tears before surgery [13]. In addition, it is very important to ensure that the guide wire is in the true lumen. Angiography should be performed before the guide wire and catheter reach the target position and before the stents are released. We should avoid the guide wire passing between the true and false lumens and confirm both ends of the stent are in the true lumen before release.
This case shows that total endovascular repair for incorrect stent-graft implantation is feasible and minimally invasive and personalized operation planning is very important to success. Further research is still needed to evaluate its clinical effect.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Chiu KW, Ghoch ME, Manenti A,Movahed A, Vaudo G S-Editor: Zhang L L-Editor: FilipodiaE-Editor: Xing YX
| 1. | Roselli EE, Bakaeen FG, Johnston DR, Soltesz EG, Tong MZ. Role of the frozen elephant trunk procedure for chronic aortic dissection. Eur J CardiothoracSurg. 2017;51:i35-i39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Chen Y, Ma WG, Zhi AH, Lu L, Zheng J, Zhang W, Liu YM, Zhu JM, Elefteriades JA, Sun LZ. Fate of distal aorta after frozen elephant trunk and total arch replacement for type A aortic dissection in Marfan syndrome. J ThoracCardiovascSurg. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Follis F, Filippone G, Stabile A, Montalbano G, Floriano M, Finazzo M, Follis M. Endovascular graft deployment in the false lumen of type B dissection. Interact CardiovascThoracSurg. 2010;10:597-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Zhang R, Kofidis T, Baus S, Klima U. Iatrogenic type A dissection after attempted stenting of a descending aortic aneurysm. Ann ThoracSurg. 2006;82:1523-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Maurel B, Delclaux N, Sobocinski J, Hertault A, Martin-Gonzalez T, Moussa M, Spear R, Le Roux M, Azzaoui R, Tyrrell M, Haulon S. The impact of early pelvic and lower limb reperfusion and attentive peri-operative management on the incidence of spinal cord ischemia during thoracoabdominal aortic aneurysm endovascular repair. Eur J VascEndovascSurg. 2015;49:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 6. | Cheng D, Martin J, Shennib H, Dunning J, Muneretto C, Schueler S, Von Segesser L, Sergeant P, Turina M. Endovascular aortic repair versus open surgical repair for descending thoracic aortic disease a systematic review and meta-analysis of comparative studies. J Am CollCardiol. 2010;55:986-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 297] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 7. | Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2417] [Cited by in RCA: 2331] [Article Influence: 93.2] [Reference Citation Analysis (0)] |
| 8. | Rocha RV, Friedrich JO, Elbatarny M, Yanagawa B, Al-Omran M, Forbes TL, Lindsay TF, Ouzounian M. A systematic review and meta-analysis of early outcomes after endovascular versus open repair of thoracoabdominal aortic aneurysms. J VascSurg. 2018;68:1936-1945.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Neuhauser B, Greiner A, Jaschke W, Chemelli A, Fraedrich G. Serious complications following endovascular thoracic aortic stent-graft repair for type B dissection. Eur J CardiothoracSurg. 2008;33:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Ma X, Fu W, Ma YT, Karmacharya U, Zhao J. Successful endoluminal rescue of an endovascular graft unintentionally deployed in the false lumen of Stanford type B aortic dissection. J ThoracCardiovascSurg. 2016;151:e41-e45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Guo B, Guo D, Shi Z, Dong Z, Fu W. Intravascular Ultrasound-Assisted Endovascular Treatment of Mesenteric Malperfusion in a Multichannel Aortic Dissection with Full True Lumen Collapse. J EndovascTher. 2019;26:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Koschyk DH, Nienaber CA, Knap M, Hofmann T, Kodolitsch YV, Skriabina V, Ismail M, Franzen O, Rehders TC, Dieckmann C, Lund G, Reichenspurner H, Meinertz T. How to guide stent-graft implantation in type B aortic dissection? Comparison of angiography, transesophageal echocardiography, and intravascular ultrasound. Circulation. 2005;112:I260-I264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Liu Z, Zhang Y, Liu C, Huang D, Zhang M, Ran F, Wang W, Shang T, Qiao T, Zhou M, Liu C. Treatment of serious complications following endovascular aortic repair for type B thoracic aortic dissection. J Int Med Res. 2017;45:1574-1584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |