Published online Mar 6, 2020. doi: 10.12998/wjcc.v8.i5.946
Peer-review started: November 29, 2019
First decision: December 30, 2019
Revised: January 6, 2020
Accepted: February 12, 2020
Article in press: February 12, 2020
Published online: March 6, 2020
Processing time: 97 Days and 17.9 Hours
Severe hyponatremia is considered a rare complication of pituitrin, which is widely used for the treatment of pulmonary hemorrhage. However, the management of pituitrin-associated hyponatremia can be challenging because a rapid correction of hyponatremia may cause the development of osmotic demyelination syndrome, resulting in life-threatening neurological injuries.
A 20-year-old Chinese man with massive hemoptysis developed symptomatic hyponatremia (116 mmol/L) after therapy by a continuous intravenous drip of pituitrin. To normalize his serum sodium, a hypertonic saline infusion was applied for 3 d, and the pituitrin administration was stopped concurrently. Then, an overly rapid increase in serum sodium level (18 mmol/L in 24 h) was detected after treatment. One day later, the patient experienced a sudden onset of generalized tonic-clonic seizures, as well as subsequent dysarthria and dystonia. Magnetic resonance imaging revealed increased signal intensity in the bilateral symmetric basal ganglia on the T2-weighted images, compatible with a diagnosis of extrapontine myelinolysis. The patient received an intravenous administration of high-dose corticosteroids, rehabilitation, and neurotrophic therapy. Finally, his clinical abnormalities were vastly improved, and he was discharged with few residual symptoms.
Physicians should be fully aware that pituitrin can cause profound hyponatremia and its correction must be performed at a controlled rate to prevent the development of osmotic demyelination syndrome.
Core tip: Osmotic demyelination syndrome (ODS) is a devastating neurologic disorder often associated with a precipitous rise in serum sodium. Here we describe a rare case of extrapontine myelinolysis that developed after a rapid correction of symptomatic hyponatremia induced by pituitrin to treat a patient with massive hemoptysis. Because ODS is mainly an iatrogenic disease, effective prevention is mandatory. Through an analysis of the previous literature, the risk factors, clinical characteristics, and outcomes of vasopressin (pituitrin)-associated ODS are discussed in this article.
- Citation: Fang LJ, Xu MW, Zhou JY, Pan ZJ. Extrapontine myelinolysis caused by rapid correction of pituitrin-induced severe hyponatremia: A case report. World J Clin Cases 2020; 8(5): 946-953
- URL: https://www.wjgnet.com/2307-8960/full/v8/i5/946.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i5.946
Massive hemoptysis, representing a life-threatening condition of pulmonary hemorrhage, always needs urgent management. Pituitrin[1,2], extracted from the posterior pituitary, comprises vasopressin and oxytocin and is widely used to treat massive hemoptysis since vasopressin possesses a strong vasoconstriction ability. In addition, vasopressin, also called antidiuretic hormone, has been shown to reduce renal free water excretion, leading to dilutional hyponatremia.
The treatment of pituitrin-induced hyponatremia is quite challenging. On the one hand, severe hyponatremia is often associated with cerebral edema, which causes neurological symptoms ranging from nausea to seizures and even death; on the other hand, an overly rapid correction of hyponatremia may cause abrupt osmotic fluctuations and pose a high risk for osmotic demyelination syndrome (ODS). ODS, first reported in alcoholic patients with malnutrition in 1959[3], comprises central pontine myelinolysis (CPM) and extrapontine myelinolysis (EPM) and is a noninflammatory demyelinating disorder affecting the pons and other regions of the central nervous system; it is characterized clinically by severe and often irreversible neurological deterioration. To date, the underlying mechanism is not fully understood, and the rapid correction of hyponatremia remains the most documented etiologic factor for ODS. It has been suggested that rapid osmotic fluctuations and osmotic-induced fluid shifts may cause cell shrinkage and apoptosis, disruption of the blood-brain barrier, and injury to the myelin sheath, resulting in demyelination[4,5]. Additionally, chronic alcoholism, malnutrition, liver transplantation, and other chronic diseases have also been regarded as risk factors according to recent studies[6].
Here, we report a case of EPM following a rapid correction of pituitrin-induced hyponatremia when treating a patient with massive hemoptysis. We review similar cases reported in the PubMed database and summarize the clinical characteristics and the underlying mechanism in this paper.
A 20-year-old Chinese man was initially admitted to the pulmonary department complaining of repeated hemoptysis for more than one week. During hospitalization, he developed symptomatic hyponatremia and experienced a generalized tonic-clonic seizure, dysarthria, and dystonia after hyponatremia correction.
The patient’s hemoptysis started one week ago but worsened, and he coughed up large amounts of bright red blood (approximately 150-200 mL) in the last 24 h. At the local hospital, he received conventional therapy with aminomethylbenzoic acid and antibiotics, which was ineffective. Then, he was transferred to our hospital for further treatment. He was diagnosed with bronchiectasis 2 years ago, and he had similar episodes in the past.
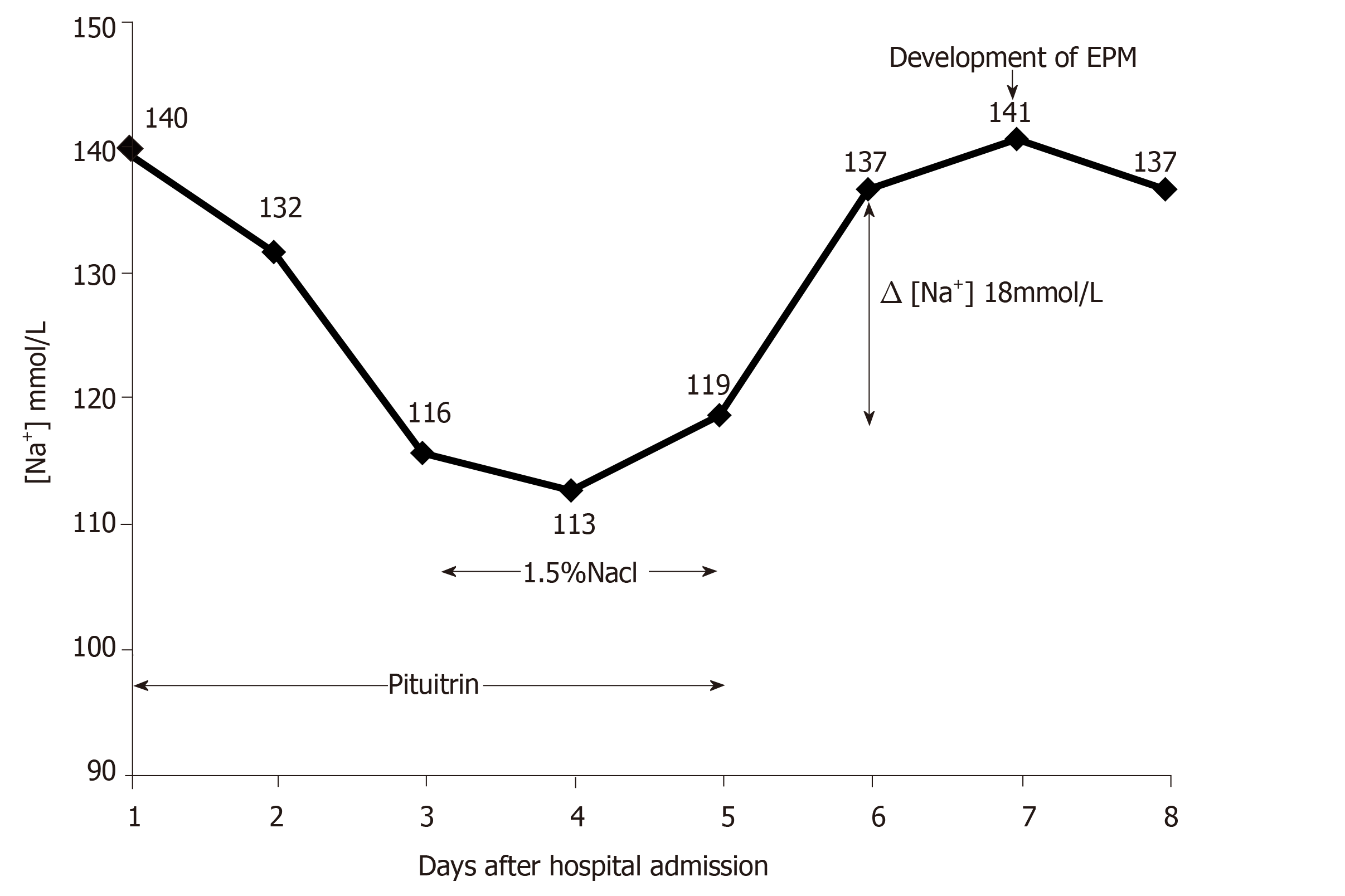
To treat the massive hemoptysis, pituitrin (24 U in 40 mL saline) was administered by intravenous drip continuously at a rate of 3-4 mL/h via a pump for a total of 5 d. In addition, he was given oxygen and empirical antibiotic therapy. Electrolyte disorders and his urine output were intensively monitored accordingly (Table 1). The hemoptysis gradually decreased, but he exhibited severe nausea and vomiting after 3 d of pituitrin infusion. A biochemical assessment revealed significant hyponatremia with a sodium level of 116 mmol/L, as well as a low serum osmolality of 248 mOsm/kg. We prescribed a daily infusion of 1.5% hypertonic saline (500-750 mL per day) from day 3 to day 5. However, it failed to normalize the serum sodium level, and the symptomatic hyponatremia sustained for more than 48 h. Meanwhile, his urine output declined to 0.9 L per day.
| Day | Serum K+ (mmol/L) | Serum Na+ (mmol/L) | Serum osmolality (mOsm/kg) | 1.5% NaCl (mL) | Urine output (L) |
| 1 | 4.12 | 140 | 298 | _ | 2.1 |
| 2 | 4.10 | 132 | 284 | _ | 1.8 |
| 3 | 3.63 | 116 | 248 | 500 | 1.6 |
| 4 | 3.29 | 113 | 240 | 750 | 1.1 |
| 5 | 3.35 | 119 | 251 | 750 | 0.9 |
| 6 | 4.07 | 137 | 288 | _ | 2.1 |
| 7 | 3.80 | 141 | 299 | _ | 2.2 |
| 8 | 4.52 | 137 | 292 | _ | 1.6 |
On day 5, his serum sodium was 119 mmol/L. Then, we stopped pituitrin therapy since we considered pituitrin to be the main cause of his severe hyponatremia. On day 6, the serum sodium level rose to 137 mmol/L [Δ(Na+) 18 mmol/L], accompanied by an obviously elevated urine output from 0.9 L to 2.1 L. Unfortunately, on day 7, the patient developed sudden-onset generalized tonic-clonic seizures. After the seizures were controlled by intravenous diazepam, the neurological symptoms worsened over the following days, presenting as severe dysarthria and movement disorders. He was unable to speak, write, and walk. The changes of the patient’s serum sodium during pituitrin treatment and correction of hyponatremia are shown in Figure 1.
The patient had a history of bronchiectasis for 2 years but had no other comorbid conditions, such as alcoholism, malnutrition, hepatic cirrhosis, or renal insufficiency.
The family history was unremarkable.
The vital signs after seizure cessation included a temperature of 37.2 °C, blood pressure of 116/63 mmHg, heart rate of 85 beats per minute, respiratory rate of 20 breaths per minute, and oxygen saturation of 97% in room air. The neurological examination revealed mildly disturbed consciousness and severe dysarthria, as well as exaggerated muscular tone. The cranial nerve functions, muscle strength, and sensation were all intact.
A routine blood test revealed a white blood cell count of 11.3 × 109/L with 67.4% of neutrophils. The red blood cell and platelet counts were normal. The serum C-reactive protein was slightly elevated at 1.0 mg/dL (normal range: < 0.8 mg/dL). The cerebral spinal fluid test was normal. The electroencephalogram suggested medium, nonspecific neurological dysfunction. Other laboratory data such as biochemistry, electrolytes, thyroid function, tumor markers, and autoimmune profiles were within the normal range. The electrocardiogram and transthoracic cardiac ultrasound were within normal limits.
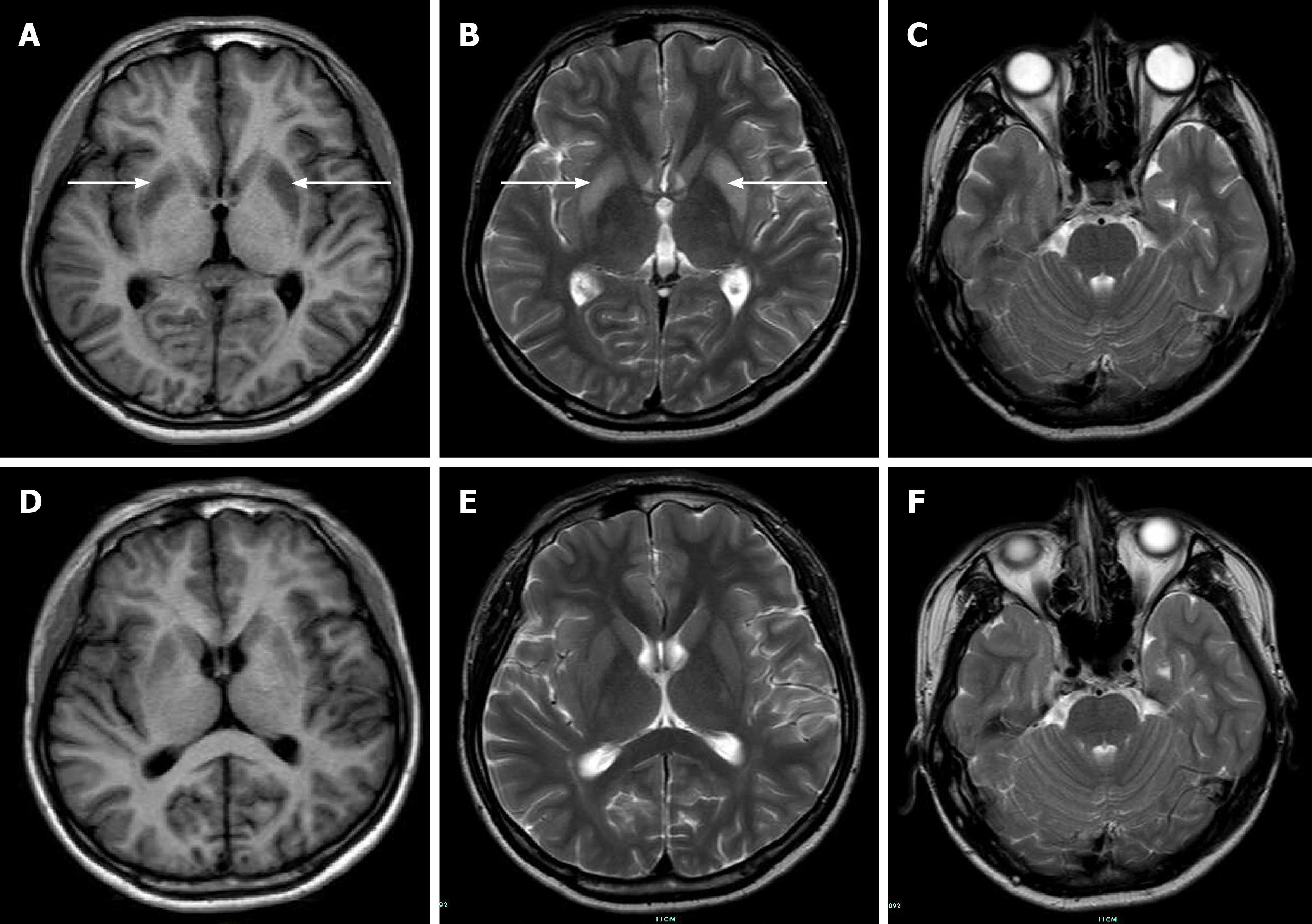
An initial imaging evaluation by head computed tomography presented normal results. Magnetic resonance imaging (MRI) of the brain revealed increased signal intensity in the bilateral symmetric basal ganglia on T2-weighted images and low signal intensity on T1-weighted images (Figure 2).
After expert consultation by neurologists, the patient was diagnosed with EPM caused by the rapid correction of pituitrin-induced severe hyponatremia.
The patient was transferred to the neurology department and was mainly treated with high-dose intravenous methylprednisolone, starting from 240 mg daily for 5 days, tapering off slowly, and finally shifting to oral glucocorticoids. In addition, rehabilitation and neurotrophic therapy were also introduced to treat EPM.
Approximately one week later, the patient began to speak and write simple words; then, progressively, he was able to construct sentences. One month later, his speech vastly improved, but his fluency was still slightly affected. Finally, this patient was discharged with few residual symptoms. Follow-up brain MRI showed reduced signal intensity in the affected regions, which correlated with clinical improvement.
Hyponatremia, defined as a plasma sodium level of less than 135 mmol/L, is the most common electrolyte abnormality encountered in hospitalized patients, resulting from a varied spectrum of conditions[7]. In this case, significant hyponatremia was detected after an intravenous infusion of pituitrin to treat pulmonary hemorrhage. As there was no history of syndromes that caused inappropriate antidiuretic hormone secretion or adrenal insufficiency in this patient, we considered pituitrin to be involved.
Arginine vasopressin in pituitrin is the main active component for the treatment of various bleeding disorders[1]. Vasopressin activates V1a receptors on vascular smooth muscle to contribute to a strong contraction of the arterioles and capillaries. However, its interaction with V2 receptors on renal collecting duct cells can exert antidiuretic effects by stimulating the cyclic adenosine monophosphate signaling cascade, leading to an increase in free water reabsorption and secondary dilutional hyponatremia[2]. As shown in our case, after pituitrin therapy, the patient's daily urine output declined gradually, and his serum sodium fell to a low level of 116 mmol/L, accompanied by severe nausea and vomiting.
The incidence of hyponatremia associated with vasopressin or its analogs (such as terlipressin and desmopressin) varies greatly according to the type of patients studied, ranging from less than 1% to more than 50%. In a retrospective study of 102 adult patients with septic shock who were given vasopressin therapy, only one patient developed hyponatremia[8]. Yim et al[9] reported a hyponatremia rate as high as 67% when terlipressin was used to treat variceal bleeding. Choi et al[10] analyzed 172 nocturnal polyuria patients treated with desmopressin and revealed that 14% of the patients developed hyponatremia, of whom 10% and 4% had mild (126-134 mmol/L) and severe (≤ 125 mmol/L) hyponatremia, respectively.
Although generally safe, it has been reported that in some severe cases, vasopressin or its analogs can reduce serum sodium to a much greater degree, resulting in cerebral edema, seizures, and death[11,12]. The management of severe hyponatremia is complex. Because, for severe patients (< 120 mmol/L), a sodium supplement is always needed to avoid the onset of hyponatremic encephalopathy, but active correction of hyponatremia may increase the risk for developing ODS.
ODS is a devastating neurologic disorder often associated with a precipitous rise in serum sodium. CPM mainly involves the central pontine, and EPM classically affects the thalamus, basal ganglia, or subcortical white matter. The difference in the location of the lesions results in different clinical presentations that are commonly delayed for 2-7 d after rapid elevations in the serum sodium level, including dysarthria, dystonia, behavioral disturbances, confusion, coma, and even death[6,13]. Brain MRI has been considered the gold standard imaging technique to reveal ODS lesions, which typically present with hypointense T1-weighted and hyperintense T2-weighted signals.
Recommendations suggest that ideally, hyponatremia should be corrected by limiting the sodium increase to no more than 8-10 mmol/L every 24 h[7]. In this case, progressive neurological injury occurred after a rapid increase in serum sodium from 119 to 137 mmol/L in 24 h. The imaging features of the brain MRI revealed a hyperintense T2-weighted signal in the bilateral symmetric basal ganglia and were in agreement with the diagnosis of EPM. We summarized several probable causes for a significant increase in serum sodium in this patient. First, an excess of hypertonic saline was transfused. Second, frequent electrolyte monitoring was absent during restoration. Third, pituitrin was withheld abruptly rather than reduced gradually.
According to the guidelines, 100 mL of 3% saline infusion is suggested for severe symptoms and can be repeated two or three times if needed[7,14]. Accordingly, the serum sodium should be checked at least every 2 h to prevent vast fluctuations in serum osmolality. In addition, care must be taken to avoid water diuresis by pituitrin withdrawal that can cause overcorrection. Because the impaired free water excretion was transitory, once the medication stopped, free water excretion could occur unabatedly, and hyponatremia could resolve spontaneously. If the drug was stopped abruptly, a rapid decline in the circulating exogenous antidiuretic hormone levels could lead to the excretion of copious amounts of free water and put the patient at a high risk of overly increasing serum sodium. In particular, when hypertonic saline was also applied, the excessive supplementation of sodium could aggravate the rapid correction of hyponatremia to a dangerous level.
Through a search of the literature, seven cases of ODS associated with vasopressin or its analogs were found in the PubMed database[15-21]. We review the reported cases and summarize the clinical characteristics in Table 2. Briefly, including our case, the mean age of these eight patients was 29.1 years (ranging from 13 to 69 years), with five adolescents younger than 25 years. Pituitrin was prescribed in two patients with massive hemoptysis, and terlipressin was prescribed in one patient with gastrointestinal bleeding. Desmopressin was used in four patients who had central diabetes insipidus. An increase in serum sodium of more than 10 mmol/L in 24 h was observed in seven (87.5%) patients. Only one patient who had a steady decline in his serum sodium level (less than 8 mmol/L per day) developed CPM 25 d after admission[21]. Six (75%) patients had a definite history of drug discontinuation and administration of hypertonic saline. This result suggested that young patients who were provided with hypertonic saline and rapid drug discontinuation were particularly predisposed to an overly rapid correction of their serum sodium levels. Additionally, another study that included 15 patients with desmopressin-associated hyponatremia showed that 13 patients with an average age of 32.5 years who were treated with hypertonic saline and discontinued desmopressin during hyponatremia correction developed significant morbidity (77%) and mortality (23%) compared to the control group[22]. This finding also indicated that cessation in a gradual manner may prevent fatal neurological injury when symptomatic patients were administered hypertonic saline.
| Ref. | Age/sex | Drug | Original disease | Treatment of hypona-tremia | Hypona-tremia improve-ment (mmol/L) | Lesions | Neuro-logical presentation | Therapy for ODS | Outcome |
| Niehaus et al[15], 2001 | 16/F | Antidiuretic hormone | Nocturnal enuresis | Discontinued drug + hypertonic saline | 10 in 24 h (128 to 138) | EPM, CPM | Conscious disturbance | Prednisolone | Recovery |
| Gutenstein[16], 2007 | 39/F | Desmopressin | Hypopituitary | Saline | 38 in 48 h (103 to 141) | EPM, CPM | Dysarthria, hemiplegia, seizures | NA | Death |
| Ranger et al[17], 2010 | 13/M | Desmopressin | Central DI | NA | 60 in 48 h (116 to 176) | EPM, CPM | Coma | NA | Death |
| Solà et al[18], 2010 | NA | Terlipressin | Gastrointestinal bleeding | Discontinued drug + hypertonic saline | 19 in 24 h (109 to 128) | ODS, | Seizures, coma | NA | Recovery |
| Zhuang et al[19], 2014 | 24/F | Pituitrin | Hemoptysis | Discontinued drug + hypertonic saline | 16 in 24 h (118 to 134) | EPM | Dysarthria, quadriparesis, dystonia | Corticosteroid | Recovery |
| Nabaei et al[20], 2015 | 23/F | Desmopressin | Central DI | Discontinued drug + hypertonic saline | 40 in 24 h (126 to166) | EPM, CPM | Quadriparesis, dysarthria, dysphagia | NA | Severe brain damage |
| Hossain et al[21], 2018 | 69/M | Desmopressin | Central DI | Discontinued drug + hypertonic saline | Less than 8 in 24 h | CPM | NA | NA | Recovery |
Concerning the treatment for ODS, supportive therapy should be provided to all patients. Some animal studies and limited case reports have shown that re-lowering the sodium at an early stage may help prevent ODS[23,24]. Chang et al[25] applied plasma exchange to successfully treat a case of CPM caused by acute hypernatremia. Glucocorticoid treatment is also suggested. In animal experiments, early dexamethasone treatment can exert a preventive effect on ODS by reducing the disruption of the blood-brain barrier[26]. High-dose intravenous methylprednisolone achieved success in the current case. In these seven reported cases, five patients recovered after treatment, and two patients died. Nevertheless, there are still no proven definite and effective therapies for ODS, so its prevention is quite essential.
We report a rare case of EPM following the rapid correction of pituitrin-induced hyponatremia. Physicians should be fully aware that the correction of hyponatremia must be performed at a controlled rate. In addition, this case provides a strong warning that hypertonic saline might be a high-risk medication and may require additional precautions when applied to treat pituitrin-induced severe hyponatremia.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vyshka G S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Xing YX
| 1. | Trimble HG, Wood JR. Control of pulmonary hemorrhage by intravenous use of pituitrin. Calif Med. 1952;76:155-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Maybauer MO, Maybauer DM, Enkhbaatar P, Traber DL. Physiology of the vasopressin receptors. Best Pract Res Clin Anaesthesiol. 2008;22:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Adams RD, Victor M, Mancall EL. Central pontine myelinolysis: a hitherto undescribed disease occurring in alcoholic and malnourished patients. AMA Arch Neurol Psychiatry. 1959;81:154-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 449] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 4. | Baker EA, Tian Y, Adler S, Verbalis JG. Blood-brain barrier disruption and complement activation in the brain following rapid correction of chronic hyponatremia. Exp Neurol. 2000;165:221-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Adler S, Martinez J, Williams DS, Verbalis JG. Positive association between blood brain barrier disruption and osmotically-induced demyelination. Mult Scler. 2000;6:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Brown WD. Osmotic demyelination disorders: central pontine and extrapontine myelinolysis. Curr Opin Neurol. 2000;13:691-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 133] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Spasovski G, Vanholder R, Allolio B, Annane D, Ball S, Bichet D, Decaux G, Fenske W, Hoorn EJ, Ichai C, Joannidis M, Soupart A, Zietse R, Haller M, van der Veer S, Van Biesen W, Nagler E; Hyponatraemia Guideline Development Group. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur J Endocrinol. 2014;170:G1-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 496] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 8. | Obritsch MD, Jung R, Fish DN, MacLaren R. Effects of continuous vasopressin infusion in patients with septic shock. Ann Pharmacother. 2004;38:1117-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Yim SY, Seo YS, Jung CH, Kim TH, Kim ES, Keum B, Kim JH, An H, Yim HJ, Yeon JE, Jeen YT, Lee HS, Chun HJ, Byun KS, Um SH, Kim CD, Ryu HS. Risk Factors for Developing Hyponatremia during Terlipressin Treatment: A Retrospective Analyses in Variceal Bleeding. J Clin Gastroenterol. 2015;49:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Choi EY, Park JS, Kim YT, Park SY, Kim GH. The risk of hyponatremia with desmopressin use for nocturnal polyuria. Am J Nephrol. 2015;41:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Guo J, Wang D, Zeng K, Xu G, Zhao Y. Generalized tonic-clonic seizures in adult patients following intravenous administration of desmopressin. Aging Clin Exp Res. 2013;25:479-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Lucchini B, Simonetti GD, Ceschi A, Lava SA, Faré PB, Bianchetti MG. Severe signs of hyponatremia secondary to desmopressin treatment for enuresis: a systematic review. J Pediatr Urol. 2013;9:1049-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Gocht A, Colmant HJ. Central pontine and extrapontine myelinolysis: a report of 58 cases. Clin Neuropathol. 1987;6:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Verbalis JG, Goldsmith SR, Greenberg A, Korzelius C, Schrier RW, Sterns RH, Thompson CJ. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126:S1-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 655] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 15. | Niehaus L, Kulozik A, Lehmann R. Reversible central pontine and extrapontine myelinolysis in a 16-year-old girl. Childs Nerv Syst. 2001;17:294-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Gutenstein M. Osmotic myelinolysis syndrome after treatment of severe deamino arginine vasopressin-associated hyponatraemia: pitfalls in emergency medicine. Emerg Med Australas. 2007;19:68-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Ranger A, Szymczak A, Levin S, Salvadori M, Fraser DD. Osmotic myelinolysis with malignant cerebellar edema occurring after DDAVP-induced hyponatremia in a child. Pediatr Neurosurg. 2010;46:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Solà E, Lens S, Guevara M, Martín-Llahí M, Fagundes C, Pereira G, Pavesi M, Fernández J, González-Abraldes J, Escorsell A, Mas A, Bosch J, Arroyo V, Ginès P. Hyponatremia in patients treated with terlipressin for severe gastrointestinal bleeding due to portal hypertension. Hepatology. 2010;52:1783-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Zhuang L, Xu Z, Li Y, Luo B. Extrapontine myelinolysis associated with pituitrin: case report and literature review. BMC Neurol. 2014;14:189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Nabaei G, Abdi S, Fatehi F. Demyelination syndrome due to rapid correction of desmopressin-associated hyponatremia in a known case of central diabetes insipidus: a case report. Acta Neurol Belg. 2015;115:819-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Hossain T, Ghazipura M, Reddy V, Rivera PJ, Mukherjee V. Desmopressin-Induced Severe Hyponatremia with Central Pontine Myelinolysis: A Case Report. Drug Saf Case Rep. 2018;5:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Achinger SG, Arieff AI, Kalantar-Zadeh K, Ayus JC. Desmopressin acetate (DDAVP)-associated hyponatremia and brain damage: a case series. Nephrol Dial Transplant. 2014;29:2310-2315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Ochiai H, Uenishi E. Early Relowering of Serum Sodium Concentration Overcomes Disturbances in Consciousness during Hyponatremia Overcorrection and Prevents Osmotic Demyelination Syndrome. Intern Med. 2018;57:2353-2357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Soupart A, Penninckx R, Stenuit A, Perier O, Decaux G. Reinduction of hyponatremia improves survival in rats with myelinolysis-related neurologic symptoms. J Neuropathol Exp Neurol. 1996;55:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Chang KY, Lee IH, Kim GJ, Cho K, Park HS, Kim HW. Plasma exchange successfully treats central pontine myelinolysis after acute hypernatremia from intravenous sodium bicarbonate therapy. BMC Nephrol. 2014;15:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Sugimura Y, Murase T, Takefuji S, Hayasaka S, Takagishi Y, Oiso Y, Murata Y. Protective effect of dexamethasone on osmotic-induced demyelination in rats. Exp Neurol. 2005;192:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |