Published online Feb 6, 2020. doi: 10.12998/wjcc.v8.i3.624
Peer-review started: December 15, 2019
First decision: December 30, 2019
Revised: January 6, 2020
Accepted: January 11, 2020
Article in press: January 11, 2020
Published online: February 6, 2020
Processing time: 52 Days and 20.3 Hours
Oncologic immunotherapy is attracting attention as an effective strategy for cancer treatment. Currently, there are two kinds of inhibitors: Anti-PD-1 antibodies and anti-PD-L1 antibodies. These inhibitors have shown significant implications in improving the outcomes of certain cancer types in recent years. However, along with its effectiveness, adverse events cannot be ignored. As an anti-PD-1 antibody, camrelizumab (SHR-1210) has some side effects in tumor immunotherapy. The most common adverse event is reactive capillary hemangioma. While it is widely reported to occur in the skin, gingival reactive capillary hemangioma is rarely reported.
A 54-year-old man complained of gingival overgrowth on the anterior aspect of the maxilla and mandible for more than 6 mo. He had been placed on SHR-1210 for lung cancer for 7 mo. A gingival mass extending from canine to canine was noted on the lingual surfaces of the mandible. Gingival enlargement was noted in the front teeth. A clinical diagnosis of gingival reactive capillary hemangioma and chronic periodontitis was made. The treatment involved a complex local treatment (repeated local applications of an antibiotic paste, scaling and root planning, and surgery). The excised tissue was sent for histopathological examination, which confirmed the diagnosis of capillary hemangioma. After the operation, most of the gingival enlargement was reduced. At the 2-mo follow-up, it was noted that the gingival overgrowth was immediately reduced after the replacement of the anti-PD-1 agent with an anti-PD-L1 agent.
As the prescription for SHR-1210 has increased considerably in recent years, the occurrence of its possible side effects, including gingival reactive capillary hemangioma, has increased. It is recommended that regular oral examinations be performed before and during the treatment of tumors with SHR-1210.
Core tip: Camrelizumab (SHR-1210) is a kind of anti-PD-1 antibody. It was reported that a unique treatment-related adverse event was reactive capillary hemangioma. Notably, most reactive capillary hemangiomas occur in the skin, and this side effect is rarely seen in oral tissues. In this report, we describe the case of a patient who experienced gingival reactive capillary hemangioma during SHR-1210 treatment for lung cancer.
- Citation: Yu Q, Wang WX. Camrelizumab (SHR-1210) leading to reactive capillary hemangioma in the gingiva: A case report. World J Clin Cases 2020; 8(3): 624-629
- URL: https://www.wjgnet.com/2307-8960/full/v8/i3/624.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i3.624
Oncologic immunotherapy is attracting attention as an effective strategy for cancer treatment. The PD-1 pathway inhibits the T cell antitumor immune response in the local tumor microenvironment[1,2]. Currently, there are two kinds of inhibitors that inhibit the PD-1 pathway: Anti-PD-1 antibodies and anti-PD-L1 antibodies[3]. These inhibitors have shown significant implications in improving the outcomes of certain cancer types in recent years, such as melanoma, renal cell carcinoma, non-small cell lung cancer, small cell lung cancer, and gastric cancer[4]. However, along with its effectiveness, adverse events cannot be ignored, including diarrhea, rash, pruritus, dry mouth, vitiligo, etc.[5-8].
Camrelizumab (SHR-1210) is a kind of anti-PD-1 antibody. It was reported that a unique treatment-related adverse event was reactive capillary hemangioma[9-11]. Notably, most reactive capillary hemangiomas occur in the skin[10], and this side effect is rarely seen in oral tissues. In this report, we describe the case of a patient who experienced gingival reactive capillary hemangioma during SHR-1210 treatment for lung cancer.
A 54-year-old man visited the Department of Periodontology, Stomatological Hospital of Shandong University, China with a complaint of gingival overgrowth on the anterior aspect of the maxilla and mandible, which had been present for more than 6 mo.
The patient had been placed on SHR-1210 (200 mg through intravenous transfusion biweekly) for lung cancer for 7 mo. During the treatment, the patient had taken nifedipine for 1 mo. Then, he noted overgrowth on the anterior aspect of the maxilla and mandible later. After the cessation of nifedipine, the enlargement decreased in size on the palatal surfaces of the maxillary anterior teeth and gradually increased in size on the lingual surfaces of the mandibular anterior teeth. He used metronidazole, which was not effective.
A review of the patient’s medical history revealed nothing significant other than lung cancer and hypertension.
The patient has no significant personal or family history.
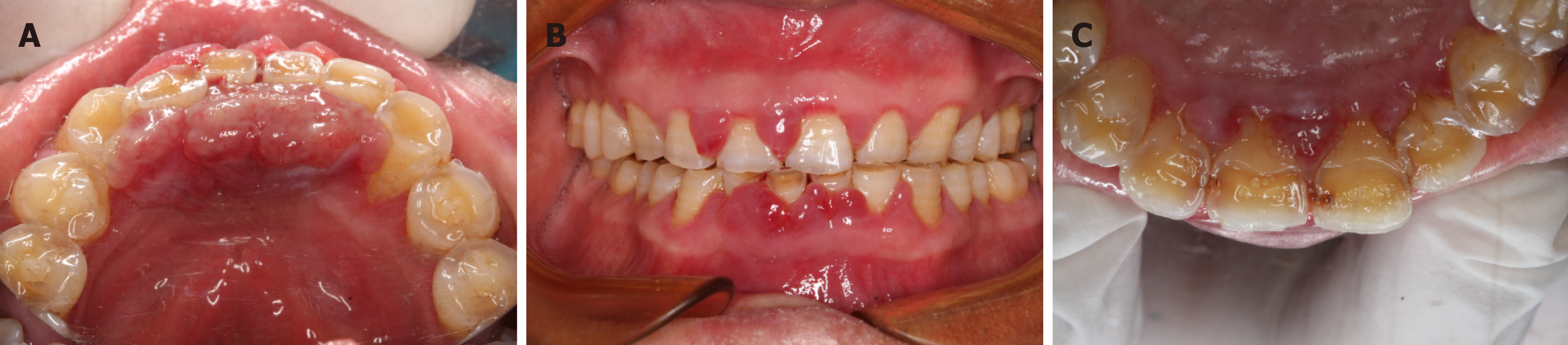
The extraoral examination was normal. The intraoral examination revealed an interdental papilla enlargement in the upper anterior region, with moderate gingival overgrowth presenting on the facial aspects of the lower anterior region and a gingival mass extending from canine to canine on the lingual surfaces of the mandible (Figure 1). The mass was magenta colored, discrete, pedunculated, and bleeding on palpation. In addition, mobility examination revealed grade I mobility in tooth 43; grade II mobility in teeth 11, 21, and 32; and grade III mobility in teeth 31, 41, and 42.
All routine blood investigations were unremarkable.
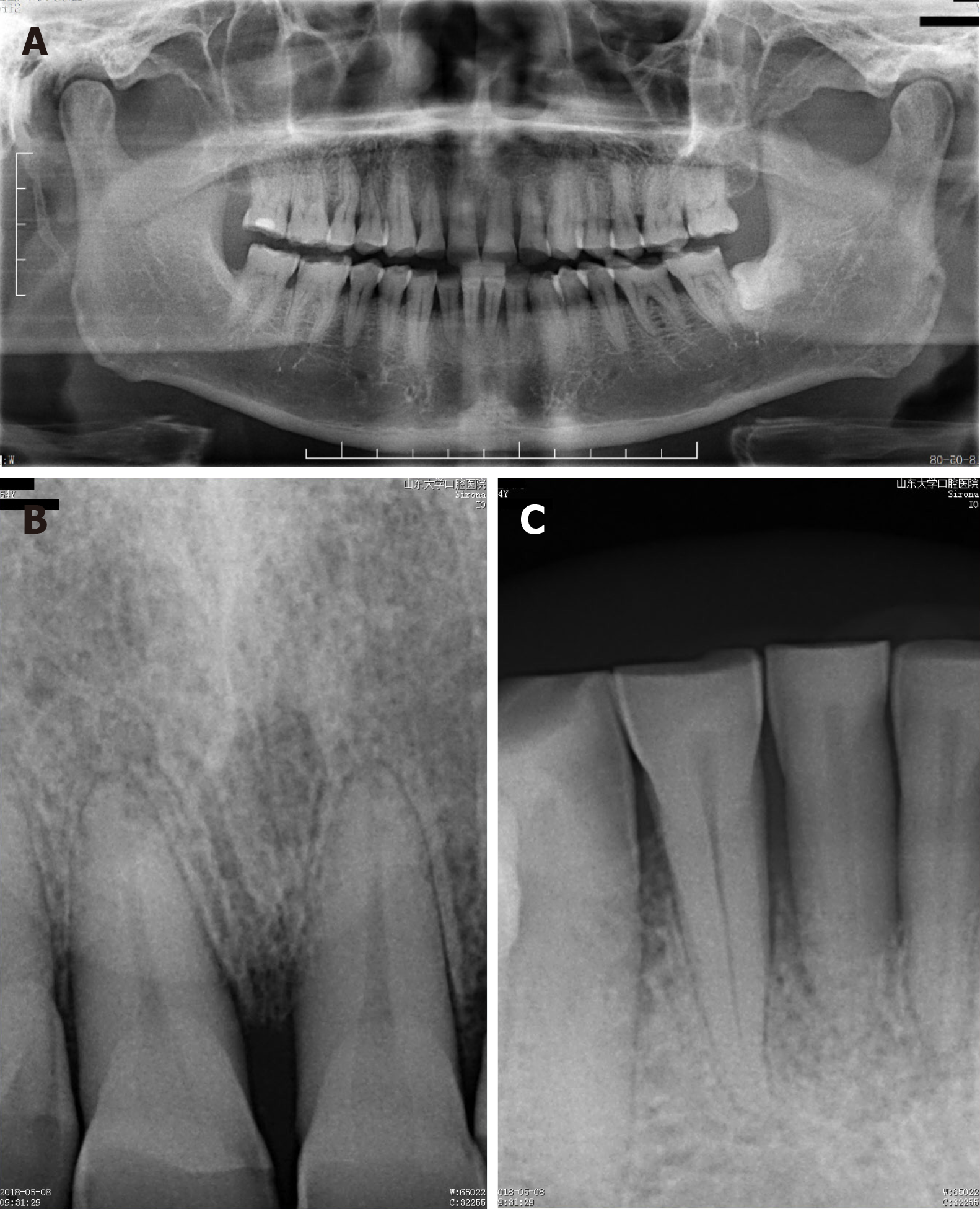
The radiographic examination showed severe horizontal bone resorption in teeth 31 and 41 and mild horizontal bone resorption in the upper front teeth. It also revealed moderate horizontal bone resorption in the upper and lower alveolar bone (Figure 2).
Reactive capillary hemangioma; chronic periodontitis.
The treatment plan was oral hygiene instructions, supra- and sub-gingival scaling, repeated local applications of an antibiotic paste (tetracycline), surgical removal of the epulis, and supportive periodontal treatment. The instructions consisted of mechanical toothbrushing and a chlorhexidine (0.12%) rinse. After obtaining informed consent from the patient, the lingual lesion around the lower anterior teeth was completely excised under local anesthesia using a semiconductor laser. The excised tissue was sent for histopathological examination.
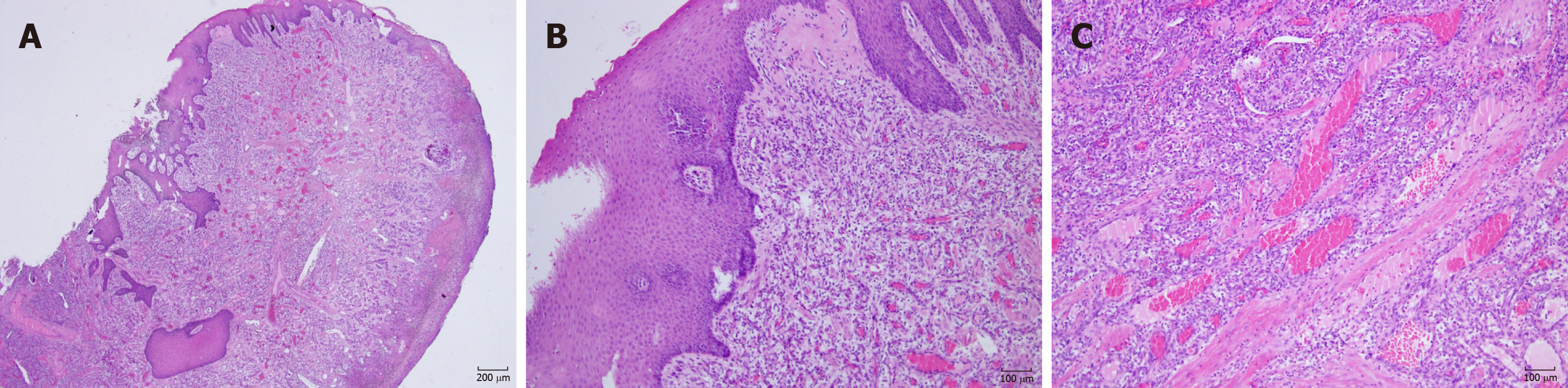
Microscopic examination showed a stratified squamous surface epithelium. The connective tissue stroma was composed of loosely arranged collagen fiber bundles interspersed with moderate chronic inflammatory cell infiltration of lymphocytes and plasma cells and many blood vessels containing red blood cells (Figure 3). The histopathological features confirmed the diagnosis of capillary hemangioma.
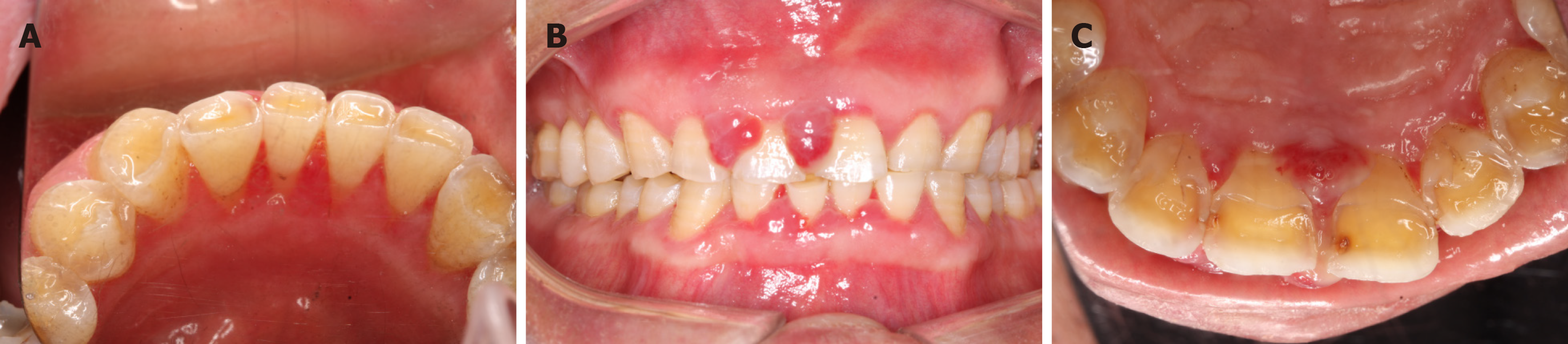
At the 2-wk postoperative appointment, the surgical site healed uneventfully with gingival color normalization and slight swelling (Figure 4A). The mobility of teeth 31, 41, and 42 improved to grade II. However, the interdental papilla overgrowth around the upper anterior teeth was magenta colored and soft in consistency (Figure 4B and C). Concerns were raised that SHR-1210 might be the cause of epulis. The patient’s oncologist changed his antineoplastic medication 2 mo later by replacing the anti-PD-1 agent with an anti-PD-L1 agent. He then reported a reduction in gingival overgrowth immediately after the cessation of SHR-1210.
Immunotherapy has been accepted as an alternative therapy to surgery, chemotherapy, and radiotherapy for tumors. Anti-PD-1 and anti-PD-L1 agents have been suggested to be effective for certain tumors[4]. However, some adverse events have been reported[8]. SHR-1210 is a kind of anti-PD-1 agent. Since it was applied to clinical management, SHR-1210 has been reported to cause skin capillary hemangioma. The average time to occurrence was 23 d[10].
The patient developed gingival enlargement after the application of SHR-1210 and nifedipine (Figure 1). As a calcium channel blocker, nifedipine can induce gingival hyperplasia. Here, an important question is what the true underlying cause of the gingival overgrowth and epulis was. It was reported that spontaneous regression of capillary hemangioma is observed after the termination of SHR-1210[10]. There is evidence that cessation of nifedipine can reduce excessive gingival growth[12,13]. The mass was still present after the patient stopped taking nifedipine. However, the patient’s gingival mass disappeared after the cessation of SHR-1210. In addition, histological examination showed capillary hemangioma (Figure 3). Thus, it could be considered that epulis was caused by SHR-1210.
Reactive capillary hemangioma is a unique adverse effect related with camrelizumab treatment[11], and a total of 85.7% of patients with advanced solid tumors who received camrelizumab monotherapy developed reactive capillary hemangioma[14]. The exact mechanisms of SHR-1210-related gingiva capillary hemangioma are still under investigation. A possible explanation is that SHR-1210 is a potent agonist of human VEGFR-2[15]. VEGFR-2 can drive hemangioma development by activating vascular endothelial cell proliferation. In addition, the incidence of reactive capillary hemangioma was 12.1% when those patients were treated with a combination of camrelizumab and apatinib (a VEGFR-2 inhibitor)[16]. Another possible reason may be oral bacterial plaque. Bacterial plaque is the initiator of oral periodontal diseases[17]. The inflammation of the gingiva induced by inadequate oral hygiene seems to enhance the interaction between the drug and gingival tissue[18].
Generally, treatment was not required in the majority of cases, because reactive capillary hemangioma lesions could spontaneously regress after the discontinuation of SHR-1210[14]. Only lesions occurred on the body area prone to friction or with a high risk of bleeding were treated with local therapy, such as laser or surgical resection[14,19]. However, in this case, the reactive capillary hemangioma lesion mainly occurred on the lingual aspects of the lower anterior teeth, which was related to difficulty in eating and speaking clearly. In addition, the patient suffered chronic periodontitis. Therefore, we performed several scaling and root planning sessions on the patient. After obtaining informed consent from the patient, the lingual lesion around the lower anterior teeth was completely excised under local anesthesia via a semiconductor laser (Figure 4A). After 2 mo, the patient’s oncologist changed his antineoplastic medication to an anti-PD-L1 agent. He then reported spontaneous regression of gingival enlargement immediately after the discontinuation of SHR-1210.
As the prescription for SHR-1210 has increased considerably in recent years, the occurrence of its possible side effects, including gingiva reactive capillary hemangioma, has increased. It is recommended that regular oral examination be performed before and during the treatment of tumors with SHR-1210.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Grawish M, Mousa HA, Man MQ S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Wu YXJ
| 1. | Lipson EJ, Forde PM, Hammers HJ, Emens LA, Taube JM, Topalian SL. Antagonists of PD-1 and PD-L1 in Cancer Treatment. Semin Oncol. 2015;42:587-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 235] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 2. | Sanmamed MF, Chen L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell. 2018;175:313-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 986] [Article Influence: 164.3] [Reference Citation Analysis (0)] |
| 3. | Constantinidou A, Alifieris C, Trafalis DT. Targeting Programmed Cell Death -1 (PD-1) and Ligand (PD-L1): A new era in cancer active immunotherapy. Pharmacol Ther. 2019;194:84-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 246] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 4. | Bardhan K, Anagnostou T, Boussiotis VA. The PD1: PD-L1/2 Pathway from Discovery to Clinical Implementation. Front Immunol. 2016;7:550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 437] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 5. | Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A; KEYNOTE-006 investigators. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521-2532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4026] [Cited by in RCA: 4485] [Article Influence: 448.5] [Reference Citation Analysis (1)] |
| 6. | Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, Hodi FS. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1801] [Cited by in RCA: 1827] [Article Influence: 166.1] [Reference Citation Analysis (0)] |
| 7. | Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbé C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3964] [Cited by in RCA: 4346] [Article Influence: 434.6] [Reference Citation Analysis (0)] |
| 8. | Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8900] [Cited by in RCA: 9903] [Article Influence: 761.8] [Reference Citation Analysis (0)] |
| 9. | Fang W, Yang Y, Ma Y, Hong S, Lin L, He X, Xiong J, Li P, Zhao H, Huang Y, Zhang Y, Chen L, Zhou N, Zhao Y, Hou X, Yang Q, Zhang L. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol. 2018;19:1338-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 357] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 10. | Mo H, Huang J, Xu J, Chen X, Wu D, Qu D, Wang X, Lan B, Wang X, Xu J, Zhang H, Chi Y, Yang Q, Xu B. Safety, anti-tumour activity, and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody in advanced solid tumours: a dose-escalation, phase 1 study. Br J Cancer. 2018;119:538-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 11. | Teng Y, Guo R, Sun J, Jiang Y, Liu Y. Reactive capillary hemangiomas induced by camrelizumab (SHR-1210), an anti-PD-1 agent. Acta Oncol. 2019;58:388-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Lederman D, Lumerman H, Reuben S, Freedman PD. Gingival hyperplasia associated with nifedipine therapy. Report of a case. Oral Surg Oral Med Oral Pathol. 1984;57:620-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 120] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Ramon Y, Behar S, Kishon Y, Engelberg IS. Gingival hyperplasia caused by nifedipine--a preliminary report. Int J Cardiol. 1984;5:195-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 114] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Chen X, Ma L, Wang X, Mo H, Wu D, Lan B, Qu D, Zhang H, Huang J, Xu B. Reactive capillary hemangiomas: a novel dermatologic toxicity following anti-PD-1 treatment with SHR-1210. Cancer Biol Med. 2019;16:173-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Finlay WJJ, Coleman JE, Edwards JS, Johnson KS. Anti-PD1 'SHR-1210' aberrantly targets pro-angiogenic receptors and this polyspecificity can be ablated by paratope refinement. MAbs. 2019;11:26-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu R, Zhang G, Zhao C, Zhang Y, Chen C, Wang Y, Yi X, Hu Z, Zou J, Wang Q. Anti-PD-1 Antibody SHR-1210 Combined with Apatinib for Advanced Hepatocellular Carcinoma, Gastric, or Esophagogastric Junction Cancer: An Open-label, Dose Escalation and Expansion Study. Clin Cancer Res. 2019;25:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 359] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 17. | Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3:17038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 920] [Cited by in RCA: 1479] [Article Influence: 184.9] [Reference Citation Analysis (0)] |
| 18. | Lertpimonchai A, Rattanasiri S, Arj-Ong Vallibhakara S, Attia J, Thakkinstian A. The association between oral hygiene and periodontitis: a systematic review and meta-analysis. Int Dent J. 2017;67:332-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 202] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 19. | Song Y, Wu J, Chen X, Lin T, Cao J, Liu Y, Zhao Y, Jin J, Huang H, Hu J, Luo J, Zhang L, Xue H, Zhang Q, Wang W, Chen C, Feng J, Zhu J. A Single-Arm, Multicenter, Phase II Study of Camrelizumab in Relapsed or Refractory Classical Hodgkin Lymphoma. Clin Cancer Res. 2019;25:7363-7369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |