Published online Feb 6, 2020. doi: 10.12998/wjcc.v8.i3.594
Peer-review started: November 8, 2019
First decision: December 12, 2019
Revised: December 31, 2019
Accepted: January 8, 2020
Article in press: January 8, 2020
Published online: February 6, 2020
Processing time: 79 Days and 15.2 Hours
Targeted treatments may greatly affect the natural history of urothelial carcinoma based on their pharmacokinetics. A phase II trial has explored the combination of cytotoxic chemotherapy with the anti-HER-2 monoclonal antibody trastuzumab in selected patients with metastatic bladder cancer, but it failed.
Here, we report a case of recurrent urothelial bladder carcinoma (UBC) in a patient who has undergone three operations, and further illuminate its diagnosis and treatment. The diagnosis of UBC was rendered according to the pathological indices. Next-generation sequencing on formalin fixed paraffin-embedded (FFPE) tissue was also performed and suggested HER2 gene amplification in the FFPE tissue. Based on HER2 gene amplification in FFPE, the patient was treated with chemotherapy in combination with trastuzumab after his third surgery. Fortunately, the patient got a clinically complete remission to trastuzumab for 34 mo.
There is not enough clinical evidence for incorporating trastuzumab in routine treatment of UBC. This case hinted that recurrent UBC patients with HER2 gene amplification may benefit from targeted trastuzumab. Further studies are needed to further investigate the status of HER2 gene and better determine trastuzumab in the management of UBC.
Core tip: Urothelial bladder carcinoma (UBC) is the most common type of urinary system tumor. Approximately 25% of patients present de novo with metastatic disease affecting long-term survival. Although cisplatin-based combination chemotherapy has become the standard first-line regimens for recurrent UBC patients, there are still no second- or third-line treatments for definite efficacy. HER2 gene amplification has been found in UBC patients, but there is not enough clinical evidence for incorporating trastuzumab for treatment of recurrent UBC. This case hinted that recurrent UBC patients with HER2 gene amplification may benefit from targeted trastuzumab, and more cases in the future are needed to confirm our findings.
- Citation: Jiang Q, Xie MX, Zhang XC. Complete response to trastuzumab and chemotherapy in recurrent urothelial bladder carcinoma with HER2 gene amplification: A case report. World J Clin Cases 2020; 8(3): 594-599
- URL: https://www.wjgnet.com/2307-8960/full/v8/i3/594.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i3.594
It has been suggested that a way forward in the treatment of advanced or metastatic urothelial carcinoma may be consistent with the progress made in the targeted therapy of advanced breast cancer, where trastuzumab-based therapy has shown substantial benefit in patients presenting tumors with overexpression and/or amplification of the ERBB2 gene, which encodes the human epidermal growth factor receptor 2 (HER2). A recent phase II clinical trial (NCT01828736) of advanced or metastatic urothelial carcinoma explored the combination of chemotherapy (gemcitabine and platinum) with trastuzumab. However, the results are similar to those achieved with cytotoxic chemotherapy alone, and the contribution of trastuzumab in this single-arm phase II trial is unclear[1]. Patients were selected for enrollment based on HER2 overexpression by immunohistochemistry, gene amplification, and/or elevated serum HER-2. Different tests and “cut-offs” for the putative predictive biomarkers may be the key reasons for the failure of this trial[2]. Herein, we present a recurrent urothelial bladder carcinoma (UBC) patient with HER2 gene amplification tested by targeted next-generation sequencing (NGS), and the patient has benefited from targeted trastuzumab up to present.
A 43-year-old Chinese man presented to the Medical Oncology Department of our hospital complaining of recurrent UBC for which he has undergone three operations.
In March 2013, the patient presented with pain and intermittent hematuria for 3 mo. On April 12, 2013, he received partial cystectomy for high-grade papillary urothelial carcinoma (WHO grade III). Pathology confirmed that the surgical margin was negative. After four cycles of gemcitabine and carboplatin (GC) as adjuvant chemotherapy, he experienced local recurrence of the bladder, and then received radical cystectomy and ureterocutaneostomy for bladder infiltrating urothelial carcinoma, classified as rpT4aN0M0 on November 22, 2013. From December 2013 to May 2014, he received six cycles of TP (paclitaxel and cisplatin) as first-line chemotherapy. On July 12, 2016, he experienced residual urethra progression and left inguinal lymph node enlargement, and then received the third operation to remove the left inguinal lymph nodes that were pathologically confirmed to have tumor infiltration.
The patient’s main previous medical history was cystolith and pollen allergy. There was a history of pancreatic carcinoma in his patient’s family.
The Eastern Cooperative Oncology Group score of this patient was 0, and the numeric pain intensity scale was 0. An old surgical scar of about 10 cm can be seen in the lower abdomen, and a bladder stoma can be seen in the right lower abdomen with a drainage bag. There was no redness, swelling, or exudation around the stoma, and the urine in the drainage bag was clear.
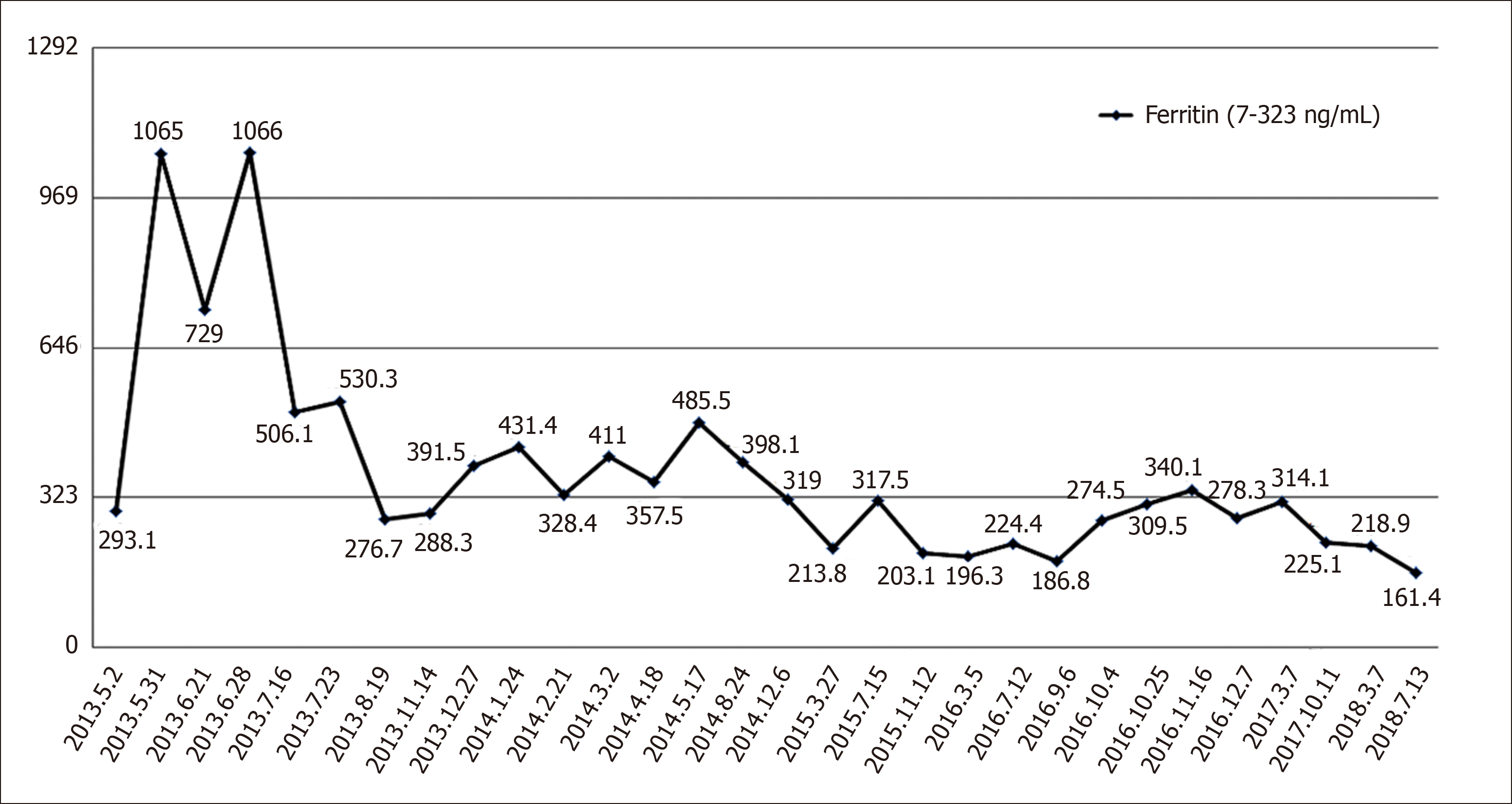
The routine blood examination, blood biochemistry, and urine analysis were normal. Electrocardiogram, chest X-ray, and arterial blood gas were also normal. Serum tumor markers including alpha-fetoprotein, carcinoembryonic antigen, cancer antigen 125, cancer antigen 19-9, and ferritin were routinely monitored, and only ferritin was higher than the upper limit of reference range and trended to be associated with tumor burden. Detailed monitoring values are shown in Figure 1. Left inguinal lymph nodes were resected during the third operation, and the pathology suggested urothelial carcinoma metastasis, Immunohistochemistry showed hepatocyte (-), GPC-3 (-), PSA (-), TTF-1 (-), CK7 (+), CK20 (+), P63 (+), GATA-3 (+), CK5/6 (+), P504S (part +), and CD44 (+).
Pelvic magnetic resonance indicated postoperative changes of bladder cancer (after the third operation).
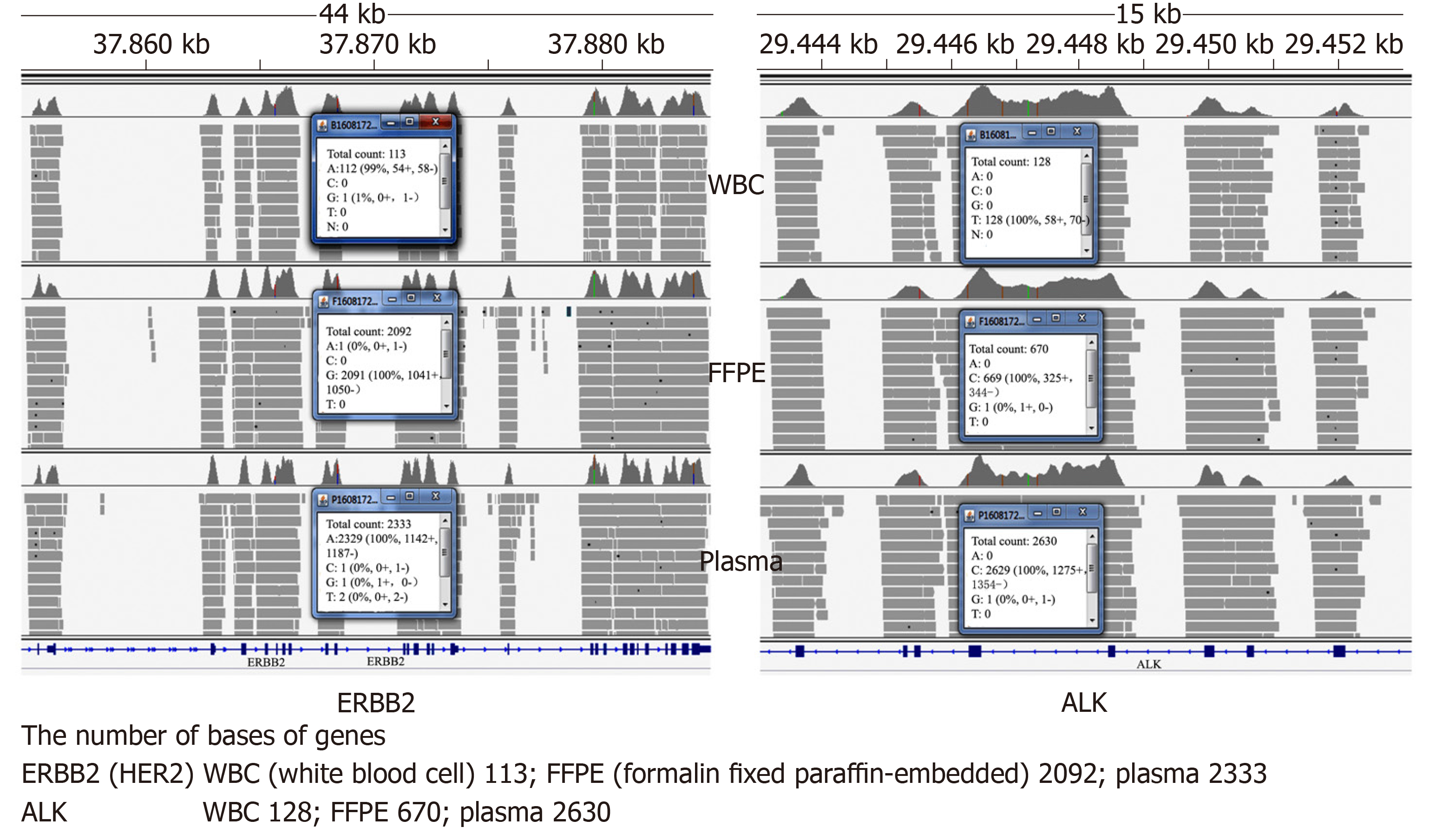
A customized NGS panel targeting 416 genes was further carried out on formalin fixed paraffin-embedded sample, with white blood cells used as a negative control. The sequencing results suggested ERBB2 (HER2) amplification in formalin fixed paraffin-embedded sample (Figure 2).
Recurrent urothelial bladder carcinoma (TNM stage: rpT4aN0M1a).
After a multidisciplinary consultation, a second-line regimen was decided with trastuzumab 6 mg/kg every three weeks after a loading dose of 8 mg/kg and cisplatin 75 mg/m2 every three weeks, from September 2016. After five cycles, this treatment was disrupted because of the patient’s economic condition.
Serum tumor markers including alpha-fetoprotein, carcinoembryonic antigen, cancer antigen 125, cancer antigen 19-9, and ferritin were routinely monitored, and only ferritin was higher than the upper limit of reference range and trended to lower (Figure 1). Fortunately, there was no recurrence until now. The patient got a clinically complete remission to trastuzumab for 34 mo.
At diagnosis, approximately 25% of patients with UBC present de novo with metastatic disease[3]. Unfortunately, approximately 11% of patients with UBC have regional or distant metastases at initial presentation, with 5-year survival rates of about 35% and 5%, respectively[4]. Compared with other solid tumors, UBC is a chemosensitive malignancy characterized by relatively high response rates to combination chemotherapy. Cisplatin-based combination chemotherapy has been shown to improve survival benefit, not only in adjuvant and neoadjuvant therapy for patients with locally advanced carcinoma but also in the patients with metastatic disease. Methotrexate, vinblastine, doxorubicin, cisplatin, and GC are currently the standard first-line regimens (Table 1) for locally advanced or metastatic disease[2]. Available therapies for management of platinium-refractory metastatic UBC are not satisfactory with a response rate that is low in second-line therapy with some agents (Table 2) and with no validated third-line therapy[2].
| Regimen | No of patients | OR (%) | mOS (mo) | P value |
| MVAC | 246 | 36 | 12.5 | < 0.01 |
| Cisplatin | 11 | 8.2 | ||
| MVAC | 110 | 65 | 12.6 | < 0.05 |
| CISCA | 46 | 10 | ||
| MVAC | 169 | 59 | 12.5 | > 0.05 |
| FAP | 42 | 12.5 | ||
| MVAC | 263 | 58 | 14.1 | > 0.05 |
| HD-MVAC | 72 | 5.5 | ||
| MVAC | 408 | 46 | 14.8 | > 0.05 |
| GC | 50 | 13.8 | ||
| MVAC | 220 | 54 | 14.2 | < 0.05 |
| DC | 37 | 9.3 | ||
| MVAC | 85 | 40 | 14.2 | > 0.05 |
| PC | 28 | 13.8 | ||
| GC | 627 | 46 | 12.8 | > 0.05 |
| GCP | 57 | 15.7 |
| Regimen | No of patients | OR% (95%CI) |
| Paclitaxel | 31 | 10 (0-26) |
| Docetaxel | 30 | 13 (4-30) |
| Ifosfamide | 56 | 20 (10-32) |
| Gemcitabine | 35 | 20 (10-36) |
| Paclitaxel and carboplatin | 44 | 16 (7-30) |
| Vinflunine | 51 | 18 (8-31) |
| Pemetrexed | 13 | 8 (NR) |
| Pemetrexed | 47 | 28 (16-43) |
HER2 gene plays a critical role in the pathogenesis of UBC. Several studies have reported that the overexpression rate of HER2 protein is between 5% and 80% in UBC[3]. Laé et al[5] analyzed the HER2 status of tissue specimens from 1005 patients with muscle-invasive bladder cancer and found that overexpression of HER2 protein and HER2 gene amplification accounted for 11.4% and 5.1%, respectively[5]. Several clinical trials have explored inhibitors of the HER2 pathway in selected patients with metastatic UBC (Table 3)[1,6,7]. A phase II trial of patients with metastatic bladder cancer explored the combination of cytotoxic chemotherapy (GC and paclitaxel) with the anti-HER-2 monoclonal antibody trastuzumab. The patients were selected for enrollment based HER-2 overexpression by immunohistochemistry, gene amplification, and/or elevated serum HER-2. Thirty-one of forty-four (70%) patients achieved objective responses (5 complete and 26 partial). However, these results are similar to the results achieved with cytotoxic chemotherapy alone, and the contribution of trastuzumab in this single-arm phase II trial is unclear. Another phase II clinical trial explored lapatinib [the dual HER-2/ epidermal growth factor receptor (EGFR) pathway inhibitor] as second-line therapy in metastatic BC patients. The patients were eligible provided that they had 1+, 2+, or 3+ expression of either EFGR or HER-2 by immunohistochemistry (from either primary or metastatic tumor samples). An objective response to treatment was observed in 1.7% (95% confidence interval: 0.0%–9.1%) of patients; however, 18 (31%; 95% confidence interval: 19%–44%) patients had a stable disease. Further analysis revealed that clinical benefit was associated with EGFR overexpression, and, to some extent, HER-2 overexpression. The same pathway inhibitors were used in these trials, but the patients were selected for enrollment based on HER-2 overexpression by immunohistochemistry, gene amplification, and/or elevated serum HER-2. Different tests and ‘‘cut-offs’’ for the putative predictive biomarkers may be the critical factors in the era of targeted therapeutics. The rate of HER2 gene mutation is about 2% in breast cancer, but it was not reported in urinary epithelial carcinoma[8]. Studies have shown that HER2 gene mutation is one of the mechanisms of anti-HER2 therapy (e.g., herceptin and lapatinib) for drug resistance[9,10]. In this case, the patient harbored HER2 gene amplification but no HER2 mutation tested by NGS achieved more than two years of disease-free progression after his third surgery, which might be the important factor in the effectiveness of trastuzumab.
| Regimen | Phase | No of patients | Prior chemotherapy | Target enrichment | OR (%) | mOS (mo) |
| Carboplatin/ paclitaxel/ gemcitabine/trastuzmab | I/II | 57 | No | Yes | 70 | 14.1 |
| Lapatinib | II | 59 | Yes | No | 1.7 | 4.5 |
| Lapatinib maintenance after first-line chemotherapy | II/III | 116 | No | Yes | 14 | 12.6 |
This case hinted that recurrent UBC patients with HER2 gene amplification may benefit from targeted trastuzumab. Further studies and cases are needed to further investigate the status of HER2 gene and better determine trastuzumab in the management of UBC, particularly after failure of routine therapy.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bettaieb A, Garg M, Garg PK, Vynios D S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Liu MY
| 1. | Oudard S, Culine S, Vano Y, Goldwasser F, Théodore C, Nguyen T, Voog E, Banu E, Vieillefond A, Priou F, Deplanque G, Gravis G, Ravaud A, Vannetzel JM, Machiels JP, Muracciole X, Pichon MF, Bay JO, Elaidi R, Teghom C, Radvanyi F, Beuzeboc P. Multicentre randomised phase II trial of gemcitabine+platinum, with or without trastuzumab, in advanced or metastatic urothelial carcinoma overexpressing Her2. Eur J Cancer. 2015;51:45-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 2. | Galsky MD, Hall SJ. Bladder cancer: current management and opportunities for a personalized approach. Mt Sinai J Med. 2010;77:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Cheetham PJ, Petrylak DP. New agents for the treatment of advanced bladder cancer. Oncology. 2016;30:571-579, 588. [PubMed] |
| 4. | Galsky MD, Pal SK, Lin SW, Ogale S, Zivkovic M, Simpson J, Derleth C, Schiff C, Sonpavde G. Real-World Effectiveness of Chemotherapy in Elderly Patients with Metastatic Bladder Cancer in the United States. Bladder Cancer. 2018;4:227-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Laé M, Couturier J, Oudard S, Radvanyi F, Beuzeboc P, Vieillefond A. Assessing HER2 gene amplification as a potential target for therapy in invasive urothelial bladder cancer with a standardized methodology: results in 1005 patients. Ann Oncol. 2010;21:815-819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 159] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 6. | Wülfing C, Machiels JP, Richel DJ, Grimm MO, Treiber U, De Groot MR, Beuzeboc P, Parikh R, Pétavy F, El-Hariry IA. A single-arm, multicenter, open-label phase 2 study of lapatinib as the second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma. Cancer. 2009;115:2881-2890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 169] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 7. | Yong HG, Yoo J, Noh JH, Chan K. Emerging targeted therapies in advanced bladder cancer. Transl Cancer Res. 2017;6:S666-S676. |
| 8. | Martin V, Cappuzzo F, Mazzucchelli L, Frattini M. HER2 in solid tumors: more than 10 years under the microscope; where are we now? Future Oncol. 2014;10:1469-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Fang Y, Jiang Y, Wang X, Yang X, Gao Y, Wang J. Somatic mutations of the HER2 in metastatic breast cancer. Tumour Biol. 2014;35:11851-11854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Park YH, Shin HT, Jung HH, Choi YL, Ahn T, Park K, Lee A, Do IG, Kim JY, Ahn JS, Park WY, Im YH. Role of HER2 mutations in refractory metastatic breast cancers: targeted sequencing results in patients with refractory breast cancer. Oncotarget. 2015;6:32027-32038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |