Published online Feb 6, 2020. doi: 10.12998/wjcc.v8.i3.577
Peer-review started: October 12, 2019
First decision: December 23, 2019
Revised: January 7, 2020
Accepted: January 11, 2020
Article in press: January 11, 2020
Published online: February 6, 2020
Processing time: 116 Days and 22.5 Hours
Peutz-Jeghers syndrome (PJS) and mesenteric fibromatosis (MF) are rare diseases, and PJS accompanying MF has not been previously reported. Here, we report a case of a 36-year-old man with both PJS and MF, who underwent total colectomy and MF surgical excision without regular follow-up. Two years later, he sought treatment for recurrent acute abdominal pain. Emergency computed tomography showed multiple soft tissue masses in the abdominal and pelvic cavity, and adhesions in the small bowel and peritoneum. Partial intestinal resection and excision of the recurrent MF were performed to relieve the symptoms.
A 36-year-old male patient underwent total colectomy for PJS with MF. No regular reexamination was performed after the operation. Two years later, due to intestinal obstruction caused by MF enveloping part of the small intestine and peritoneum, the patient came to our hospital for treatment. Extensive recurrence was observed in the abdomen and pelvic cavity. The MF had invaded the small intestine and could not be relieved intraoperatively. Finally, partial bowel resection, proximal stoma, and intravenous nutrition were performed to maintain life.
Regular detection is the primary way to prevent deterioration from PJS. Although MF is a benign tumor, it has characteristics of invasive growth and ready recurrence. Therefore, close follow-up of both the history of MF and gastrointestinal surgery are advisable. Early detection and early treatment are the main means of improving patient prognosis.
Core tip: Peutz-Jeghers syndrome with mesenteric fibromatosis is extremely rare and has not been previously reported. Resection of the diseased bowel and mesenteric fibromatosis may be the best therapy. Regular review and timely intervention may improve patient prognosis. This paper analyzed the characteristics of Peutz-Jeghers syndrome and mesenteric fibromatosis in detail, and summarized this case, with the aim of providing a reference for future clinical diagnosis and treatment.
- Citation: Cai HJ, Wang H, Cao N, Wang W, Sun XX, Huang B. Peutz-Jeghers syndrome with mesenteric fibromatosis: A case report and review of literature. World J Clin Cases 2020; 8(3): 577-586
- URL: https://www.wjgnet.com/2307-8960/full/v8/i3/577.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i3.577
Peutz-Jeghers syndrome (PJS) is a rare autosomal dominant genetic disease with an incidence of approximately 1 in 200000[1]. Some researchers have shown that truncating mutation of the LKB1/STK11 in chromosome 19p13.3 is the main cause of PJS[2]. The clinical features manifest mainly as mucocutaneous pigmentation and multiple polyps in the gastrointestinal tract, and family history is common. Mesenteric fibromatosis (MF) refers to fibroid lesions originating from the mesentery, which form a rare benign mesenchymal tissue tumor with an incidence of approximately 1 in 2 million[3]. MF is a fibroblastic clonal hyperplastic lesion characterized primarily by invasive growth and common local recurrence, but distant metastasis is rare[4]. At present, the pathogenesis of MF remains unclear, but it may be closely associated with trauma, surgery, genetics, and endocrine and other factors. The incidence of MF in familial adenomatous polyposis (FAP) is 7%-12%[5], but no case of PJS combined with MF has been reported to date. For PJS combined with MF, R0 resection to the greatest extent as possible is the best treatment, and regular follow-up and timely treatment may be important factors to delay disease progression[6]. We report this case to provide guidance for future research of the malignancy risk and to inform the development of monitoring methods for PJS and the clinical characteristics of MF. We hope that experience in clinical encounters with similar cases will accumulate.
On March 29, 2016, a 36-year-old male patient came to our hospital for treatment of recurrent diarrhea for more than 1 mo.
He had undergone local lobectomy for left pulmonary hemangioma compression 1 year prior.
His brother had a history of gastrointestinal polyps.
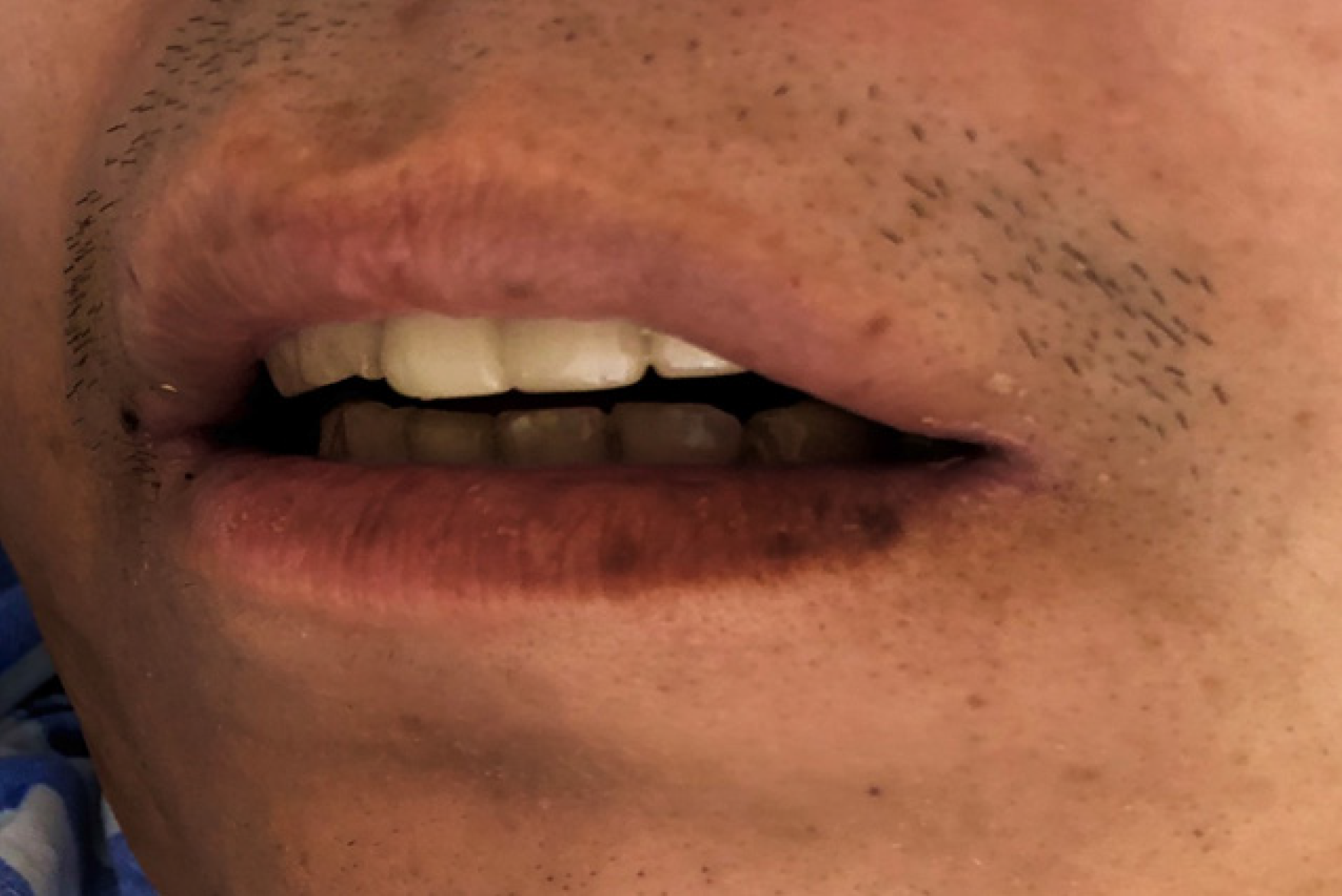
No abnormalities were noted on physical examination, with the exception of multiple pigmented maculae on the lips and oral mucosa (Figure 1) and a surgical scar on the left chest wall of up to 10 cm.
No abnormalities were found in laboratory testing, including routine blood testing, liver function, blood amylase, and tumor markers.
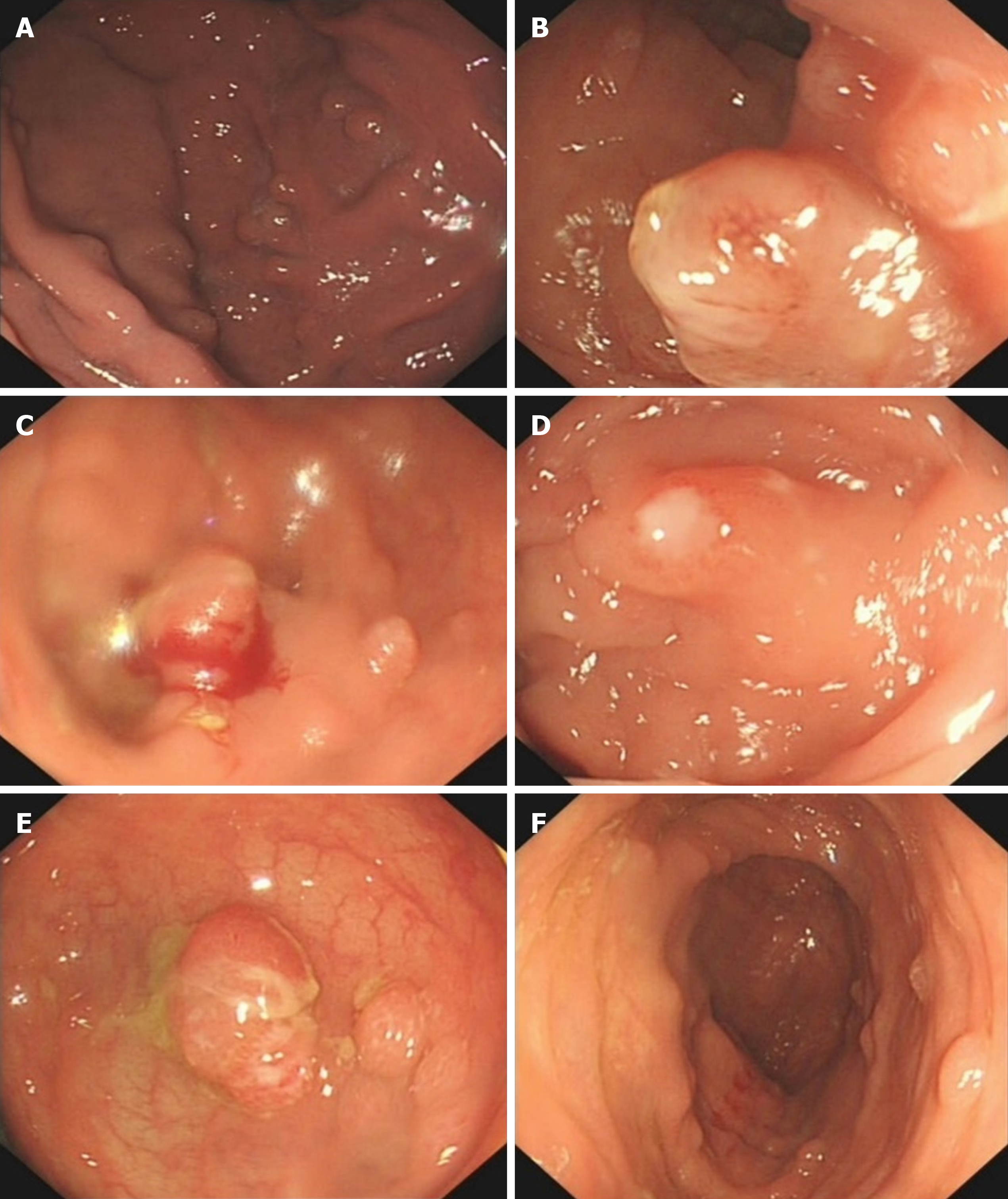
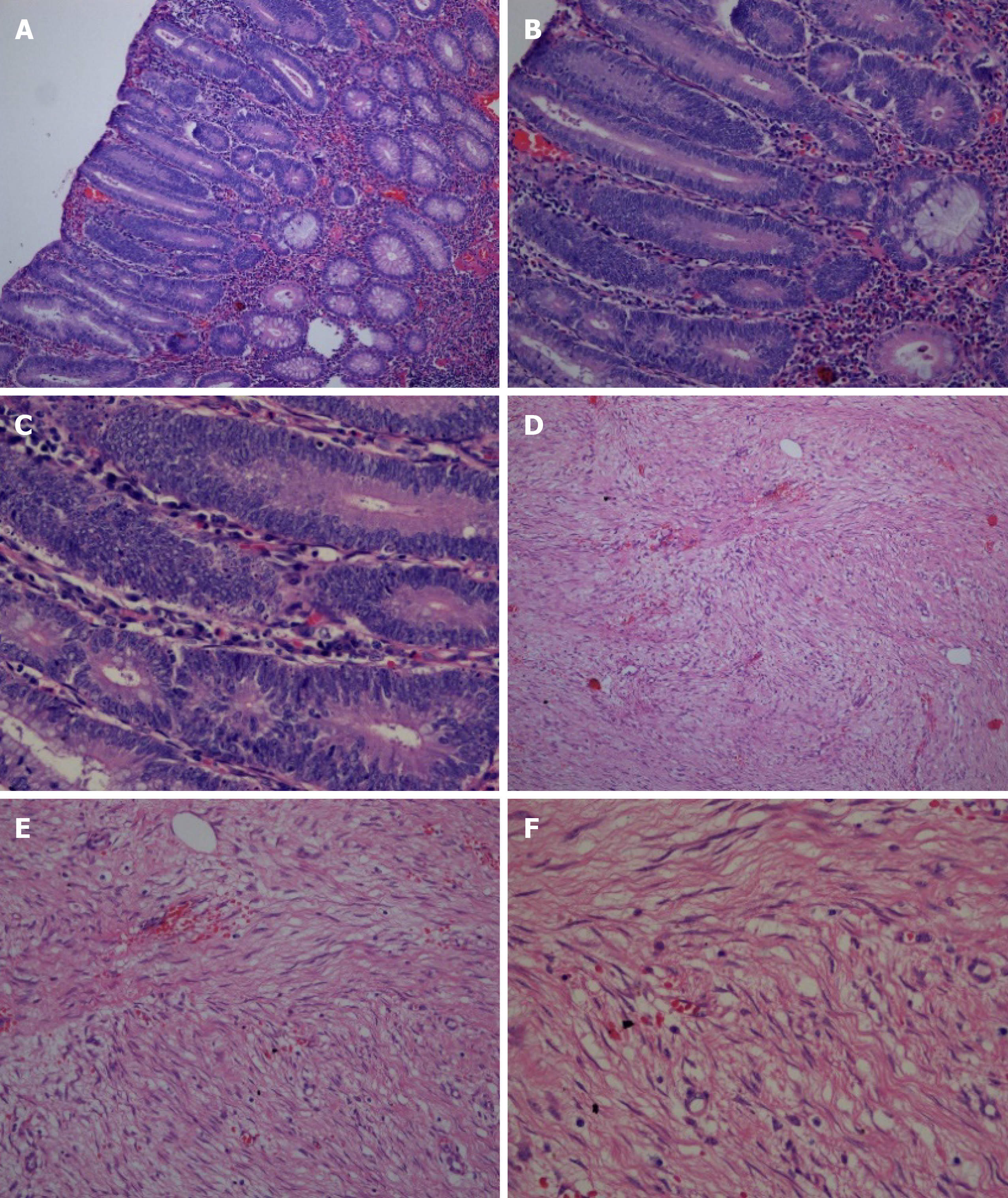
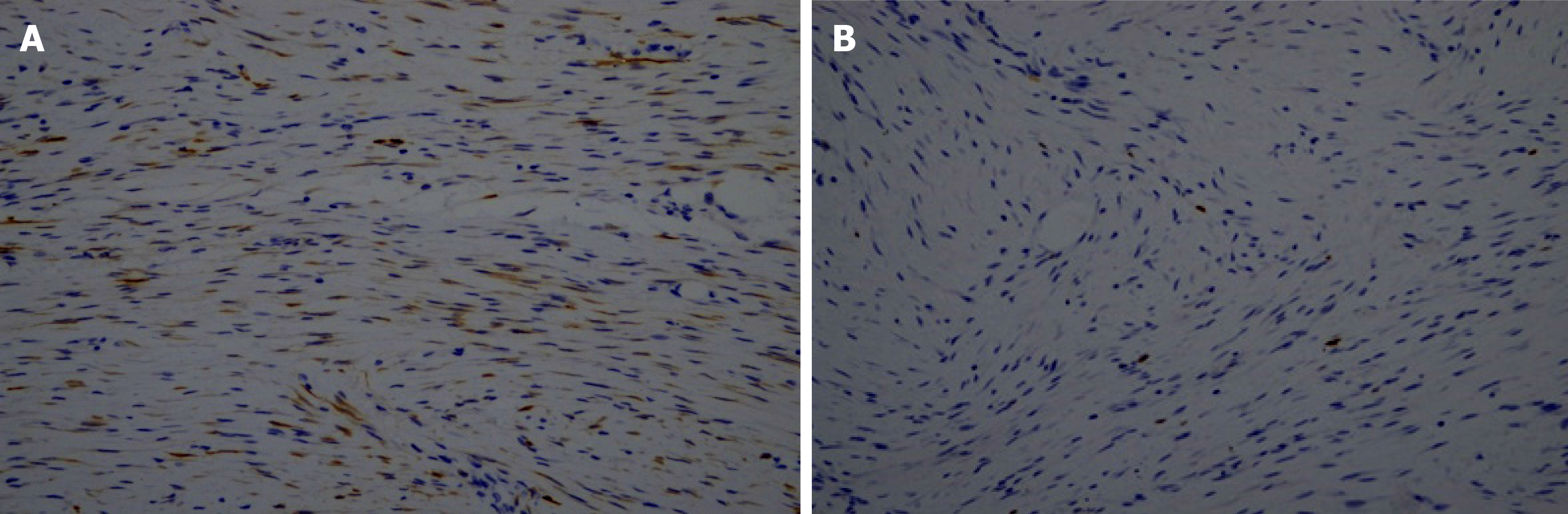
Electronic gastroscopy revealed that the gastric fundus mucosa was rough, with multiple local soybean-sized smooth flattened polyps (Figure 2A). Electronic colonoscopy showed that the colonic mucosa was hyperemic, and revealed edema, multiple hyperplasia and swelling with irregular morphology, and ulcer hemorrhage in some polyps (Figure 2B-2F). Abdominal computed tomography (CT) examination revealed a soft tissue density shadow under the right semi-transverse colon, of approximately 3.3 cm × 2.1 cm with an unclear boundary. The tumor was slightly strengthened after the enhanced scan, fat density was seen at the edge, the surrounding fat gap was cloudy, and no abnormal soft tissue tumor was observed on the inside and outside of the intestinal cavity (Figure 3). The pathological findings suggested multiple tubularvillous adenomas with moderate dysplasia in the entire colon, and fusiform cell tumor-like hyperplasia was found on the mesenteric side of the colon (Figure 4). Immunohistochemistry revealed that the sample was CD34-, Des-, S100-, SM-, CD117-, Dog-1-, Calponin+, and 2%Ki-67+, in agreement with fibromatosis (Figure 5).
PJS with MF.
Because of the extensive blood vessels and high mobility of the mesentery, ultrasound-guided biopsy carried a risk of bleeding, and the patient's colon polyps were extensive and at risk of cancer formation. Therefore, we performed laparoscopic total colectomy under general anesthesia after discussion. Masses in the hepatic curvature of the colon enclosed part of the mesentery and transverse colon, and some of the intestines were stiff and congested. After routine anti-infection rehydration treatment, the patient was discharged from the hospital with an order for reexamination by electronic gastroscopy and abdominal CT.
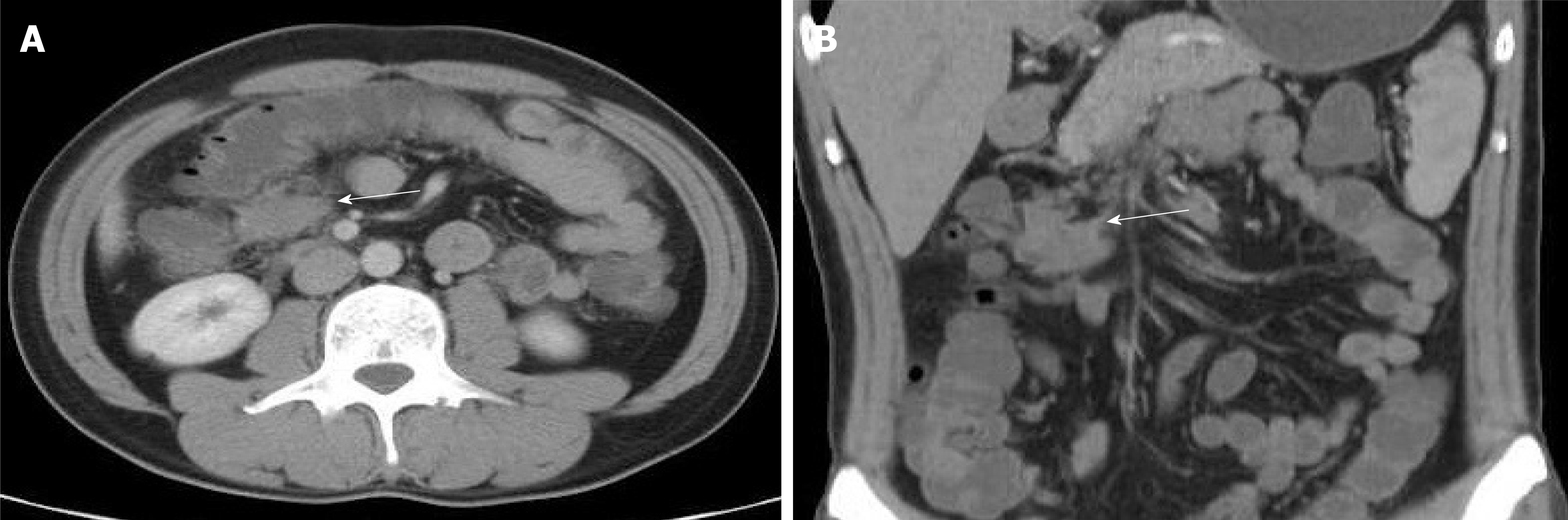
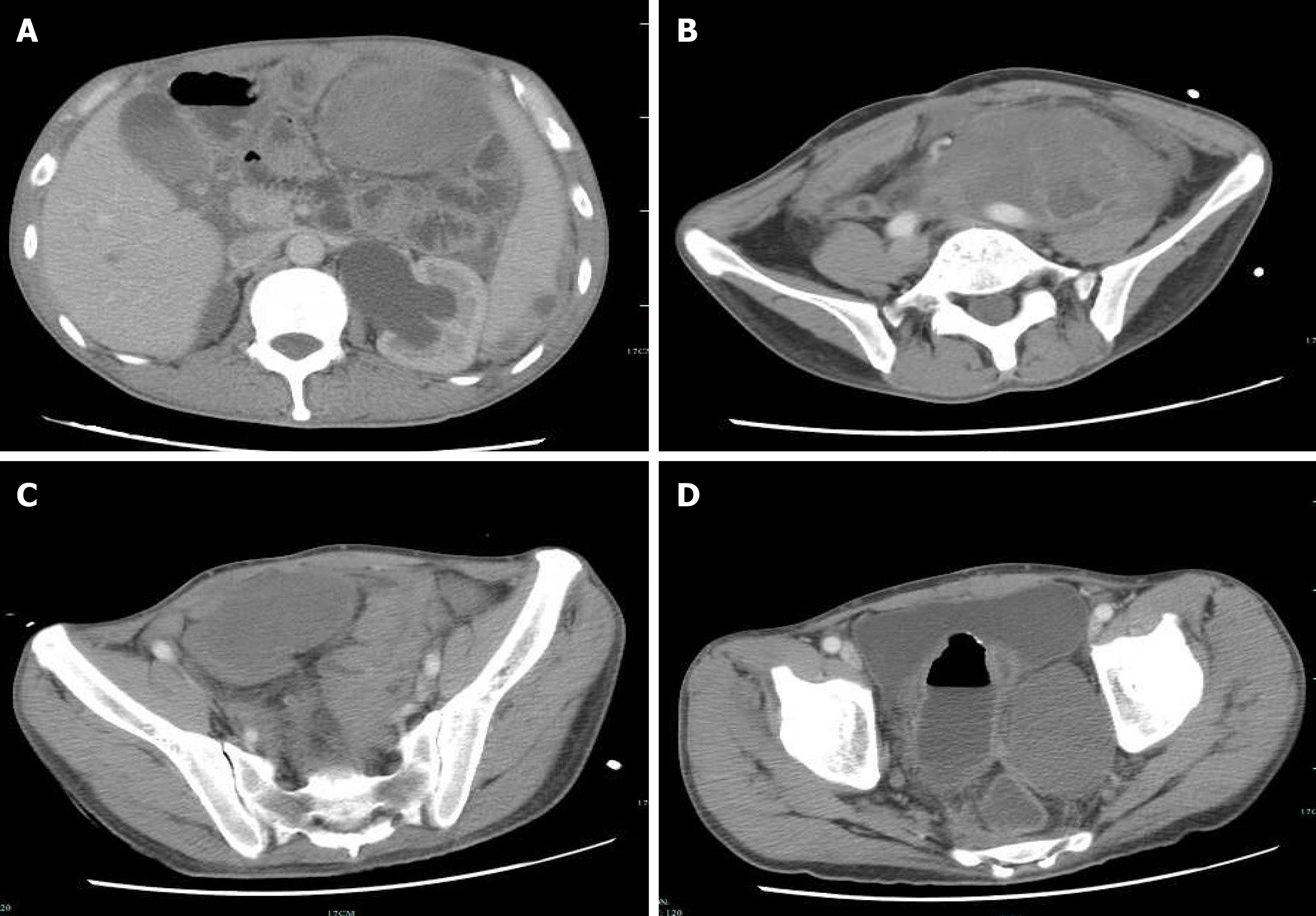
On August 10, 2018, because of recurrent abdominal pain and diarrhea for more than 3 mo, and acute aggravation for 3 d, the patient came to our hospital for treatment. Emergency enhanced CT showed thickening of the intestinal wall, peritoneum and omentum; multiple masses with mixed density in the abdomen and pelvis, with the largest mass located in the left middle abdomen within liquefaction and necrosis; an unclear boundary of 9.9 cm × 14 cm; a patchy high density shadow in and around the lesion; heterogeneous enhancement; and an unclear boundary between the masses and the adjacent intestine after an enhanced scan (Figure 6).
After general discussion, we considered the recurrence of fibromatosis and intestinal obstruction caused by adhesion. After exclusion of contraindications, laparotomy under general anesthesia was performed. Multiple abdominal tumors were found during the operation, the largest of which was located in the small mesentery, 60 cm from Treitz’s ligament, with a size of approximately 14 cm × 12 cm. The mass in the distal intestine adhered closely to the pelvic cavity of the lower abdominal wall, and some of the intestine was ruptured and difficult to separate from the surrounding tissues in a frozen state. Thus, the surgical procedure comprised resection of the masses and adhesive intestinal tube, proximal enterostomy as well as anal anastomosis. Due to breakage of part of the patient's small intestine, edema exudation, the large scope of the operation, and the long operation time, the patient was sent to the intensive care unit for further anti-infection treatment after the operations. Finally, the pathological results confirmed the recurrence of fibromatosis.
At present, patients with short bowel syndrome are given parenteral nutrition to maintain their vital signs; because of limited economic capacity, no further radiotherapy or chemotherapy is performed for MF.
PJS is a rare autosomal dominant genetic disease, which was first reported by Peutz in 1921 and first described by Jeghers in 1949, according to PubMed[7]. Chen et al[8] have found that germline mutation of the LKB1 gene is the main cause of familial PJS in China. The primary mechanism through which the LKB1 gene regulates cell proliferation is modulation of Rb protein phosphorylation through a p53 dependent pathway. AMP-activated protein kinase is the main kinase downstream of LKB1, which can sense the energy state of the body and maintain the homeostasis of energy metabolism[9]. LKB1 may be a signal transduction molecule that regulates p53-dependent apoptosis, and its dysfunction may lead to defects in intestinal epithelial cell apoptosis, which consequently initiates multiple benign hamaromatous polyps in the gastrointestinal tract in patients with PJS and ultimately leads to cancerization[10]. PJS mucosal pigment spots mainly manifest as uniformly light brown areas, usually less than 5 mm, which are common in the lip (94%), gingiva, buccal mucosa, mouth, nose, and eyes; among these, the pigment maculae on the lips are unusual in that they are vermilion and are usually darker and denser than freckles. At present, there are no reports of malignant changes in the skin and mucous pigmentation in PJS. FAP and PJS are clinically similar, and skin and mucous pigment spots may be one of the important differentiators.
PJS was diagnosed on the basis of clinical features, family history, electronic gastroenteroscopy and imaging. In this case, the patient's symptoms and family history are relatively typical for diagnosis. For small intestinal polyps smaller than 1 cm, detection with CT and electronic gastroenteroscopy may be difficult; capsule endoscopy plays an important role, and, combined with CT, can greatly improve the detection rate[11].
Endoscopic polypectomy is currently the main treatment, but in the following cases, surgery is necessary[12]: Intestinal polyps larger than 2 cm or colon polyps larger than 1.5 cm; intussusception, acute intestinal obstruction and other related complications; polyps with irregular morphology and malignant tendency; gastrointestinal bleeding; and polyps spread throughout the entire intestine. In our patient, there were multiple polyps in the entire colon, and some polyps were irregular with hemorrhage. Therefore, surgical total colectomy was the best treatment.
According to the ACG guidelines in the United States, the malignancy risks of PJS include colorectal cancer, breast cancer, pancreatic cancer, gynecological cancer, small bowel cancer, lung cancer and gastric esophageal cancer. Therefore, regular reexamination is important, as is the time and quality of follow-up. Previous studies in this field are reviewed and summarized in Table 1[13-20].
| Cancer site | General population risk, % | Syndrome risk, % | Average age of diagnosis in yr | Age at the start of monitoring | Monitoring interval in yr | Monitoring mode | Ref. |
| Pancreas | 1.5 | 11-36 | 41-52 | 30 | 1-2 | MRCP or US | [11,14-17] |
| Stomach | < 1 | 29 | 30-40 | 8.181 | 3 | Gastroscopy | [11,14,15,18] |
| Small bowel | < 1 | 13 | 37-42 | 8.181 | 3 | Capsule endoscopy | [11,14,15] |
| Colon | 4.5 | 39 | 42-46 | 8.181 | 3 | Colonoscopy | [11] |
| Testicle (Sertoli cell tumor) | < 1 | 9 | 6-9 | 14 | 1 | Testicular examination or US | [11,14,15] |
| Breast | 12.4 | 32-54 | 37-59 | 25 | 1 | MRI or mammography | [11,14-16,19] |
| Uterus | 2.7 | 9 | 43 | 25 | 1 | Pelvic examination or vaginal US | [14,15] |
| Cervix (adenoma malignum) | < 1 | 10 | 34-40 | 25 | 1 | Cervical smear | [11,14,15,20] |
| Ovary (mostly SCTAT (sex cord tumor with annular tubules)) | 1.6 | 21 | 28 | 25 | 1 | Pelvic examination or vaginal US | [11,15] |
| Lung | 6.9 | 7-17 | 47 | – | – | Quit smoking | [14-16] |
MF, also known as desmoid tumor[21], is a rare benign tumor with rapid and invasive growth. It can occur at any age, most commonly in women 30 to 40 years of age, although cases have been reported in children. Its pathogenesis remains unclear, and many researchers believe that its pathogenesis is associated with genetic factors, trauma, surgical stress, pregnancy, sex and endocrine factors[22-26]. The recurrent MF tumors in this case were larger, more numerous and more extensive than those during the first occurrence; we therefore inferred that the recurrence of MF may correlate with surgical stress. Vitellaro et al[27] also confirmed that the risk of developing MF after laparoscopic excision is significantly lower than that after laparotomy. Therefore, decreasing surgical stress is a focus of surgical research. Strategies include performing careful preoperative work; understanding the locations of masses and the surrounding anatomical structure; and ensuring that an experienced surgeon does the work.
The probability of MF recurrence after surgery for FAP is higher than that for other gastrointestinal malignancies[28]. More MF images should be collected and analyzed, because the treatment of MF after recurrence of malignant gastrointestinal tumors is completely different from that of MF after recurrence of benign gastrointestinal tumors.
In this case, the first CT scan indicated that the masses showed soft tissue density, the tumors were slightly enhanced, the fat density was seen at the edge, and the boundary between tumors and the surrounding fat space was unclear and could be easily misdiagnosed. Therefore, MF should be distinguished from the following diseases. The first is liposarcoma, which occurs primarily in retroperitoneal, extremities and deep soft tissue of the trunk in people above 60 years of age[29]. On CT, the lumps are mainly fat density and irregular septa, and focal enhancement can be seen in internal bleeding. Because magnetic resonance imaging has strong soft tissue resolution, it is necessary when liposarcoma is suspected[30]. Magnetic resonance imaging can be used to identify the composition of the mass and analyze the relationship with the surrounding soft tissue. Gastrointestinal stromal tumor is also a differential diagnosis that often occurs in the elderly; the smaller tumors have homogeneous density, and the larger ones usually have cysts, necrosis and calcification; most are linked with the intestinal cavity. Because of abundant blood supply, the contrast agent in the arterial phase enters the tumor quickly, shows clear enhancement, and clears quickly in the venous phase and the delayed phase, showing "fast in and fast out" enhancement characteristics. The third differential diagnosis is metastases: The imaging findings of metastases are similar to those of the primary foci. Therefore, understanding the history of tumors is the most critical step in the diagnosis of the disease. Metastases of gastrointestinal malignancies are more aggressive than MF accompanied by local lymph node metastasis and distant metastasis. At this time, fluorine18-fluorodeoxyglucose positron emission tomography/CT (PET/CT) can be used to comprehensively evaluate the number and nature of masses, and aid in distinguishing PJS. As in this case, PET/CT can be used to observe whether gastrointestinal polyps and MF exhibit increased radioactive uptake and to distinguish the relationship between polyps and MF, thus providing clinical diagnostic value[31].
MF often has no clear clinical symptoms, and patients often seek medical treatment after complications such as abdominal pain, intestinal obstruction, peritonitis, hydronephrosis or other symptoms[32,33]; thus, the optimal diagnosis and treatment times for MF recurrence are often missed. Although MF is a benign tumor, it has the characteristics of invasive growth and ready recurrence. In the second reexamination of the case, the masses had widely invaded the small intestine, thus resulting in intestinal obstruction due to adhesion. In the emergency treatment, the wrapped small intestine could only be resected extensively. Although obstruction was solved, it could be sustained only by long-term intravenous nutrition. At present, there is no unified understanding of the treatment for MF, whereas close follow-up, early diagnosis and early treatment are the best ways to improve the prognosis of patients. If a patient is reviewed in time and treated early, as in our case, most of the small intestine can be saved, and the prognosis of the patient can be greatly improved. Some researchers have shown that postoperative radiotherapy, chemotherapy, and endocrine therapy may be beneficial[34]. The use of hormonal therapy for the treatment of these tumors is based on the association of these tumors with pregnancy or contraceptive pills and reports of regression after menopause or oophorectomy. Success rates of approximately 50% have been obtained with hormonal treatments and other agents such as warfarin, vitamin C, and nonsteroidal anti-inflammatory drugs. Palliative ultrasound-guided high-intensity focused ultrasound ablation is also an option for patients who cannot undergo complete resection[35]. In addition, some researchers believe that a "wait-and-see" approach may provide the best outcome, rather than radical surgical resection; the ability to improve patients' quality of life is essential[36,37].
In summary, PJS accompanied by MF is extremely rare, and thus there is no unified treatment standard at present. Consequently, early detection and individualized treatment are highly important. In addition, MF is a recurrent and invasive benign tumor, which should be considered highly important by both patients and doctors.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: González-González R, Moustaki M S-Editor: Dou Y L-Editor: Filipodia E-Editor: Liu MY
| 1. | Duan SX, Wang GH, Zhong J, Ou WH, Fu MX, Wang FS, Ma SH, Li JH. Peutz-Jeghers syndrome with intermittent upper intestinal obstruction: A case report and review of the literature. Medicine (Baltimore). 2017;96:e6538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Daniell J, Plazzer JP, Perera A, Macrae F. An exploration of genotype-phenotype link between Peutz-Jeghers syndrome and STK11: a review. Fam Cancer. 2018;17:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 3. | Sakorafas GH, Nissotakis C, Peros G. Abdominal desmoid tumors. Surg Oncol. 2007;16:131-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Bn A, Cd JK, Ps S, M M, Urs R. Giant aggressive mesenteric fibromatosis- a case report. J Clin Diagn Res. 2015;9:PD07-PD08. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Chaudhary P. Mesenteric fibromatosis. Int J Colorectal Dis. 2014;29:1445-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Huang K, Wang CM, Chen JG, Du CY, Zhou Y, Shi YQ, Fu H. Prognostic factors influencing event-free survival and treatments in desmoid-type fibromatosis: analysis from a large institution. Am J Surg. 2014;207:847-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | JEGHERS H, McKUSICK VA, KATZ KH. Generalized intestinal polyposis and melanin spots of the oral mucosa, lips and digits; a syndrome of diagnostic significance. N Engl J Med. 1949;241:993, illust; passim. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 332] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Chen C, Zhang X, Wang D, Wang F, Pan J, Wang Z, Liu C, Wu L, Lu H, Li N, Wei J, Shi H, Wan H, Zhu M, Chen S, Zhou Y, Zhou X, Yang L, Liu J. Genetic Screening and Analysis of LKB1 Gene in Chinese Patients with Peutz-Jeghers Syndrome. Med Sci Monit. 2016;22:3628-3640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Hou L, Liu T, Wang J. Isoflurane suppresses the self-renewal of normal mouse neural stem cells in a p53-dependent manner by activating the Lkb1-p53-p21 signalling pathway. Mol Med Rep. 2015;12:7412-7418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | George SH, Milea A, Sowamber R, Chehade R, Tone A, Shaw PA. Loss of LKB1 and p53 synergizes to alter fallopian tube epithelial phenotype and high-grade serous tumorigenesis. Oncogene. 2016;35:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Beggs AD, Latchford AR, Vasen HF, Moslein G, Alonso A, Aretz S, Bertario L, Blanco I, Bülow S, Burn J, Capella G, Colas C, Friedl W, Møller P, Hes FJ, Järvinen H, Mecklin JP, Nagengast FM, Parc Y, Phillips RK, Hyer W, Ponz de Leon M, Renkonen-Sinisalo L, Sampson JR, Stormorken A, Tejpar S, Thomas HJ, Wijnen JT, Clark SK, Hodgson SV. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 457] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 12. | Ferlitsch M, Moss A, Hassan C, Bhandari P, Dumonceau JM, Paspatis G, Jover R, Langner C, Bronzwaer M, Nalankilli K, Fockens P, Hazzan R, Gralnek IM, Gschwantler M, Waldmann E, Jeschek P, Penz D, Heresbach D, Moons L, Lemmers A, Paraskeva K, Pohl J, Ponchon T, Regula J, Repici A, Rutter MD, Burgess NG, Bourke MJ. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49:270-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 764] [Article Influence: 95.5] [Reference Citation Analysis (0)] |
| 13. | Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW; American College of Gastroenterology. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110:223-62; quiz 263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 957] [Cited by in RCA: 1087] [Article Influence: 108.7] [Reference Citation Analysis (0)] |
| 14. | van Lier MG, Wagner A, Mathus-Vliegen EM, Kuipers EJ, Steyerberg EW, van Leerdam ME. High cancer risk in Peutz-Jeghers syndrome: a systematic review and surveillance recommendations. Am J Gastroenterol. 2010;105:1258-64; author reply 1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 329] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 15. | Giardiello FM, Brensinger JD, Tersmette AC, Goodman SN, Petersen GM, Booker SV, Cruz-Correa M, Offerhaus JA. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 961] [Cited by in RCA: 868] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 16. | Hearle N, Schumacher V, Menko FH, Olschwang S, Boardman LA, Gille JJ, Keller JJ, Westerman AM, Scott RJ, Lim W, Trimbath JD, Giardiello FM, Gruber SB, Offerhaus GJ, de Rooij FW, Wilson JH, Hansmann A, Möslein G, Royer-Pokora B, Vogel T, Phillips RK, Spigelman AD, Houlston RS. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res. 2006;12:3209-3215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 508] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 17. | Chen KT. Female genital tract tumors in Peutz-Jeghers syndrome. Hum Pathol. 1986;17:858-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 38] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Kumar S, Long JM, Ginsberg GG, Katona BW. The role of endoscopy in the management of hereditary diffuse gastric cancer syndrome. World J Gastroenterol. 2019;25:2878-2886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Faughnan ME, Palda VA, Garcia-Tsao G, Geisthoff UW, McDonald J, Proctor DD, Spears J, Brown DH, Buscarini E, Chesnutt MS, Cottin V, Ganguly A, Gossage JR, Guttmacher AE, Hyland RH, Kennedy SJ, Korzenik J, Mager JJ, Ozanne AP, Piccirillo JF, Picus D, Plauchu H, Porteous ME, Pyeritz RE, Ross DA, Sabba C, Swanson K, Terry P, Wallace MC, Westermann CJ, White RI, Young LH, Zarrabeitia R; HHT Foundation International - Guidelines Working Group. International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J Med Genet. 2011;48:73-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 698] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 20. | Brownstein MH, Wolf M, Bikowski JB. Cowden's disease: a cutaneous marker of breast cancer. Cancer. 1978;41:2393-2398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Kumar A, Kumar S, Sinha R, Kumari V. Largest size of extra-abdominal fibromatosis of axilla in a young man. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | van Broekhoven DL, Verhoef C, Grünhagen DJ, van Gorp JM, den Bakker MA, Hinrichs JW, de Voijs CM, van Dalen T. Prognostic value of CTNNB1 gene mutation in primary sporadic aggressive fibromatosis. Ann Surg Oncol. 2015;22:1464-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 23. | Saito Y, Hinoi T, Ueno H, Kobayashi H, Konishi T, Ishida F, Yamaguchi T, Inoue Y, Kanemitsu Y, Tomita N, Matsubara N, Komori K, Kotake K, Nagasaka T, Hasegawa H, Koyama M, Ohdan H, Watanabe T, Sugihara K, Ishida H. Risk Factors for the Development of Desmoid Tumor After Colectomy in Patients with Familial Adenomatous Polyposis: Multicenter Retrospective Cohort Study in Japan. Ann Surg Oncol. 2016;23:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Eastley N, McCulloch T, Esler C, Hennig I, Fairbairn J, Gronchi A, Ashford R. Extra-abdominal desmoid fibromatosis: A review of management, current guidance and unanswered questions. Eur J Surg Oncol. 2016;42:1071-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Church J, Xhaja X, LaGuardia L, O'Malley M, Burke C, Kalady M. Desmoids and genotype in familial adenomatous polyposis. Dis Colon Rectum. 2015;58:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Radhakrishna V, Patil RS. Mesenteric Fibromatosis. Kathmandu Univ Med J (KUMJ). 2017;15:350-351. [PubMed] |
| 27. | Vitellaro M, Sala P, Signoroni S, Radice P, Fortuzzi S, Civelli EM, Ballardini G, Kleiman DA, Morrissey KP, Bertario L. Risk of desmoid tumours after open and laparoscopic colectomy in patients with familial adenomatous polyposis. Br J Surg. 2014;101:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Calogero A, Sagnelli C, Carlomagno N, Tammaro V, Candida M, Vernillo A, Peluso G, Minieri G, Santangelo M, Dodaro CA. Familial Polyposis Coli: The Management of Desmoid Tumor Bleeding. Open Med (Wars). 2019;14:572-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Vijay A, Ram L. Retroperitoneal liposarcoma: a comprehensive review. Am J Clin Oncol. 2015;38:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 30. | Knebel C, Neumann J, Schwaiger BJ, Karampinos DC, Pfeiffer D, Specht K, Lenze U, von Eisenhart-Rothe R, Rummeny EJ, Woertler K, Gersing AS. Differentiating atypical lipomatous tumors from lipomas with magnetic resonance imaging: a comparison with MDM2 gene amplification status. BMC Cancer. 2019;19:309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Honma Y, Terauchi T, Tateishi U, Kano D, Nagashima K, Shoji H, Iwasa S, Takashima A, Kato K, Hamaguchi T, Boku N, Shimada Y, Yamada Y. Imaging peritoneal metastasis of gastric cancer with 18F-fluorothymidine positron emission tomography/computed tomography: a proof-of-concept study. Br J Radiol. 2018;91:20180259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Schattner A, Huszar M, Adi M. Unexpected Hydronephrosis: Mesenteric Fibromatosis. Am J Med. 2018;131:e383-e384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Ng EP, Kwok SY, Lok KF, Chow MP, Lau PY. Mesenteric fibromatosis: a rare cause of peritonitis. Hong Kong Med J. 2018;24:84-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 34. | Jenayah AA, Bettaieb H, Saoudi S, Gharsa A, Sfar E, Boudaya F, Chelli D. Desmoid tumors: clinical features and treatment options: a case report and a review of literature. Pan Afr Med J. 2015;21:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Zhao WP, Han ZY, Zhang J, Yu XL, Cheng ZG, Zhou X, Liang P. Early experience: high-intensity focused ultrasound treatment for intra-abdominal aggressive fibromatosis of failure in surgery. Br J Radiol. 2016;89:20151026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Otero S, Moskovic EC, Strauss DC, Benson C, Miah AB, Thway K, Messiou C. Desmoid-type fibromatosis. Clin Radiol. 2015;70:1038-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 37. | Zeng WG, Zhou ZX, Liang JW, Hou HR, Wang Z, Zhou HT, Zhang XM, Hu JJ. Prognostic factors for desmoid tumor: a surgical series of 233 patients at a single institution. Tumour Biol. 2014;35:7513-7521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |