Published online Feb 6, 2020. doi: 10.12998/wjcc.v8.i3.517
Peer-review started: December 5, 2019
First decision: December 23, 2019
Revised: January 9, 2020
Accepted: January 15, 2020
Article in press: January 15, 2020
Published online: February 6, 2020
Processing time: 62 Days and 18.8 Hours
Pathological complete response (pCR) is rare in hormone receptor-positive (HR+) HER2-negative breast cancer (BC) treated with either endocrine therapy (ET) or chemotherapy. Radical resection of locoregional relapse, although potentially curative in some cases, is challenging when the tumor invades critical structures. The oral cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with ET has obtained a significant increase in objective response rates and progression-free survival in patients with advanced BC and is now being evaluated in the neoadjuvant setting. We present a clinical case of a patient with an inoperable locoregional relapse of HR+ HER2-negative BC who experienced pCR after treatment with palbociclib.
We report the clinical case of a 60-year-old patient who presented with an inoperable locoregional relapse of HR+, HER2-negative BC 10 years after the diagnosis of the primary tumor. During a routine follow-up visit, breast magnetic resonance imaging and positron emission tomography/computed tomography revealed a 4-cm lesion in the right subclavicular region, infiltrating the chest wall and extending to the subclavian vessels, but without bone or visceral involvement. Treatment was begun with palbociclib plus letrozole, converting the disease to operability over a period of 6 mo. Surgery was performed and a pCR achieved. Of note, during treatment the patient experienced a very uncommon toxicity characterized by burning tongue and glossodynia associated with dysgeusia, paresthesia, dysesthesia, and xerostomia. A reduction in the dose of palbociclib did not provide relief and treatment with the inhibitor was thus discontinued, resolving the tongue symptoms. Laboratory exams were unremarkable. Given that this was a late relapse, the tumor was classified as endocrine-sensitive, a condition associated with high sensitivity to palbociclib.
This case highlights the potential of the cyclin-dependent kinase 4/6 inhibitor plus ET combination to achieve pCR in locoregional relapse of BC, enabling surgical resection of a lesion initially considered inoperable.
Core tip: The rate of pathological complete response after endocrine therapy in hormone receptor-positive breast cancer is low, limiting the value of pathological complete response as a surrogate endpoint for the effectiveness of this treatment. Moreover, radical resection of locoregional recurrence is difficult to achieve when the tumor invades critical structures, e.g., blood vessels. Several studies have evaluated whether endocrine therapy could also be used as a research platform for testing novel drugs in patients with ER-positive disease.
- Citation: Palleschi M, Maltoni R, Barzotti E, Melegari E, Curcio A, Cecconetto L, Sarti S, Manunta S, Rocca A. Can cyclin-dependent kinase 4/6 inhibitors convert inoperable breast cancer relapse to operability? A case report. World J Clin Cases 2020; 8(3): 517-521
- URL: https://www.wjgnet.com/2307-8960/full/v8/i3/517.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i3.517
Pathological complete response (pCR) occurs infrequently in hormone receptor positive (HR+), HER2-negative breast cancer (BC) treated with endocrine therapy (ET) or chemotherapy. A recent meta-analysis reported similar clinical responses in H+ BC treated with neoadjuvant ET or chemotherapy, but lower toxicity for the former[1]. The use of neoadjuvant therapy potentially facilitates breast conservation and permits the assessment in vivo of biomarkers to identify responsive or resistant subgroups of tumors. Radical resection of locoregional relapse, albeit potentially curative, may be problematic when the tumor invades critical structures.
In November 2018, a 60-year-old woman in follow-up for BC at our institute [Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS] experienced a locoregional relapse.
In June 2008 the patient underwent mastectomy, with a diagnosis of moderately differentiated (G2) infiltrating ductal carcinoma of the right breast [estrogen receptor (ER) 80%, progesterone receptor 50%, HER2-, MiB1 15%), pT1cpN0 M0. She was referred to our institute (IRST IRCCS) and, based on the disease stage and prognostic factors, began adjuvant hormone therapy with tamoxifen in September 2008. Given her premenopausal status, a luteinizing hormone-releasing hormone analog was added. The patient completed 5 years of hormone therapy.
The medical history of the patient was unremarkable.
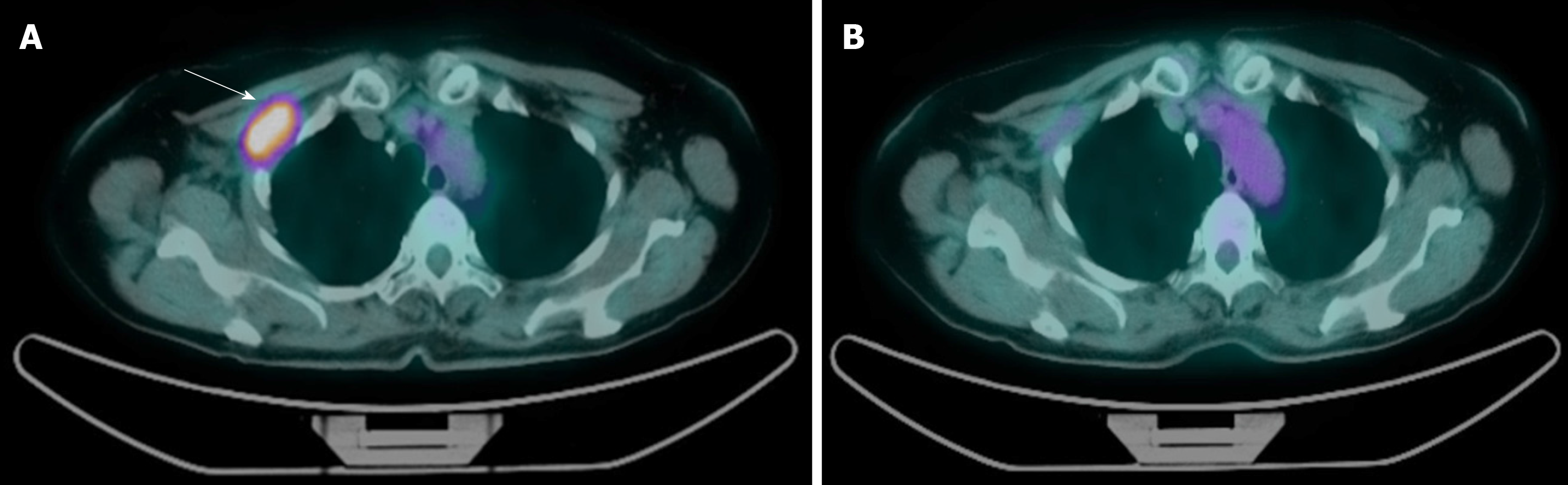
In November 2018, after a disease-free interval of 125 mo, the patient reported pain in the right subclavicular region. A targeted ultrasound scan and subsequent breast magnetic resonance imaging (MRI) revealed the presence of a 4-cm lesion infiltrating the muscle and fat tissue of the right subclavicular region and extending to the subclavian vein and artery. A positron emission tomography/computed tomography scan confirmed a locoregional relapse, without, however, involvement of viscera or bone (Figure 1A). The lesion was biopsied and histology confirmed a metastasis of breast adenocarcinoma with immunophenotypical features of ductal carcinoma of the breast (ER 100%, progesterone receptor 90%, HER2- and Ki67 25%). The multidisciplinary team excluded the option of surgery due to the involvement of axillary vessels.
In November 2018, the patient started first-line therapy with letrozole 2.5 mg/d administered orally continually and palbociclib 125 mg/d orally taken on a 21-d-on, 7-d-off basis. After the first cycle, the patient reported several adverse events (AEs) i.e., grade 3 neutropenia, burning tongue and glossodynia associated with dysgeusia, paresthesia, dysesthesia, and xerostomia. A neurological examination was negative. The dose of palbociclib was reduced without, however, an improvement in the patient’s condition. In February 2019, after 3 cycles of therapy, a breast MRI confirmed a partial response of disease. In May, palbociclib was definitively interrupted, leading to a complete resolution of the tongue symptoms, while letrozole was continued.
Laboratory exams were unremarkable, including vitamin B12, folates, and iron. Neurological antibodies were also negative (anti-amphiphysin, anti-CV2.1, anti-PNMA2 (Ma-2/TA), anti-Ri, anti-Yo, anti-Hu).
Six months after starting treatment, breast MRI and positron emission tomography/computed tomography showed radiologic complete response of the disease (RECIST 1.1) (Figure 1B).
The multidisciplinary team met once again, this time proposing a surgical evaluation.
Hormone receptor-positive BC.
On July 9th 2019, the patient underwent right axillary and interpectoral node dissection.
Histology showed a pCR, with fibrotic areas representing the tumor bed (ypT0ypN0). The patient is still undergoing treatment with letrozole and has a good quality of life. She is currently awaiting to start radiotherapy.
The use of targeted therapies has changed the landscape of cancer treatments. Palbociclib is a first-class oral cyclin-dependent kinase 4/6 inhibitor (CDK 4/6i) which, in combination with ET, has led to a significant increase in objective response rates and progression-free survival in patients with advanced HR+, HER2-negative BC, also showing excellent tolerability[2,3].
In terms of efficacy, the addition of palbociclib to a treatment regimen confers a progression‐free benefit. CDK 4 and 6 promote cell - cycle entry from the G1 phase to the S phase by phosphorylating Rb protein, and palbociclib works by inhibiting them, thus limiting tumor growth. In particular, this kinase inhibitor has shown high activity in advanced ER-positive, HER2 - BC. Cyclin D1 is needed for BC growth and couples CDK 4/6, promoting cell cycling.
Palbociclib has also been evaluated in the neoadjuvant setting[3]. In HR-positive disease, a decrease in baseline values of proliferation marker Ki67 (protein encoded by the MKI67 gene) following ET has been validated as a marker of treatment benefit and a predictor of recurrence-free survival. Given the predominantly antiproliferative effects of palbociclib, suppression of Ki67 is a rational endpoint for estimating the advantage of adding palbociclib to an aromatase inhibitor with respect to aromatase inhibitor alone in neoadjuvant patients.
In particular, pCR was investigated as an endpoint in the NeoPalana single-arm trial in which BC patients received neoadjuvant anastrozole with the addition of palbociclib on cycle 1 day 1 (C1D1). Patients left the study on C1D15 if Ki67 was > 10%. No cases of pCR were observed[4]. Recently, the PALLET trial aroused widespread international interest with its investigation of palbociclib in the same setting. Patients were randomized to letrozole monotherapy or letrozole plus palbociclib for 14 wk. The combination group showed substantial superiority over letrozole monotherapy in terms of change in Ki67, but pCR in the breast occurred infrequently and there was no evidence of a difference in clinical efficacy between letrozole and palbociclib plus letrozole[5] . The results from the ACOSOG Z1031 trial indicated that patients with endocrine-resistant tumors achieved very low pCR rates with chemotherapy, whereas those who obtained a preoperative endocrine prognostic index of 0 had an excellent long-term prognosis, despite not undergoing antiblastic treatment[6]. Cottu et al[7] compared the neoadjuvant letrozole-palbociclib combination with chemotherapy in patients with high-risk luminal BC, concluding that the combination was associated with poor pathological response[7]. In the N007 study, Chow et al[8] reported that pCR was achieved in only one of the 20 patients treated with letrozole plus palbociclib.
The optimal duration of treatment with letrozole-palbociclib is a much-debated issue because, although prolonging neoadjuvant ET increases the possibility of clinical response and breast conservation, there is no proof that it improves pCR rates. A phase IV clinical trial suggested that neoadjuvant letrozole administered for 7.5 mo was more effective at achieving beneficial shrinkage in tumor volume and facilitating conservative surgery than a 4-mo treatment[9].
The most common AEs reported in palbociclib trials are neutropenia, leukopenia, fatigue, nausea, and headache. Mucositis occurs in around 14% of patients[10]. The question arises as to whether there is a correlation between toxicity and response to treatment. Such a correlation has been reported in other tumor types, e.g., skin rash in patients receiving EGFR inhibitors[11,12]. Although there would not appear to be any AEs associated with a better response to CDK4/6i, it has been seen that side-effects such as neutropenia and diarrhea are not correlated with a poorer response to treatment.
Our clinical case highlights the potential of CDK4/6i plus ET combination to achieve pCR in BC patients with locoregional relapse. Despite the need for a dose reduction and the early interruption of palbociclib, our patient was able to undergo surgical resection of a lesion that was initially considered inoperable. Given that this was a late relapse, the disease was classified as endocrine-sensitive, a condition associated with sensitivity to palbociclib. We also describe, for the first time, an uncommon toxicity (burning tongue syndrome) associated with this treatment.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alkan A S-Editor: Dou Y L-Editor: A E-Editor: Qi LL
| 1. | Spring LM, Gupta A, Reynolds KL, Gadd MA, Ellisen LW, Isakoff SJ, Moy B, Bardia A. Neoadjuvant Endocrine Therapy for Estrogen Receptor-Positive Breast Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2016;2:1477-1486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 272] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 2. | Turner NC, Ro J, André F, Loi S, Verma S, Iwata H, Harbeck N, Loibl S, Huang Bartlett C, Zhang K, Giorgetti C, Randolph S, Koehler M, Cristofanilli M; PALOMA3 Study Group. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N Engl J Med. 2015;373:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1002] [Cited by in RCA: 1165] [Article Influence: 116.5] [Reference Citation Analysis (0)] |
| 3. | Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, Gauthier E, Lu DR, Randolph S, Diéras V, Slamon DJ. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016;375:1925-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1472] [Cited by in RCA: 2001] [Article Influence: 222.3] [Reference Citation Analysis (0)] |
| 4. | Ma CX, Gao F, Luo J, Northfelt DW, Goetz M, Forero A, Hoog J, Naughton M, Ademuyiwa F, Suresh R, Anderson KS, Margenthaler J, Aft R, Hobday T, Moynihan T, Gillanders W, Cyr A, Eberlein TJ, Hieken T, Krontiras H, Guo Z, Lee MV, Spies NC, Skidmore ZL, Griffith OL, Griffith M, Thomas S, Bumb C, Vij K, Bartlett CH, Koehler M, Al-Kateb H, Sanati S, Ellis MJ. NeoPalAna: Neoadjuvant Palbociclib, a Cyclin-Dependent Kinase 4/6 Inhibitor, and Anastrozole for Clinical Stage 2 or 3 Estrogen Receptor-Positive Breast Cancer. Clin Cancer Res. 2017;23:4055-4065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 271] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 5. | Johnston S, Puhalla S, Wheatley D, Ring A, Barry P, Holcombe C, Boileau JF, Provencher L, Robidoux A, Rimawi M, McIntosh SA, Shalaby I, Stein RC, Thirlwell M, Dolling D, Morden J, Snowdon C, Perry S, Cornman C, Batten LM, Jeffs LK, Dodson A, Martins V, Modi A, Osborne CK, Pogue-Geile KL, Cheang MCU, Wolmark N, Julian TB, Fisher K, MacKenzie M, Wilcox M, Huang Bartlett C, Koehler M, Dowsett M, Bliss JM, Jacobs SA. Randomized Phase II Study Evaluating Palbociclib in Addition to Letrozole as Neoadjuvant Therapy in Estrogen Receptor-Positive Early Breast Cancer: PALLET Trial. J Clin Oncol. 2019;37:178-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 151] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 6. | Ellis MJ, Suman VJ, Hoog J, Goncalves R, Sanati S, Creighton CJ, DeSchryver K, Crouch E, Brink A, Watson M, Luo J, Tao Y, Barnes M, Dowsett M, Budd GT, Winer E, Silverman P, Esserman L, Carey L, Ma CX, Unzeitig G, Pluard T, Whitworth P, Babiera G, Guenther JM, Dayao Z, Ota D, Leitch M, Olson JA, Allred DC, Hunt K. Ki67 Proliferation Index as a Tool for Chemotherapy Decisions During and After Neoadjuvant Aromatase Inhibitor Treatment of Breast Cancer: Results From the American College of Surgeons Oncology Group Z1031 Trial (Alliance). J Clin Oncol. 2017;35:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 252] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 7. | Cottu P, D'Hondt V, Dureau S, Lerebours F, Desmoulins I, Heudel PE, Duhoux FP, Levy C, Mouret-Reynier MA, Dalenc F, Frenel JS, Jouannaud C, Venat-Bouvet L, Nguyen S, Ferrero JM, Canon JL, Grenier J, Callens C, Gentien D, Lemonnier J, Vincent-Salomon A, Delaloge S. Letrozole and palbociclib versus chemotherapy as neoadjuvant therapy of high-risk luminal breast cancer. Ann Oncol. 2018;29:2334-2340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 8. | Chow LWC, Morita S, Chow CYC, Ng WK, Toi M. Neoadjuvant palbociclib on ER+ breast cancer (N007): clinical response and EndoPredict's value. Endocr Relat Cancer. 2018;25:123-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Fontein DB, Charehbili A, Nortier JW, Meershoek-Klein Kranenbarg E, Kroep JR, Putter H, van Riet Y, Nieuwenhuijzen GA, de Valk B, Terwogt JM, Algie GD, Liefers GJ, Linn S, van de Velde CJ. Efficacy of six month neoadjuvant endocrine therapy in postmenopausal, hormone receptor-positive breast cancer patients--a phase II trial. Eur J Cancer. 2014;50:2190-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Cazzaniga ME, Danesi R, Girmenia C, Invernizzi P, Elvevi A, Uguccioni M; NetworkER+. Management of toxicities associated with targeted therapies for HR-positive metastatic breast cancer: a multidisciplinary approach is the key to success. Breast Cancer Res Treat. 2019;176:483-494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Fiala O, Pesek M, Finek J, Krejci J, Ricar J, Bortlicek Z, Benesova L, Minarik M. Skin rash as useful marker of erlotinib efficacy in NSCLC and its impact on clinical practice. Neoplasma. 2013;60:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3767] [Cited by in RCA: 3708] [Article Influence: 176.6] [Reference Citation Analysis (1)] |