Published online Feb 6, 2020. doi: 10.12998/wjcc.v8.i3.504
Peer-review started: September 3, 2019
First decision: October 14, 2019
Revised: December 14, 2019
Accepted: December 21, 2019
Article in press: December 21, 2019
Published online: February 6, 2020
Processing time: 155 Days and 17.6 Hours
The development of biodegradable surgical staples is desirable as non-biodegradable Ti alloy staples reside in the human body long after wound healing, which can cause allergic/foreign-body reactions, adhesion, or other adverse effects. In order to develop a biodegradable alloy suitable for the fabrication of surgical staples, we hypothesized that Zn, a known biodegradable metal, could be alloyed with various elements to improve the mechanical properties while retaining biodegradability and biocompatibility. Considering their biocompatibility, Mg, Ca, Mn, and Cu were selected as candidate alloying elements, alongside Ti, the main material of clinically available surgical staples.
To investigate the in vitro mechanical properties and degradation behavior and in vivo safety and feasibility of biodegradable Zn alloy staples.
Tensile and bending tests were conducted to evaluate the mechanical properties of binary Zn alloys with 0.1–6 wt.% Mg, Ca, Mn, Cu, or Ti. Based on the results, three promising Zn alloy compositions were devised for staple applications (wt.%): Zn-1.0Cu-0.2Mn-0.1Ti (Zn alloy 1), Zn-1.0Mn-0.1Ti (Zn alloy 2), and Zn-1.0Cu-0.1Ti (Zn alloy 3). Immersion tests were performed at 37 °C for 4 wk using fed-state simulated intestinal fluid (FeSSIF) and Hank's balanced salt solution (HBSS). The corrosion rate was estimated from the weight loss of staples during immersion. Nine rabbits were subjected to gastric resection using each Zn alloy staple, and a clinically available Ti staple was used for another group of nine rabbits. Three in each group were sacrificed at 1, 4, and 12 wk post-operation.
Additions of ≤1 wt.% Mn or Cu and 0.1 wt.% Ti improved the yield strength without excessive deterioration of elongation or bendability. Immersion tests revealed no gas evolution or staple fracture in any of the Zn alloy staples. The corrosion rates of Zn alloy staples 1, 2, and 3 were 0.02 mm/year in HBSS and 0.12, 0.11, and 0.13 mm/year, respectively, in FeSSIF. These degradation times are sufficient for wound healing. The degradation rate is notably increased under low pH conditions. Scanning electron microscopy and energy dispersive spectrometry surface analyses of the staples after immersion indicated that the component elements eluted as ions in FeSSIF, whereas corrosion products were produced in HBSS, inhibiting Zn dissolution. In the animal study, none of the Zn alloy staples caused technical failure, and all rabbits survived without complications. Histopathological analysis revealed no severe inflammatory reaction around the Zn alloy staples.
Staples made of Zn-1.0Cu-0.2Mn-0.1Ti, Zn-1.0Mn-0.1Ti, and Zn-1.0Cu-0.1Ti exhibit acceptable in vitro mechanical properties, proper degradation behavior, and in vivo safety and feasibility. They are promising candidates for biodegradable staples.
Core tip: Biodegradable Zn alloy can be prepared with adequate strength, ductility, biodegradability, and biocompatibility for use as surgical staples by alloying with ≤1 wt.% Mn or Cu and 0.1 wt.% Ti. Immersion tests reveal no gas evolution or staple fracture. In vivo, none of the Zn alloy staples cause technical failure, and all rabbits survive without complications. The degradation times are sufficient for wound healing. The degradation rate is increased under low pH conditions, suggesting staples used for resection or anastomosis of gastrointestinal tracts would degrade faster, as they are exposed to acidic digestive fluids.
- Citation: Amano H, Miyake K, Hinoki A, Yokota K, Kinoshita F, Nakazawa A, Tanaka Y, Seto Y, Uchida H. Novel zinc alloys for biodegradable surgical staples. World J Clin Cases 2020; 8(3): 504-516
- URL: https://www.wjgnet.com/2307-8960/full/v8/i3/504.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i3.504
Surgical staples are widely used in surgery as alternatives to sutures when anastomosing bowels or resecting parts of the organs. However, while absorbable sutures have gained popularity, surgical staples are still primarily composed of non-biodegradable Ti or Ti alloys, meaning they reside in the human body long after wound healing. This has resulted in reports of adverse effects including allergic and foreign-body reactions and adhesion[1-6]. It is therefore desirable that biodegradable surgical staples are developed to overcome these issues.
Biodegradable metal alloys containing Mg and Fe have been intensively investigated over the last two decades for use in implantable medical devices. Fe alloys exhibit excellent mechanical properties, but their degradation rate is low and they suffer from severe corrosion that produces a voluminous iron oxide layer that can cause inflammation[7,8]. On the contrary, Mg alloys exhibit superior biocompatibility[9] and have already been used clinically as vascular stents and orthopedic screws[10-13]. However, the number of studies on Mg-based staples is limited[14,15], mainly owing to their inadequate ductility[16]. It has also been reported that Mg staples can easily fracture or degrade at the corners of the B-shape after stapling[14] owing to their vulnerability to stress corrosion[17-19]. Another concern is the release of hydrogen gas caused by the rapid corrosion of Mg in physiological environments[20].
In light of these concerns, Zn has recently been investigated as a novel implant metal owing to its good biocompatibility and biodegradability. However, its weak mechanical strength has so far limited the development of Zn alloys suitable for surgical staples, despite extensive development for vascular stents and orthopedic screws[21,22]. In this study, we developed novel Zn alloys with superior mechanical properties suitable for fabricating surgical staples by alloying with various elements. Considering their biocompatibility, and the fact that they are essential elements for maintaining physiological homeostasis processes in the human body, Mg, Ca, Mn, and Cu were selected as candidate alloying elements. Ti, which is the main material of clinically available surgical staples, was also examined as an alloying element. Mechanical tests were conducted to determine the optimum amounts of each alloying element. Subsequently, three Zn alloy compositions were devised and the degradation behavior of these Zn alloy staples was investigated by immersion tests using simulated body fluids. Finally, the safety and feasibility of using these staples during surgery and healing were evaluated using rabbit gastric resection models.
Ingots of Zn-based binary alloys were prepared from high purity raw materials: electrolytic Zn (99.99 wt.% purity; Mitsui Mining & Smelting Co., Ltd., Tokyo, Japan), Mg (99.9 wt.% purity; Mitsui Bussan Metals Co., Ltd., Tokyo, Japan), sponge Ca (95 wt.% purity; Hitachi Alloy, Ltd, Saitama, Japan), Cu wire (99.9 wt.% purity; The Nilaco Corporation, Tokyo, Japan), Mn (99 wt.% purity; Kanto Chemical Co., Inc., Tokyo, Japan), and sponge Ti (99 wt.% purity; FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). The chemical compositions of the alloys were analyzed via inductively coupled plasma optical emission spectrometry (ICP-OES: SPECTRO ARCOS, SPECTRO Analytical Instruments GmbH, Kleve, Germany), and are listed in Table 1. The raw materials were placed in a graphite crucible and melted using a high frequency induction furnace in an argon atmosphere of 9.80 × 10-2 MPa. The melt (650 °C) was cast into an iron mold with a thickness of 20 mm, width of 170 mm, and height of 135 mm. The metal ingots were subsequently processed into sheets or wire according to the purpose of evaluation.
| Alloy designation | Element (wt.%) | |||||
| Mg | Ca | Ti | Mn | Cu | Zn | |
| Zn | - | - | - | - | - | Bal. |
| Zn–0.3Mg | 0.30 | - | - | - | - | Bal. |
| Zn–1.0Mg | 1.00 | - | - | - | - | Bal. |
| Zn–3.0Mg | 2.98 | - | - | - | - | Bal. |
| Zn–0.1Ca | - | 0.09 | - | - | - | Bal. |
| Zn–0.5Ca | - | 0.43 | - | - | - | Bal. |
| Zn–0.1Ti | - | - | 0.10 | - | - | Bal. |
| Zn–0.5Ti | - | - | 0.47 | - | - | Bal. |
| Zn–0.5Mn | - | - | - | 0.43 | - | Bal. |
| Zn–1.0Mn | - | - | - | 0.97 | - | Bal. |
| Zn–0.1Cu | - | - | - | - | 0.10 | Bal. |
| Zn–1.0Cu | - | - | - | - | 1.01 | Bal. |
| Zn–3.0Cu | - | - | - | - | 2.89 | Bal. |
| Zn–6.0Cu | - | - | - | - | 5.95 | Bal. |
Zn alloy sheets were made for mechanical testing. The ingots were first machined into plates with a thickness of 10 mm, and subsequently annealed at 200 °C in air. The annealed plates were repeatedly rolled and annealed at 200 °C until a thickness of 2.0 mm was reached. The rolling reduction was approximately 0.5 mm per pass, and annealing was conducted after each 2 mm reduction. Thereafter, the plates were cold rolled from 2.0 to 0.5 mm.
The ingots were first machined to form cylinders 18 mm in diameter, which were then swaged to 6.8 mm in diameter. The rods were then repeatedly cold rolled with grooved rolls until a diameter of 1.0 mm was reached. Annealing was carried out at 200 °C for 60 min in air when the rod diameter reached 6.0, 2.7, and 1.0 mm. Subsequently, the rods were cold drawn from 1.0 to 0.4 mm in diameter.
Next, U-shaped staples were fabricated using a numerical control wire forming machine. The diameter, width, height, weight, and surface area of the staples were 0.4 mm, 5 mm, 4 mm, 8.6 mg, and 14.8 mm2, respectively.
The staples were employed in immersion tests and animal experiments after ultrasonic cleansing and sterilization by ultraviolet radiation.
Tensile and bending tests were conducted to evaluate the mechanical properties of the binary Zn alloys. Tensile testing samples were prepared according to JIS Z 2241. The alloy sheets were cut into the shape of No.13B, with the rolling direction parallel to the tensile direction. Tensile tests were performed using a universal testing machine (Model No.5582, Instron Corp., MA, USA) at room temperature with a strain rate of 10 mm/min.
Ninety-degree bending tests were conducted to determine the ductility or resistance to fracture of the binary Zn alloys. The alloy sheets were cut to a size of 10 mm × 50 mm with the rolling direction parallel to the major axis; that is, the samples were bent perpendicular to the rolling direction. Specimens were bent to 90° with a bend radius of 0.5 mm using a pressing machine. After the bending test, the presence of wrinkles or cracks at the corners was evaluated.
Based on the results of the mechanical tests on binary Zn-based alloys, three Zn alloys were devised: Zn–1.0Cu–0.2Mn–0.1Ti (Zn alloy 1), Zn–1.0Mn–0.1Ti (Zn alloy 2), and Zn–1.0Cu–0.1Ti (Zn alloy 3). Wires of 0.4 mm diameter were produced from these alloys. Then, tensile tests were performed at a strain rate of 1 mm/min at room temperature.
The biodegradable behavior of staples fabricated from the three devised zinc alloys was assessed by immersion testing. To simulate a physiological environment, two types of test mediums were prepared as simulated body fluids: Fed-state simulated intestinal fluid (FeSSIF) (Biorelevant, Croydon, U.K.); and Hank's balanced salt solution (HBSS) (+) without phenol red (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). FeSSIF was prepared according to the manufacturer’s preparation protocol by dissolving FaSSIF/FeSSIF/FaSSGF (fasted state simulated intestinal fluids/fed state simulated intestinal fluids/fasted state simulated gastric fluids) powder (11.2 g/L) in buffer solution (pH 5). Consequently, the FeSSIF solution contained 15 mmol/L taurocholate, 3.75 mmol/L phospholipids, 319 mmol/L sodium, 203 mmol/L chloride, and 144 mmol/L acetic acid. The product specification of HBSS (+) without phenol red is 400 mg/L KCl, 8000 mg/L NaCl, 350 mg/L NaHCO3, 60 mg/L KH2PO4, 47.8 mg/L anhydrous Na2HPO4, 140 mg/L anhydrous CaCl2, 100 mg/L MgCl2 6H2O, 100 mg/L MgSO4 7H2O, and 1000 mg/L D-Glucose. The pH of the resultant solution was 5.42.
Zn alloys staples were immersed in each solution at a ratio of 0.23 mL/mm2 and held at 37 °C for 4 wk. Ten of the same type of staple were placed into a 50 mL bottle along with 35 mL of solution before sealing. Eight of these bottles were prepared for each Zn alloy. The solution was replaced twice a week. During the immersion tests, the general appearance of the Zn alloy staples was observed. After two and 4 wk of immersion, staples were extracted from four of the eight bottles for each alloy type for evaluation. The corroded surface morphology and chemical composition of the corrosion products were analyzed using field-emission scanning electron microscopy (FE-SEM: JSM-7001F, JEOL Ltd., Tokyo, Japan) and energy dispersive spectrometry (EDS: ULTRA DRY, Thermo Fisher Scientific, MA, USA).
The corrosion rate was estimated from the weight loss of staples during immersion. After the staples were removed from the solution, they were gently rinsed with distilled water and dried in air. The corrosion products were removed by a chemical reagent (a solution of 200 g/L CrO3). An average of four measurements were taken for each combination of staple material/solution. The in vitro corrosion rate was calculated by Eq., according to ASTM NACE TM0169/G31-12a Standard Guide for Laboratory Immersion Corrosion Testing of Metals: Corrosion rate = (K × W)/(A × t × D) where K is a constant (taken as 8.76 × 104 mm/year), W is mass loss (g), A is area (cm2), t is exposure time (h), and D is density (g/cm3).
Thirty-six male New Zealand White Rabbits with a mean weight of 2.4 kg ± 0.1 kg were used for this experiment. The rabbits were randomly separated into four groups of 9 rabbits according to staple material: Zn alloy 1, Zn alloy 2, Zn alloy 3, and Ti alloy (clinically available Ti alloy staple; Takasago Medical Industry Co., Ltd., Tokyo, Japan). The animals were acclimatized to laboratory conditions (23 °C, 12 h/12 h light/dark, 55% humidity, ad libitum access to food and water) for 1 wk prior to experimentation. The rabbits were anesthetized by inhaling a mixture of isoflurane gas and air. After laparotomy, a part of the stomach on the greater curvature side was resected and closed using staples. After resection, hemostasis was confirmed, and the integrity and quality of the staple line were assessed visually. Then, the abdominal incision was closed in two layers.
The general condition was closely evaluated daily to detect any signs of complications. Three rabbits in each group were sacrificed under nonpainful conditions for confirmation of sequential change at 1, 4, and 12 wk post-operation. On sacrifice, the presence of anastomotic leakage and hematoma were evaluated. In addition, tissue samples of stomachs closed by each type of staple were explanted for histopathological analysis. They were formalin-fixed, embedded in resin, and cut into 30-µm thick slices. These consecutive resin embedded sections were stained with hematoxylin–eosin (HE) and the presence of inflammation around the staples was evaluated by pathologists blinded to material on the staples.
In order to evaluate the in vivo biodegradable behavior, the remaining staples were retrieved from the stomach on sacrifice and were examined using FE-SEM and EDS.
This experimental protocol was approved by the Animal Experiment Committee of Nagoya University (Approval ID: 31354). All experiments were performed in accordance with ARRIVE Guidelines.
Experimental values are expressed as mean ± SD. The statistical significance of the difference in the corrosion rates between FeSSIF and HBSS was analyzed using the t-test. The statistical significance of the differences in staple shape before and after implantation was analyzed using the paired t-test. Differences with P < 0.05 were considered statistically significant. All statistical analyses were performed using the SAS (V.9.4; SAS Institute, Cary, North Carolina, USA).
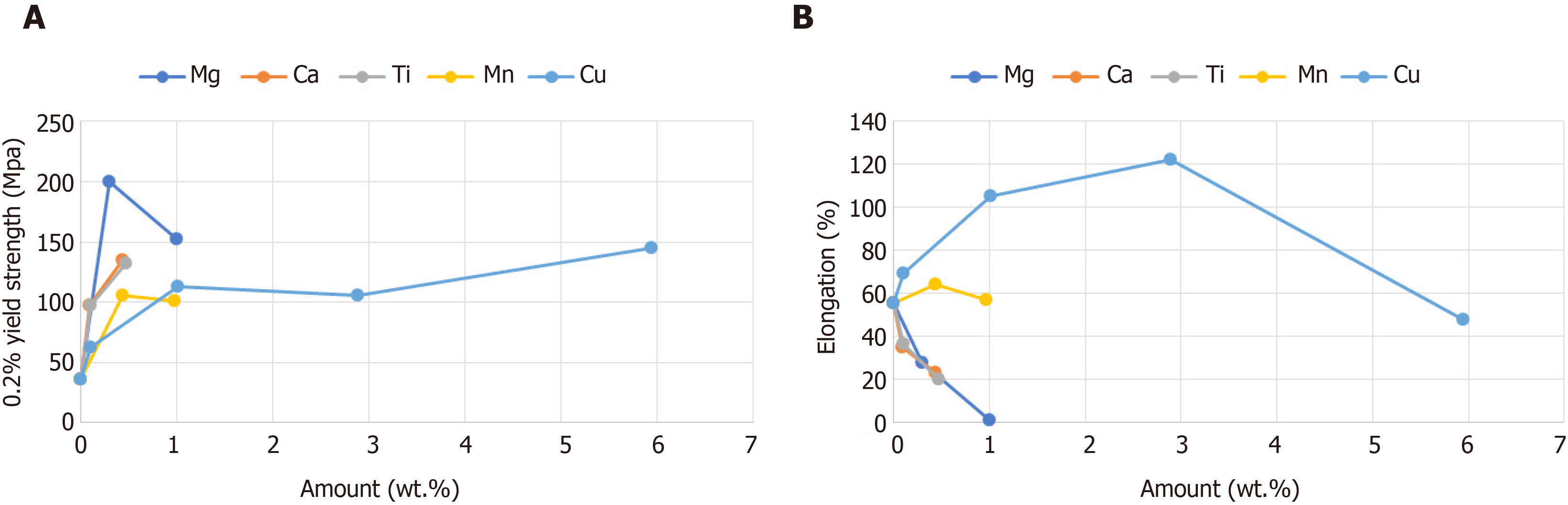
The mechanical properties of the Zn alloys are given in Figure 1 and Table 2. Additions of Cu, Ti, Mn, Ca, or Mg improved the 0.2% yield strength of Zn. Mg was the most strengthening additive, with a 0.3 wt.% Mg addition improving the 0.2% yield strength of Zn by more than 5 times, from 36 to 200 MPa. However, Mg also caused a significant reduction in elongation and bendability, and the Zn–3.0Mg alloy could not be processed into sheets with a thickness of 0.5 mm because of fracture. Additions of 0.1 and 0.5 wt.% Ca improved the 0.2% yield strength of Zn from 36 MPa to 98 and 135 MPa, respectively, while the elongation decreased from 55% to 35% and 23%, respectively. However, a deterioration in bendability occurred with more than 0.5 wt.% Ca. In contrast, while the addition of Ti also improved the 0.2% yield strength and reduced elongation, it showed little impact on bendability. The addition of Mn showed a lesser influence on the 0.2% yield strength compared with Mg, Ca, or Ti, but it had little influence on elongation or bendability. Cu improved the 0.2% yield strength as the addition amount increased, whereas the elongation was greatest with 3 wt.% Cu, and the bendability was greatest with 1.0 wt.% Cu.
| Visual appearance | |
| Zn | Wrinkles |
| Zn–0.3Mg | Broken |
| Zn–1.0Mg | Broken |
| Zn–3.0Mg | - |
| Zn–0.1Ca | Smooth |
| Zn–0.5Ca | Small cracks |
| Zn–0.1Ti | Small wrinkles |
| Zn–0.5Ti | Wrinkles |
| Zn–0.5Mn | Smooth |
| Zn–1.0Mn | Smooth |
| Zn–0.1Cu | Small wrinkles |
| Zn–1.0Cu | Smooth |
| Zn–3.0Cu | Large cracks |
| Zn–6.0Cu | Large cracks |
The compositions of the Zn alloys selected for testing as surgical staples were determined from the results of the above mechanical tests of Zn-based binary alloys. Three Zn alloys were selected: Zn–1.0Cu–0.2Mn–0.1Ti (Zn alloy 1), Zn–1.0Mn–0.1Ti (Zn alloy 2), and Zn–1.0Cu–0.1Ti (Zn alloy 3). The chemical compositions of Zn alloys 1–3, analyzed via ICP-OES, are given in Table 3, and the mechanical properties of wires made from these alloys are given in Table 4.
| Ti | Mn | Cu | Zn | |
| Zn–1.0Cu–0.2Mn–0.1Ti | 0.10 | 0.20 | 1.01 | Bal. |
| Zn–1.0Mn–0.1Ti | 0.10 | 1.00 | - | Bal. |
| Zn–1.0Cu–0.1Ti | 0.09 | - | 1.02 | Bal. |
| UTS (MPa) | 0.2% YS (MPa) | EL (%) | |
| Zn alloy 1 (Zn–1.0Cu–0.2Mn–0.1Ti) | 212 | 196 | 19 |
| Zn alloy 2 (Zn–1Mn–0.1Ti) | 198 | 180 | 7 |
| Zn alloy 3 (Zn–1Cu–0.1Ti) | 200 | 177 | 21 |
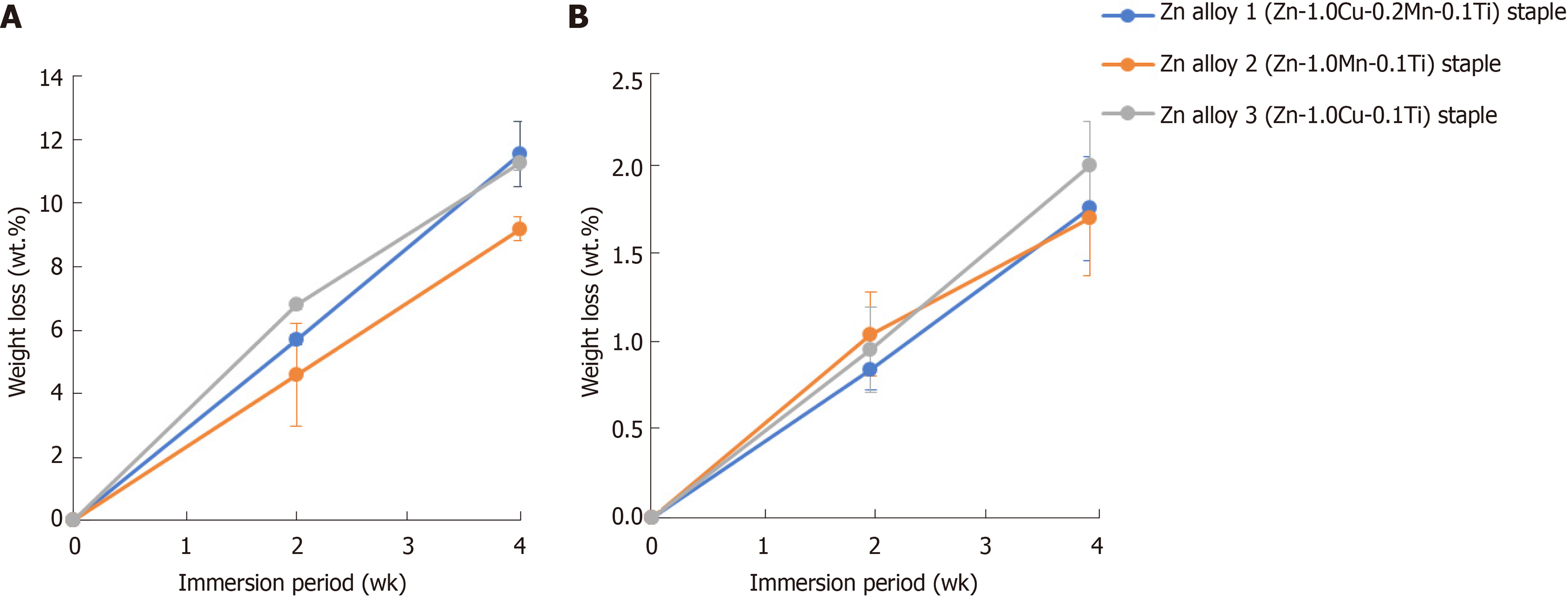
During 4 wk of immersion in FeSSIF or HBSS solution, no gas evolution or staple fracture was observed in any of the three Zn alloy staples. All three Zn alloy staples decreased in weight as the immersion time increased (Figure 2). While the corrosion rates of Zn alloy staples 1, 2, and 3 were each 0.02 mm/year in HBSS, they showed a slight difference in corrosion rate in FeSSIF, at 0.12, 0.11, and 0.13 mm/year, respectively. Notably, the corrosion rate in the acidic FeSSIF was approximately five times higher than that in the neutral HBSS (Zn alloy staple 1; P < 0.001, Zn alloy staple 2; P < 0.001, Zn alloy staple 3; P < 0.001).
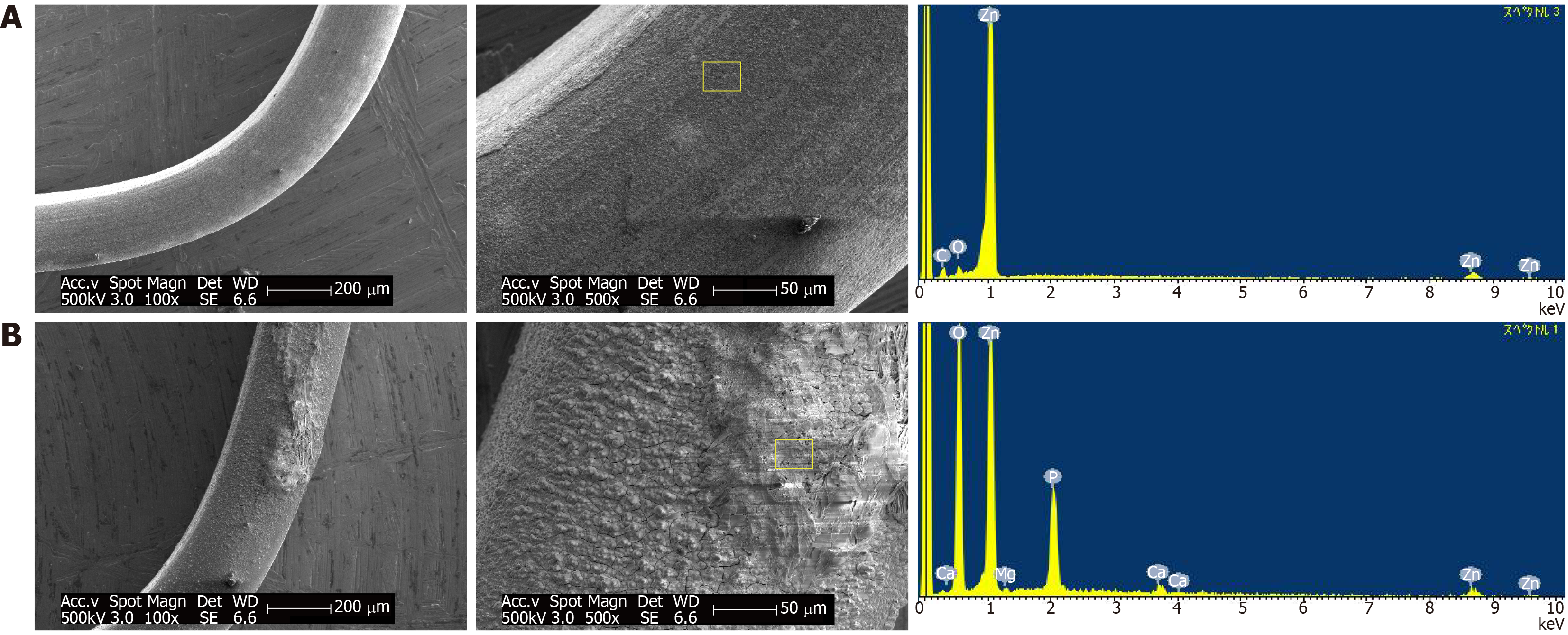
The corrosion morphologies of Zn alloy 1 staples after 4 wk of immersion are shown in Figure 3. The SEM images indicate that the staple immersed in FeSSIF developed a rough surface while that in HBSS was covered with a corrosion layer. The EDS profile of the corroded surface identified weak peaks corresponding to C and O in addition to component elements on the surface of the staple immersed in FeSSIF; while Mg, P, and Ca were identified in the corrosion products after immersion in HBSS. The fact that these elements were not present in the original alloys reveals that the HBSS solution interacted with the Zn alloy to form corrosion products. The lack of corrosion products on the staple immersed in FeSSIF shows that the component elements eluted as ions.
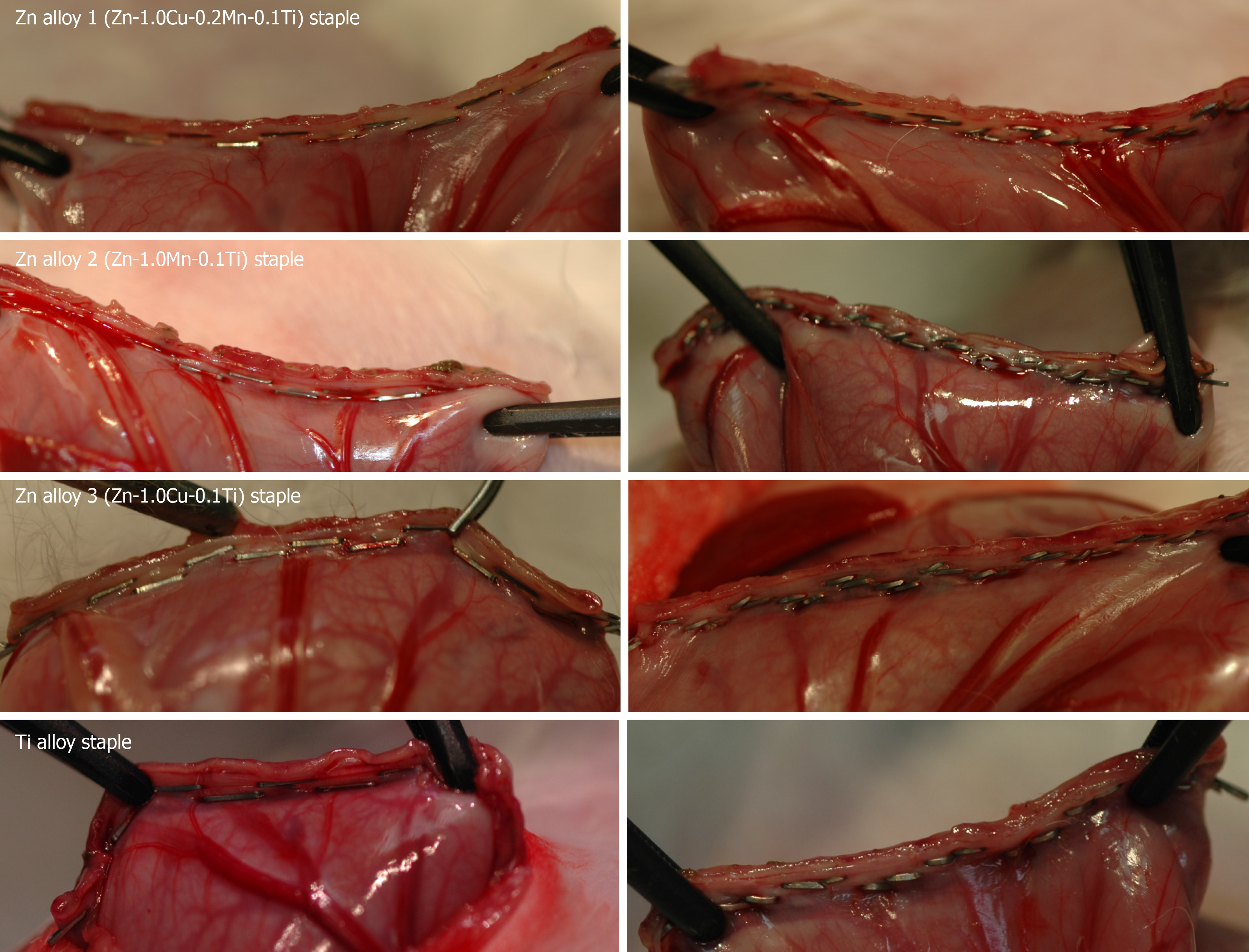
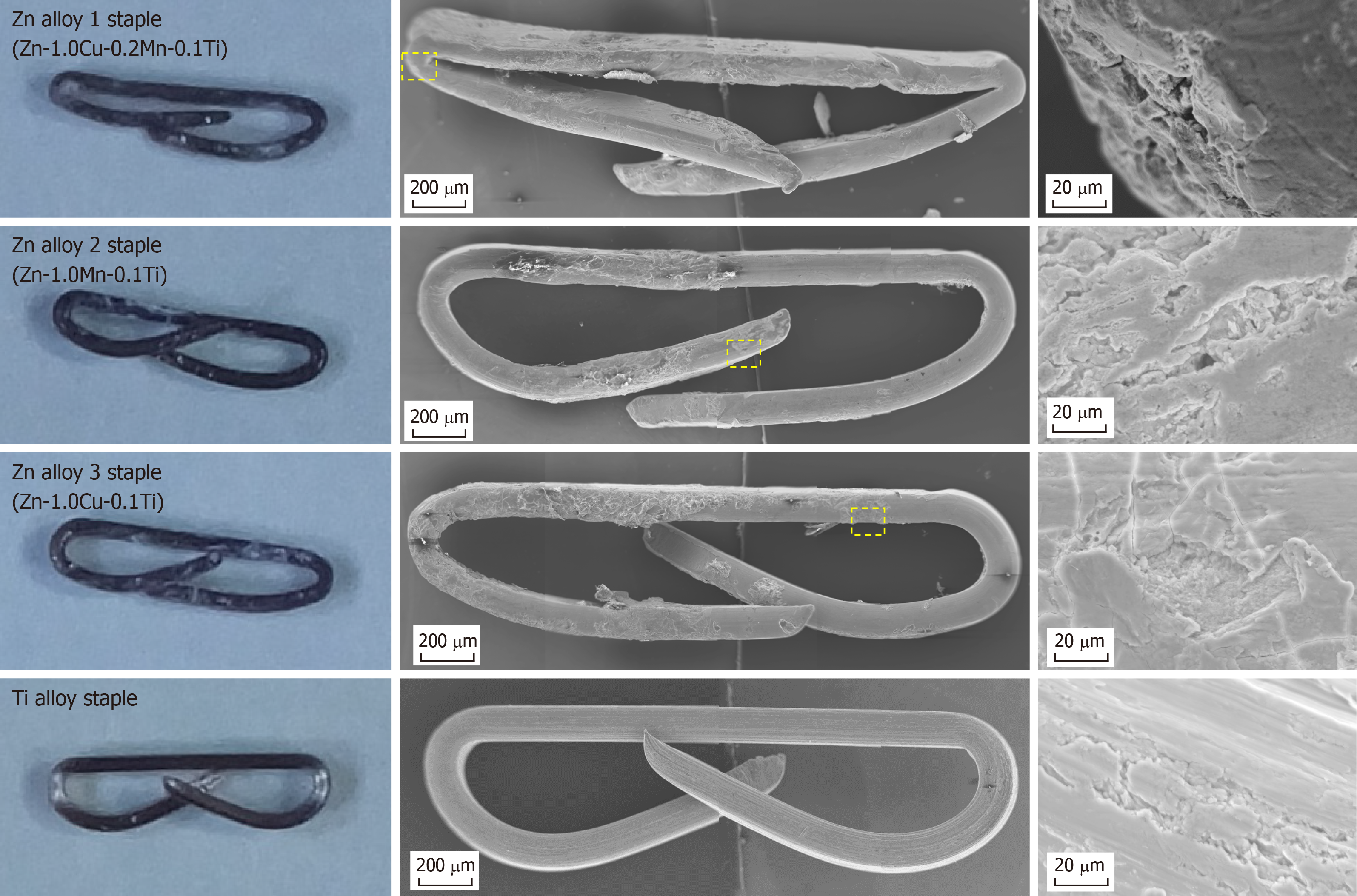
None of the three types of Zn alloy staple nor the Ti alloy staples caused any technical failure during gastric resection procedures. All staples were closed to B-shapes without fracture and all the resected stomachs were smoothly closed (Figure 4). All the rabbits survived for the predefined duration without any complications. Postoperative oral intake was favorable in all rabbits.
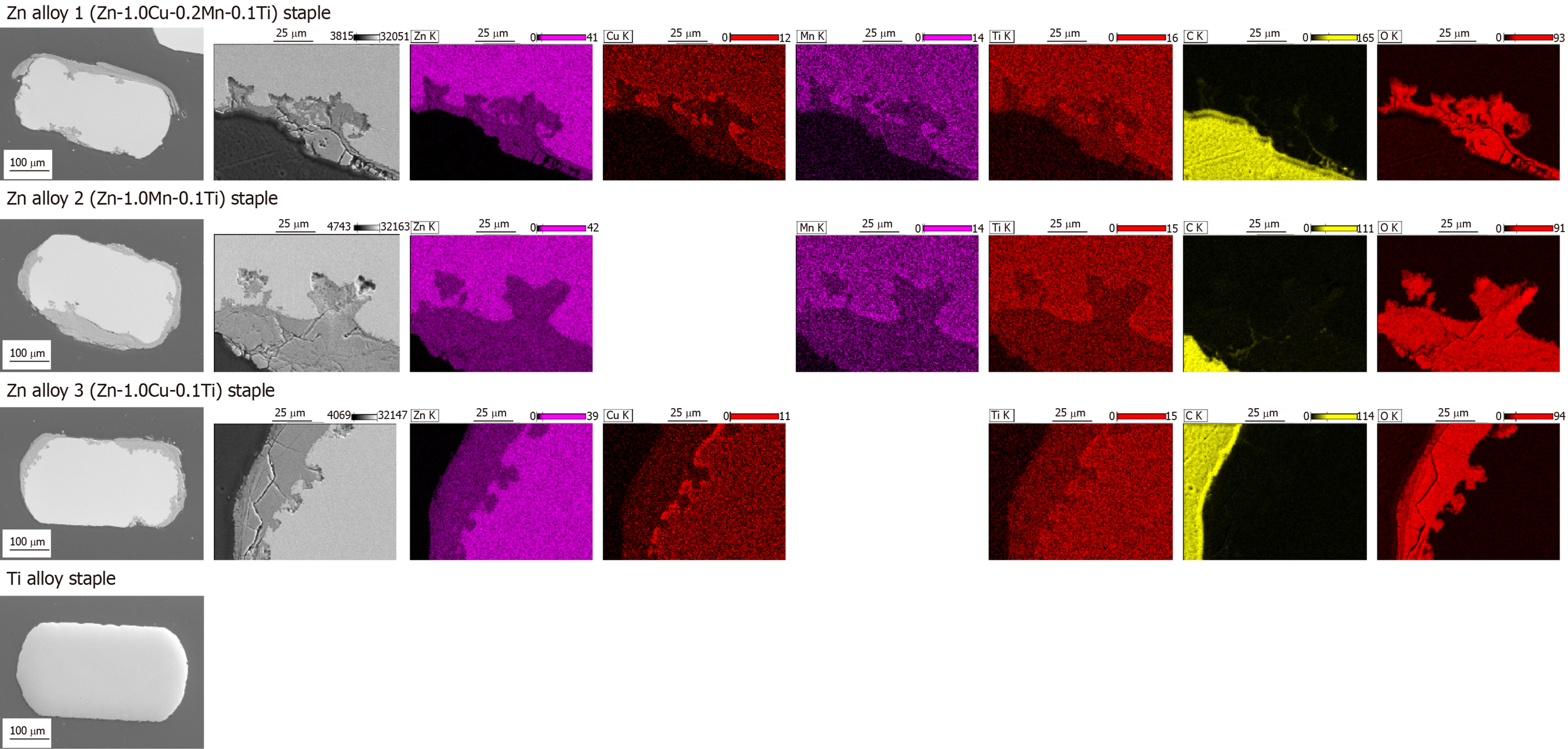
On sacrifice, all the gastric stumps were observed to have healed well, with no leakage or hematoma observed in any group. All the Zn alloy staples retained their B-shape without fracture until 12 wk after surgery. The appearance of the staples removed from the gastric tissue after 12 weeks’ implantation is shown in Figure 5 alongside the microscopic surface morphology. No significant degradation was seen by macroscopic observation, but SEM analyses revealed rough and partly corroded surfaces for all the Zn alloy staples. Figure 6 shows representative SEM images and EDS elemental maps of the staple cross-sections after 12 weeks’ implantation, showing clearly that corrosion of the Zn alloy staples began at the surface and developed inward. The corrosion products were mainly composed of Zn and O, suggesting that these products mainly comprise hydroxide compounds.
The weights of Zn alloy staples 1, 2, and 3, and the Ti alloy staples after 12 weeks’ implantation (vs the weight before implantation, P value) were 8.2 mg ± 0.1 mg (vs 8.6 mg ± 0.0 mg, P = 0.0004), 9.2 mg ± 0.9 mg (vs 9.9 mg ± 0.0 mg, P = 0.1617), 8.9 mg ± 0.1 mg (vs 9.3 mg ± 0.0 mg, P = 0.0002), and 6.7 mg ± 0.1 mg (vs 6.5 mg ± 0.0 mg, P = 0.0367).
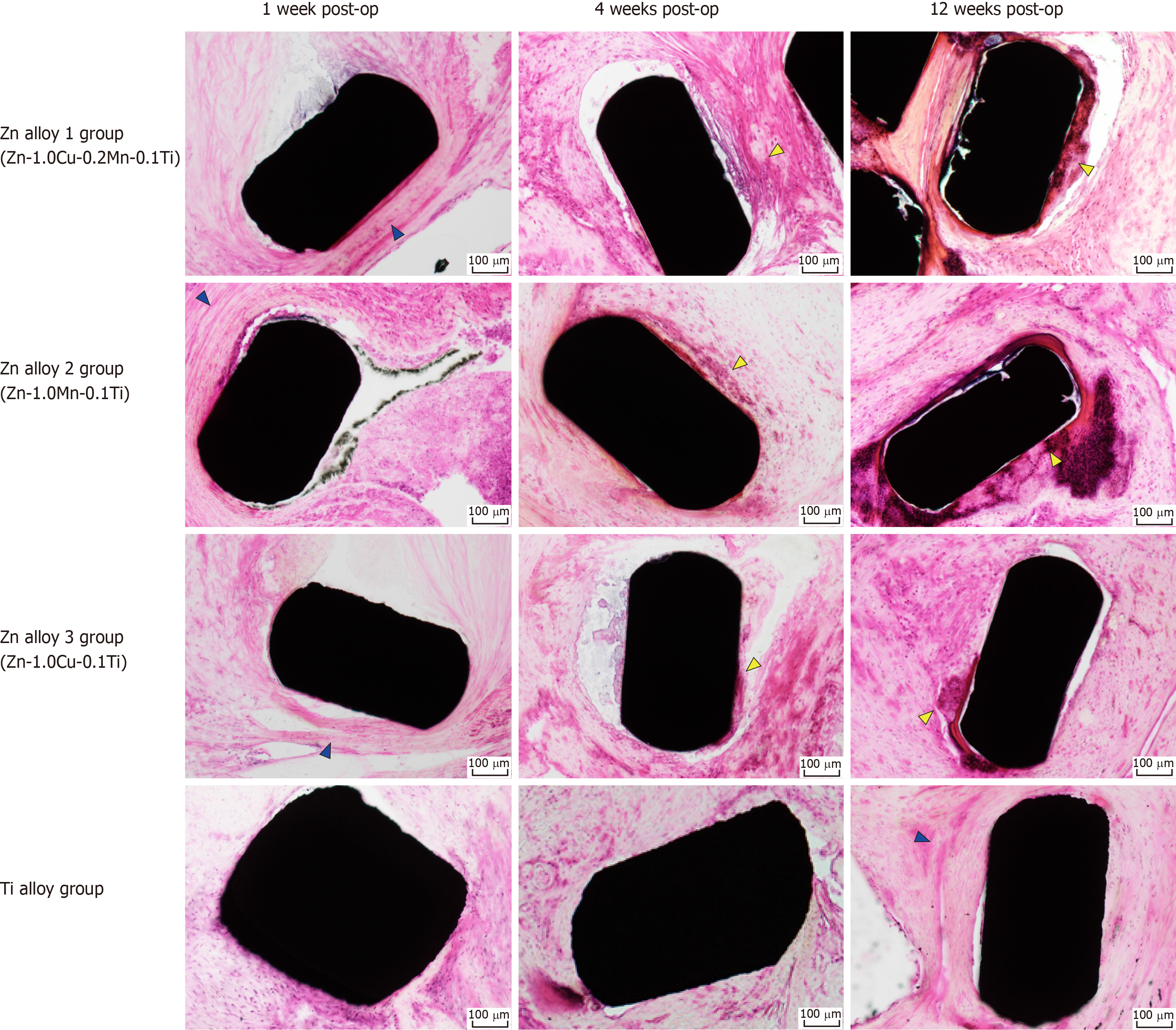
Histopathological images are shown in Figure 7. One wk after surgery, minimal inflammatory cell infiltrations—mostly segmented neutrophils and lymphocytes—were localized around only the Zn alloy staples. After 4-12 wk post-surgery, mild-to-moderate inflammatory infiltrates, composed of eosinophils, lymphocytes, and macrophages, were observed around the Zn alloy staples, along with foreign body giant cells and/or phagocytosis.
We developed novel Zn alloys with mechanical properties suitable for surgical staples for the first time. First, we determined a suitable Zn alloy composition from the results of mechanical tests on binary Zn-based alloys. Balancing the combination of strength and elongation in the same alloy is challenging, especially for Zn alloys. In most cases, a higher ultimate tensile strength is associated with an increase in yield strength, but also a drop in ductility[23]. In our study, the additions of Cu, Ti, Mn, Ca, and Mg all improved the ultimate tensile strength of Zn, but their effect on bendability and elongation varied. These properties are crucial for forming staples and ensuring that stapling can be carried out without fracture. The mechanical tests revealed that Mg was an inadequate alloying element despite improving the strength the most, because it decreased the elongation and bendability. On the contrary, Mn and Cu additions of 1 wt.% or less did not deteriorate elongation or bendability, so they were selected as candidate alloying elements. Similarly, the Ti addition improved the yield strength while showing little impact on bendability. Therefore, we determined the three zinc alloy compositions as Zn–1.0Cu–0.2Mn–0.1Ti, Zn–1.0Mn–0.1Ti, and Zn–1Cu–0.1Ti. These Zn alloys demonstrated excellent mechanical properties and were successfully fabricated into staples.
To examine the degradation behavior of the staples, we first conducted in vitro immersion tests in simulated body fluids. Mg alloy staples have been reported to evolve hydrogen gas during the process of degradation, which may interfere with the healing process, resulting in abscess formation and anastomotic leakage[20]. This was not the case with the Zn alloy staples. Immersion in FeSSIF yielded 5 times faster degradation than that in HBSS, which implies that the degradation rate is increased under low pH conditions. SEM and EDS surface analyses of one of the Zn alloy staples (Zn alloy 1) after immersion for 4 wk in FeSSIF indicated that the component elements eluted as ions and corrosion products were not produced. On the contrary, the staples immersed in neutral HBSS were covered by corrosion products, mainly based on a mixture of zinc hydroxides, calcium phosphates, and carbonates. It appears that the dissolution of Zn may have been inhibited by these corrosion products. As the in vitro degradation rate varied with the pH of the solution[24], the in vivo degradation rate is expected to differ depending on the type of tissue. For example, staples used for resection or anastomosis of gastrointestinal tracts would degrade faster as they are exposed to acidic digestive fluids. A simple calculation assuming a stable rate of degradation reveals that the complete degradation of Zn alloy staples 1, 2, and 3 would take 1.7, 1.8, and 1.5 years, respectively, according to the corrosion rate in FeSSIF. In gastrointestinal anastomosis, approximately 1–2 wk are necessary for wound healing and strength recovery of the gastrointestinal tissue with angiogenesis and collagen synthesis[25,26]. Therefore, these degradation times are sufficient for wound healing.
As far as systemic toxicity is concerned, the constituent elements of these Zn alloys are essential elements for maintaining physiological homeostasis processes, other than Ti, which is the raw material of commercial Ti alloy staples. However, the allowable range of each alloying element within the human body should be considered. Zn is the second most abundant transition metal in the human body and is essential for many biological functions[27-29]. The recommended Zn allowance for an adult is estimated at 15 mg/d[30]. Considering the use of 16 Zn alloy staples for one stapling, and assuming complete degradation periods, the amount of Zn ions released daily from Zn alloy staples 1, 2, and 3 can be calculated as approximately 0.56, 0.51, and 0.62 mg/d, respectively, which is far below the daily allowance. Similarly, the calculated daily releases of Mn and Cu ions are negligible compared to the allowed values: Zn alloys staples 1 and 2 released 0.00 and 0.01 mg/d Mn, respectively, vs 4 mg/d daily allowance; Zn alloy staples 1 and 3 each released 0.01 mg/d Cu, vs 2-3 mg/d daily allowance[30]. Therefore, systemic biosafety of the Zn alloy staples can be expected.
The safety and feasibility of the Zn alloy staples were confirmed through gastric resection in rabbit models. All the gastric resections using Zn alloy staples were successfully performed and all rabbits survived without complications (including without anastomotic leakage), and there was no distinct change in the general condition after gastric resection. In terms of local biocompatibility, histopathological analysis revealed no severe inflammatory reactions around the Zn alloy staples. Zn alloy staples are considered to have no adverse effect on local safety; nevertheless, long-term evaluation is necessary in the future. SEM images of the cross-sections of the staples showed that corrosion developed from the surface toward the center of the staples. A significant difference in the corrosion rates of Zn alloys 1 and 3 was observed—this difference was not present in the immersion tests, perhaps because the total amount of alloying elements was small (maximum 1.3 wt.%), and the observation period was short (only 4 wk). Elemental mapping of the Zn alloy staples revealed a thick layer of Cu on the surface of alloy staples 1 and 3, suggesting that micro-galvanic corrosion between Zn and Cu accelerated the corrosion rate of the staples containing Cu. However, despite this accelerated weight loss, all three Zn alloy staples kept their B-shape without fracturing in the rabbit gastric resection throughout the observational period of 12 wk. As described above, it is considered that all Zn alloy staples can provide sufficient closure for wound healing. Foreign matter that remains in a living body for a long time runs the risk of causing allergic/foreign-body reactions, adhesion, or other adverse effects. Therefore, in this respect, the use of a biodegradable Zn alloy staple is considered to be less risky than the use of a Ti alloy staple.
We developed Zn alloys with promising elemental compositions for staple applications and successfully fabricated Zn alloy staples of these compositions. However, this study has a few limitations. HBSS and FeSSIF are not completely consistent with practical environments in the body and the in vivo degradation process is more complex, which means that the in vitro results may not accurately reflect the in vivo results. Moreover, although we performed an in vivo experiment, its safety could not be fully evaluated: because only nine rabbit gastric resections were performed in each group, the incidence of anastomotic leakage was low. We also could not evaluate the corrosion timing and the effect of corrosion on the human body because the observation period was as short as 12 wk. Therefore, further studies are needed to examine long-term in vivo outcomes. It is also necessary to prepare staples of different sizes and strengths depending on the elasticity and thickness of the target tissue.
In conclusion, staples made of Zn–1.0Cu–0.2Mn–0.1Ti, Zn–1.0Mn–0.1Ti, and Zn–1.0Cu–0.1Ti exhibit acceptable in vitro mechanical properties, proper degradation behavior, and in vivo safety and feasibility. They are promising candidates for biodegradable staples.
Surgical staples are widely used in all types of surgery. However, they are manufactured from non-biodegradable Ti or Ti alloys, meaning they exist permanently within the human body; this has resulted in reports of adverse effects, including allergic and foreign-body reactions and adhesion. Therefore, the development of biodegradable surgical staples is desirable. In order to develop a biodegradable alloy suitable for the fabrication of surgical staples, we focused on Zn, which is a known biodegradable metal. In this study, we developed novel Zn alloys with superior mechanical properties compared to the conventional Zn alloys that were suitable for fabricating surgical staples.
Zn has recently been the focus of research as a novel implant metal owing to its good biocompatibility and biodegradability. However, there are no extant reports on Zn-based staples owing to its low mechanical strength.
The objective of this study is to investigate the in vitro mechanical properties and degradation behaviors as well as in vivo safety and feasibility of biodegradable Zn alloy staples.
Tensile and bending tests were conducted to evaluate the mechanical properties of binary Zn alloys with 0.1–6 wt.% Mg, Ca, Mn, Cu, or Ti. Based on the results, three promising Zn alloy compositions were evaluated for staple applications (wt.%): Zn-1.0Cu-0.2Mn-0.1Ti (Zn alloy 1), Zn-1.0Mn-0.1Ti (Zn alloy 2), and Zn-1.0Cu-0.1Ti (Zn alloy 3). Immersion tests were performed using fed-state simulated intestinal fluid (FeSSIF) and Hank's balanced salt solution (HBSS). The corrosion rates were estimated from the weight losses of the staples during immersion. Nine rabbits were used as test subjects for gastric resection using each of the Zn alloy staples, and a clinically available Ti staple was used on another group of nine rabbits. Three rabbits in each group were euthanized at 1, 4, and 12 wk post operation.
Additions of ≤1 wt.% Mn or Cu and 0.1 wt.% Ti improved the yield strength without excessive deterioration of elongation or bendability. Immersion tests revealed no staple fractures in any of the Zn alloy staples. The corrosion rates of Zn alloy staples 1, 2, and 3 were 0.02 mm/year in HBSS and 0.12, 0.11, and 0.13 mm/year, respectively, in FeSSIF. These degradation times are sufficient for wound healing. In the animal study, none of the Zn alloy staples resulted in technical failures, and all rabbits survived without complications. Histopathological analysis revealed no severe inflammatory reactions around the Zn alloy staples.
Staples made of Zn-1.0Cu-0.2Mn-0.1Ti, Zn-1.0Mn-0.1Ti, and Zn-1.0Cu-0.1Ti exhibit acceptable in vitro mechanical properties, appropriate degradation behavior, and in vivo safety and feasibility.
Zn alloy staples are proposed as promising candidates for biodegradable staples to replace the currently available Ti alloy staple.
We deeply condole the demise of Dr. Hiroshi Kishimoto, who performed the pathological analysis in this study. We express our gratitude to all those who assisted us with this study. We are especially grateful to K. Yano for her invaluable help with the animal experiments.
Manuscript source: Unsolicited Manuscript
Specialty type: Medicine, research and experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Musella M, Koch T S-Editor: Wang YQ L-Editor: A E-Editor: Xing YX
| 1. | Nihon-Yanagi Y, Ishiwatari T, Otsuka Y, Okubo Y, Tochigi N, Wakayama M, Nemoto T, Watanabe M, Kaneko H, Sumino Y, Shibuya K. A case of postoperative hepatic granuloma presumptively caused by surgical staples/clipping materials. Diagn Pathol. 2015;10:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Tomita M, Matsuzaki Y, Edagawa M, Shimizu T, Hara M, Onitsuka T. Pulmonary granuloma possibly caused by staples after video-assisted thoracoscopic surgery. Ann Thorac Cardiovasc Surg. 2003;9:123-125. [PubMed] |
| 3. | Yüksel M, Akgül AG, Evman S, Batirel HF. Suture and stapler granulomas: a word of caution. Eur J Cardiothorac Surg. 2007;31:563-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Tamai K, Mitsumori M, Fujishiro S, Kokubo M, Ooya N, Nagata Y, Sasai K, Hiraoka M, Inamoto T. A case of allergic reaction to surgical metal clips inserted for postoperative boost irradiation in a patient undergoing breast-conserving therapy. Breast Cancer. 2001;8:90-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Degidi M, Artese L, Scarano A, Perrotti V, Gehrke P, Piattelli A. Inflammatory infiltrate, microvessel density, nitric oxide synthase expression, vascular endothelial growth factor expression, and proliferative activity in peri-implant soft tissues around titanium and zirconium oxide healing caps. J Periodontol. 2006;77:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 6. | Müller K, Valentine-Thon E. Hypersensitivity to titanium: clinical and laboratory evidence. Neuro Endocrinol Lett. 2006;27 Suppl 1:31-35. [PubMed] |
| 7. | Liu B, Zheng YF. Effects of alloying elements (Mn, Co, Al, W, Sn, B, C and S) on biodegradability and in vitro biocompatibility of pure iron. Acta Biomater. 2011;7:1407-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 8. | Pierson D, Edick J, Tauscher A, Pokorney E, Bowen P, Gelbaugh J, Stinson J, Getty H, Lee CH, Drelich J, Goldman J. A simplified in vivo approach for evaluating the bioabsorbable behavior of candidate stent materials. J Biomed Mater Res B Appl Biomater. 2012;100:58-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Gu X, Zheng Y, Cheng Y, Zhong S, Xi T. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials. 2009;30:484-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 997] [Cited by in RCA: 531] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 10. | Windhagen H, Radtke K, Weizbauer A, Diekmann J, Noll Y, Kreimeyer U, Schavan R, Stukenborg-Colsman C, Waizy H. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: short term results of the first prospective, randomized, controlled clinical pilot study. Biomed Eng Online. 2013;12:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 11. | Haude M, Erbel R, Erne P, Verheye S, Degen H, Vermeersch P, Weissman N, Prati F, Bruining N, Waksman R, Koolen J. Safety and performance of the DRug-Eluting Absorbable Metal Scaffold (DREAMS) in patients with de novo coronary lesions: 3-year results of the prospective, multicentre, first-in-man BIOSOLVE-I trial. EuroIntervention. 2016;12:e160-e166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Seitz JM, Lucas A, Kirschner M. Magnesium-based compression screws: A novelty in the clinical use of implants. JOM. 2016;68:1177-1182. [DOI] [Full Text] |
| 13. | Haude M, Ince H, Kische S, Abizaid A, Tölg R, Alves Lemos P, Van Mieghem NM, Verheye S, von Birgelen C, Christiansen EH, Wijns W, Garcia-Garcia HM, Waksman R. Sustained safety and clinical performance of a drug-eluting absorbable metal scaffold up to 24 months: pooled outcomes of BIOSOLVE-II and BIOSOLVE-III. EuroIntervention. 2017;13:432-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 14. | Wu H, Zhao C, Ni J, Zhang S, Liu J, Yan J, Chen Y, Zhang X. Research of a novel biodegradable surgical staple made of high purity magnesium. Bioact Mater. 2016;1:122-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Qu S, Xia J, Yan J, Wu H, Wang H, Yi Y, Zhang X, Zhang S, Zhao C, Chen Y. In vivo and in vitro assessment of the biocompatibility and degradation of high-purity Mg anastomotic staples. J Biomater Appl. 2017;31:1203-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Somekawa H, Mukai T. Effect of grain refinement on fracture toughness in extruded pure magnesium. Scr Mater. 2005;53:1059-1064. [DOI] [Full Text] |
| 17. | Zhang Y, You J, Lu J, Cui C, Jiang Y, Ren X. Effects of laser shock processing on stress corrosion cracking susceptibility of AZ31B magnesium alloy. Surf Coat Technol. 2010;204:3947-3953. [DOI] [Full Text] |
| 18. | Denkena B, Köhler J, Stieghorst J, Turger A, Seitz J, Fau DR, Wolters L, Angrisani N, Reifenrath J, Helmecke P. Influence of stress on the degradation behavior of Mg LAE442 implant systems. Procedia CIRP. 2013;5:189-195. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Bobby Kannan M, Singh Raman RK. Evaluating the stress corrosion cracking susceptibility of Mg–Al–Zn alloy in modified-simulated body fluid for orthopaedic implant application. Scr Mater. 2008;59:175-178. [DOI] [Full Text] |
| 20. | Kuhlmann J, Bartsch I, Willbold E, Schuchardt S, Holz O, Hort N, Höche D, Heineman WR, Witte F. Fast escape of hydrogen from gas cavities around corroding magnesium implants. Acta Biomater. 2013;9:8714-8721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 21. | Bowen PK, Drelich J, Goldman J. Zinc exhibits ideal physiological corrosion behavior for bioabsorbable stents. Adv Mater. 2013;25:2577-2582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 327] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 22. | Vojtěch D, Kubásek J, Serák J, Novák P. Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation. Acta Biomater. 2011;7:3515-3522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 234] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 23. | Seitz JM, Durisin M, Goldman J, Drelich JW. Recent advances in biodegradable metals for medical sutures: a critical review. Adv Healthc Mater. 2015;4:1915-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 24. | Mostaed E, Sikora-Jasinska M, Drelich JW, Vedani M. Zinc-based alloys for degradable vascular stent applications. Acta Biomater. 2018;71:1-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 177] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 25. | Robson MC, Steed DL, Franz MG. Wound healing: biologic features and approaches to maximize healing trajectories. Curr Probl Surg. 2001;38:72-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 220] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 26. | Hendriks T, Mastboom WJ. Healing of experimental intestinal anastomoses. Parameters for repair. Dis Colon Rectum. 1990;33:891-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 244] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Hambidge KM, Krebs NF. Zinc deficiency: a special challenge. J Nutr. 2007;137:1101-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 220] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 28. | Plum LM, Rink L, Haase H. The essential toxin: impact of zinc on human health. Int J Environ Res Public Health. 2010;7:1342-1365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 750] [Cited by in RCA: 771] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 29. | Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci. 2005;6:449-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1348] [Cited by in RCA: 1355] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 30. | Chen Q, Thouas GA. Metallic implant biomaterials. Mater Sci Eng R Rep. 2015;87:1-57. [DOI] [Full Text] |