Published online Oct 6, 2020. doi: 10.12998/wjcc.v8.i19.4512
Peer-review started: March 25, 2020
First decision: July 25, 2020
Revised: August 4, 2020
Accepted: August 20, 2020
Article in press: August 20, 2020
Published online: October 6, 2020
Processing time: 188 Days and 18.9 Hours
Duodenal obstruction is a common clinical scenario that can either be mechanical or a pseudo-obstruction. Clinical management of intestinal obstruction starts from localization and proceeds to histological examination of the stenotic intestine. Systemic factors and dysfunction of distant organs might contribute to the development of intestinal obstruction. Here, we report a unique case of idiopathic mechanical duodenal obstruction, which resolved spontaneously after 3 mo of conservative treatment, but was followed by intestinal pseudo-obstruction.
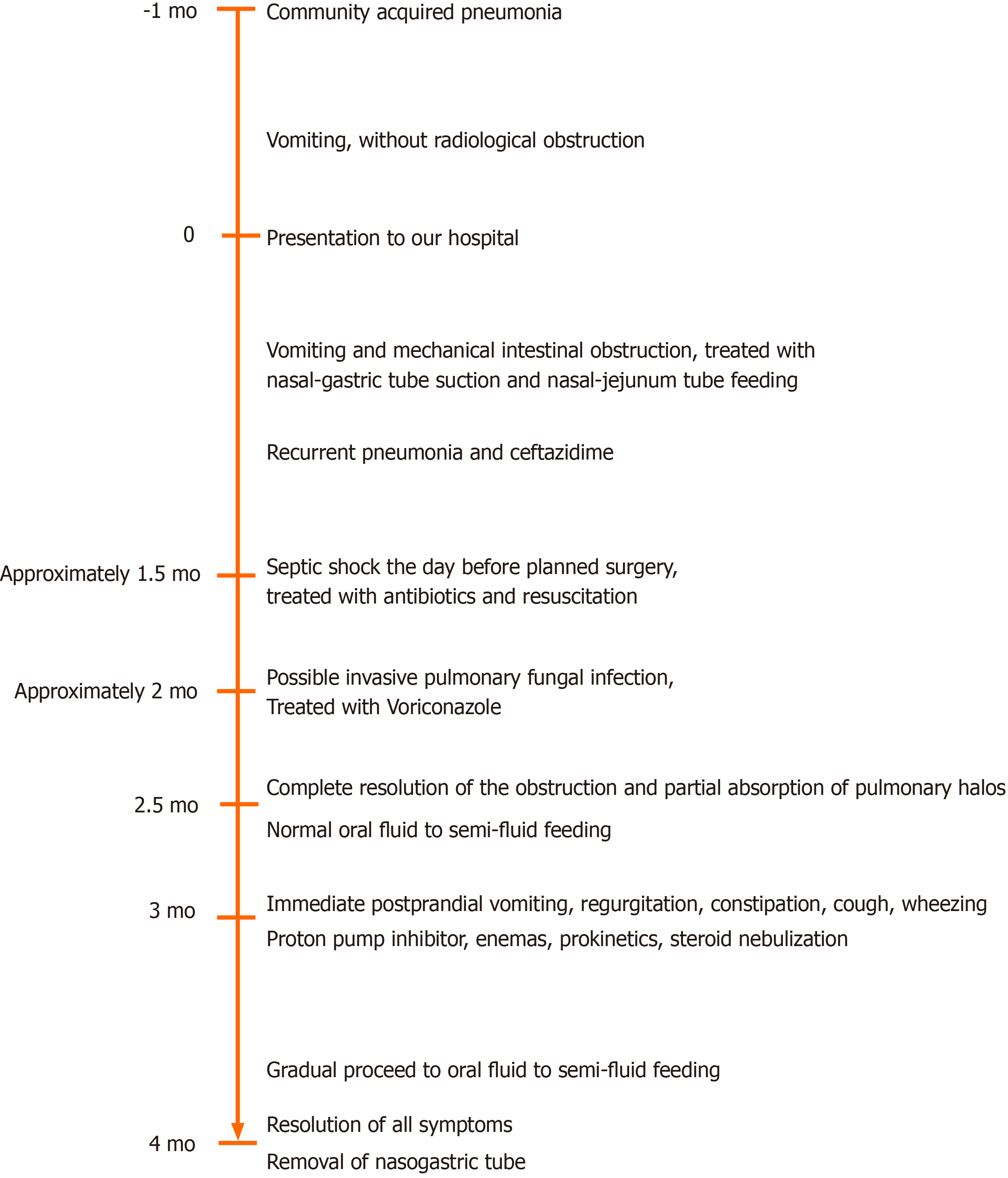
An 84-year-old woman presented with worsened postprandial vomiting accompanied by prolonged pneumonia. Thorough noninvasive investigations revealed complete circumferential stenosis in the descending duodenum without known cause. Exploratory surgery was postponed due to septic shock and possible pulmonary fungal infection. Conservative treatment for 3 mo for ileus and control of pulmonary infection resolved the intestinal obstruction completely. Unfortunately, 2 wk later, she had regurgitation and postprandial vomiting again, complicated by deteriorating wheezing and dyspnea. Computed tomography revealed a dilated stomach and proximal duodenum without new intestinal stricture or pulmonary infiltration. The patient fully recovered after combined treatment with antireflux agents, enema, prokinetics, and bronchodilators.
This complicated case highlights the inter-relationship of local and systemic contributions to ileus and gut dysfunction, which requires multidisciplinary treatment.
Core Tip: We report an 84-year-old female patient with idiopathic mechanical duodenal obstruction during recovery from a community-acquired pneumonia. Her condition deteriorated with strenuous investigations and conservative treatment. Furthermore, she had persistent pulmonary infection leading to septic shock, followed by probable fungal infection. Along with the control of pulmonary infection, her intestinal obstruction resolved spontaneously. Two weeks later, her symptoms of ileus relapsed without mechanic factors, complicated by airway hyperresponsiveness. Finally, the patient had full recovery with concomitant treatment involving both intestine and pulmonary dysfunction.
- Citation: Zhang BQ, Dai XY, Ye QY, Chang L, Wang ZW, Li XQ, Li YN. Spontaneous resolution of idiopathic intestinal obstruction after pneumonia: A case report. World J Clin Cases 2020; 8(19): 4512-4520
- URL: https://www.wjgnet.com/2307-8960/full/v8/i19/4512.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i19.4512
Duodenal obstruction, which accounts for 1%-2% of all intestinal obstructions[1], is characterized by recurrent bilious vomiting shortly after eating, early satiety, and radiological evidence of air–fluid levels in the distended bowel loops[2]. Although malignant lesions and peptic ulcers are the primary causes of duodenal obstruction[1], other local or systemic abnormalities can also cause duodenal stricture[2], which require multiple radiological evaluations, histological examination, and even surgical exploration. Additionally, there are cases of intestinal pseudo-obstruction in which no stricture can be localized[3]. Intestinal pseudo-obstruction is a heterogeneous disorder caused by neuromuscular damage[4], which may result from inherited gene mutations, connective tissue disease, paraneoplastic syndrome, metabolic and endocrine disorders, neuromuscular syndromes, surgery, medication, and infection, including systemic sepsis and pneumonia, and chronic obstructive pulmonary diseases[5,6].
Here we present an 84-year-old female patient with idiopathic mechanical duodenal obstruction, and spontaneous resolution was achieved after 3 mo of conservative treatment. Soon afterwards, she developed pseudo-intestinal obstruction. She had a full recovery with multidisciplinary treatment focusing on maintaining general homeostasis.
An 84-year-old Chinese woman was admitted to our hospital with persistent nausea and vomiting for 2 mo after an episode of community-acquired pneumonia (CAP).
Two months before admission, the patient had CAP. During treatment with intravenous moxifloxacin, she developed progressive nausea, vomiting, and constipation without hematemesis, heartburn or abdominal pain. Upper gastrointestinal (GI) radiography demonstrated decreased GI motility without obstruction. Gastroscopy at that time was normal. Positron emission tomography/computed tomography (PET/CT) showed no abnormalities. She was treated with nasogastric decompression and total parenteral nutrition. However, her condition deteriorated, with immediate postprandial vomiting, persistent low-grade fever, and dyspnea. She was then referred to our hospital.
Her previous medical history was notable for renal transplantation, hypertension, and paroxysmal atrial fibrillation, all of which were well controlled with cyclosporin A, mycophenolate mofetil, amlodipine and amiodarone.
The patient appeared weak. Vital signs were normal except for a low fever of 37.5°C. She had normal sinus rhythm and clear respiratory sounds. The abdomen was flat and soft and no GI pattern or peristaltic wave or mass was observed. Active bowel sound was auscultated.
Complete blood cell count, coagulation, liver and renal functions, as well as thyroid function were normal. Serum pH and electrolytes, including Na, K, Ca, Mg, Cl, and P, were well monitored during the entire disease course. C-reactive protein level was 19 mg/L (normal 0-8 mg/L). Serum carbohydrate antigen 19-9 (CA19-9) was elevated to 119 U/mL, but was gradually decreased to normal level during follow-up. Sputum cultures showed Pseudomonas aeruginosa. Other sputum pathogens, including fungi, mycobacteria and viruses, were negative. Repeated cultures of peripheral blood, stools, gastric drainage and urine, as well as serum pathogenic antibodies were also negative.
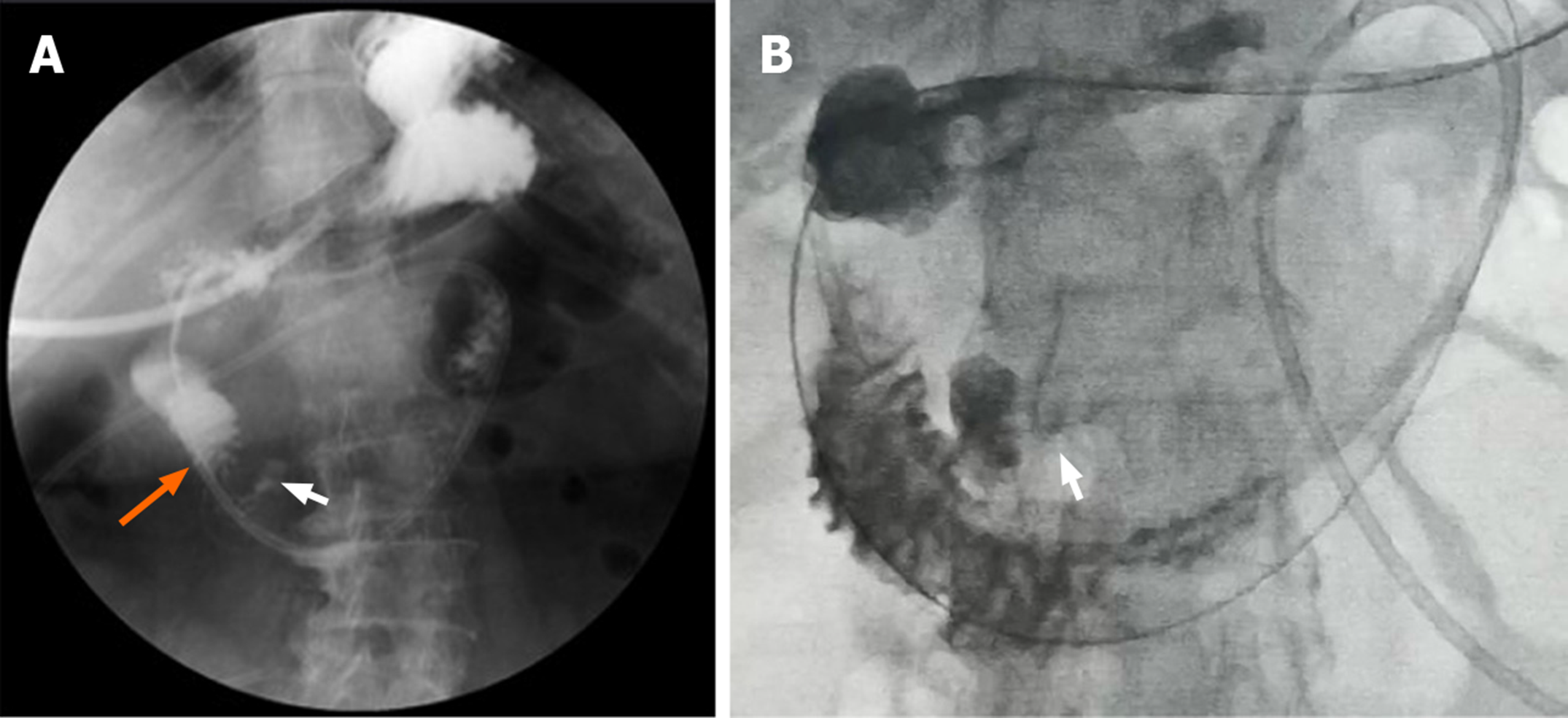
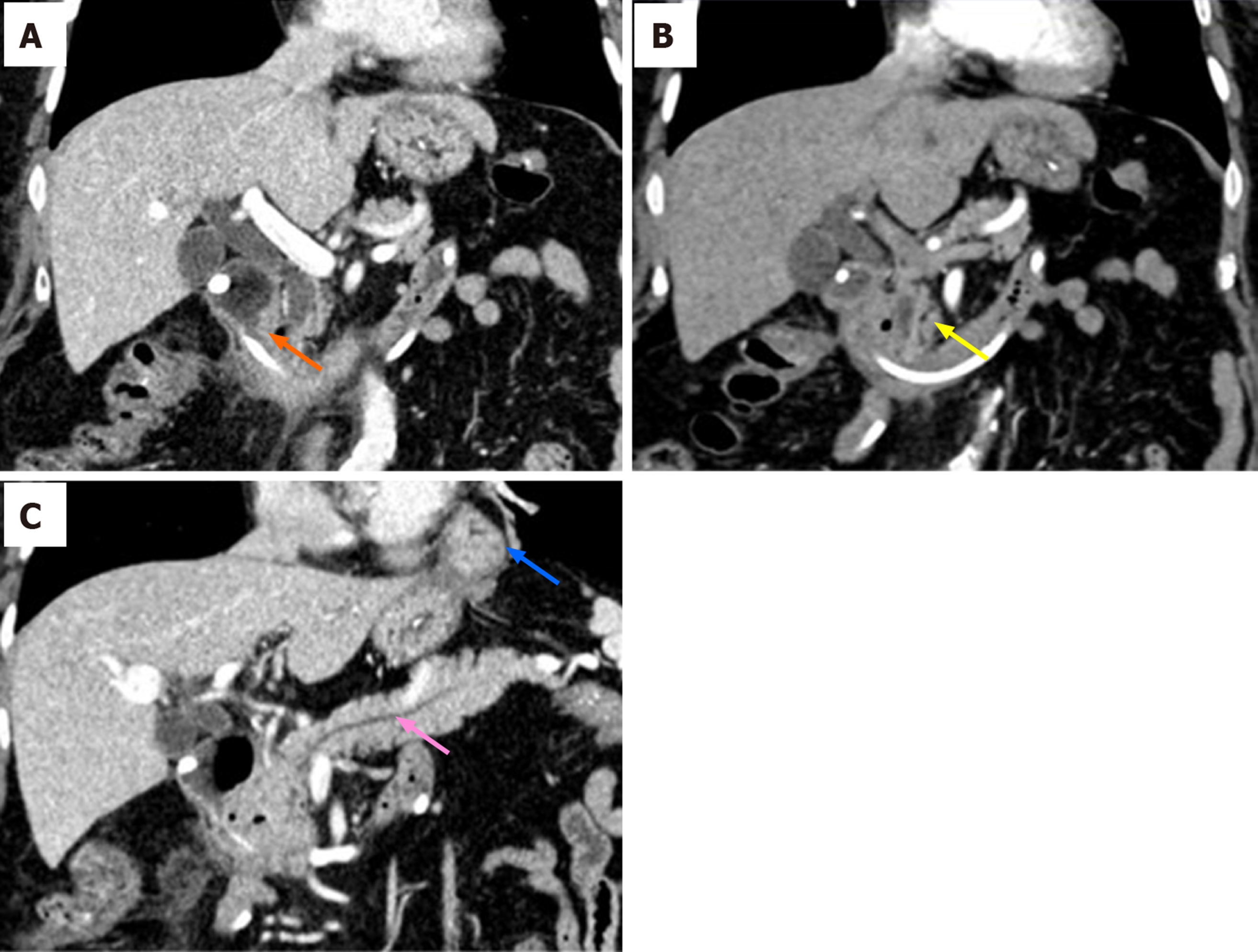
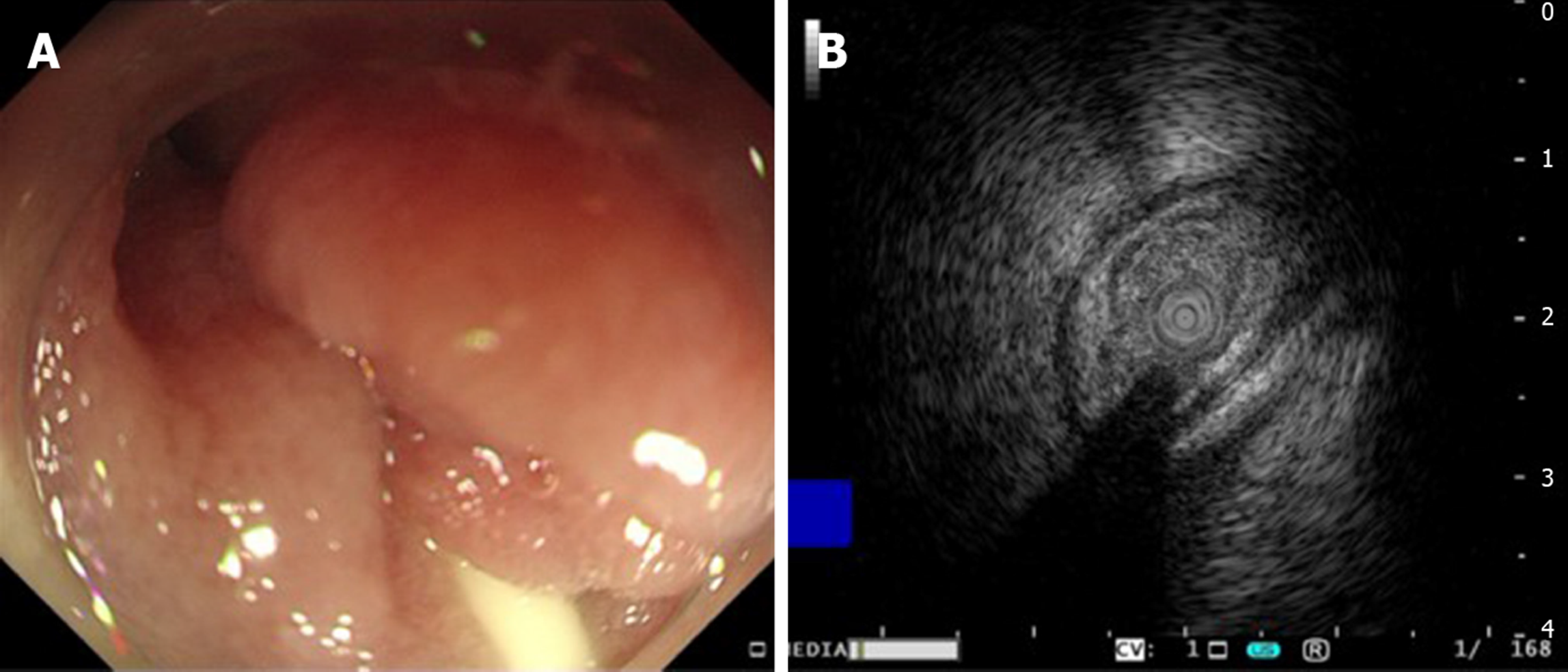
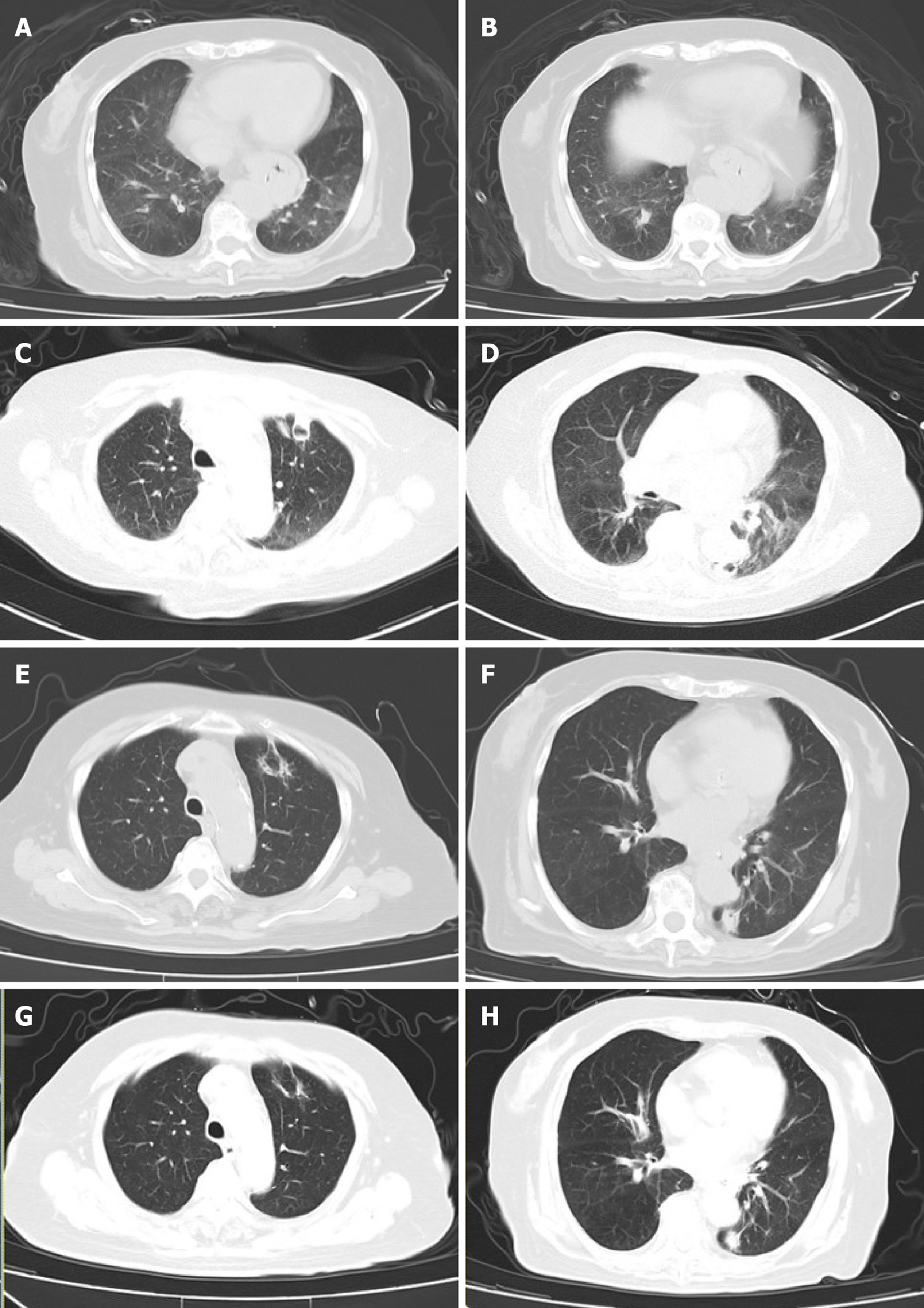
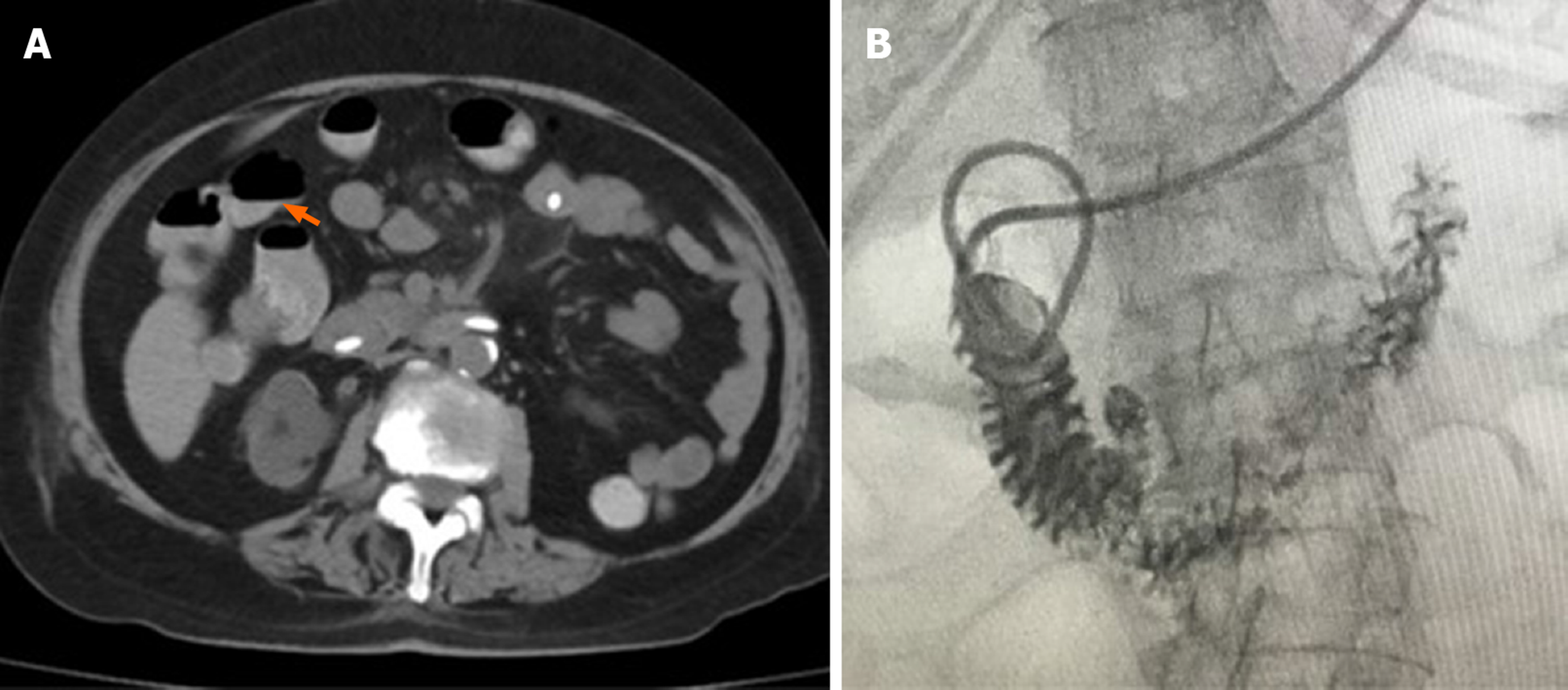
Upper GI radiography in our hospital showed intestinal stricture in the descending duodenum, which merely allowed the passage of a nasojejunal tube (Figure 1A). Axial CT with intravenous contrast demonstrated postbulbar stenosis (Figure 2A) and a thin-walled extraluminal diverticulum distal to the stenosis, with surrounding mesenteric fat stranding (Figure 2B). Other features on axial CT included hiatal hernia and a slightly dilated main pancreatic duct without mass or edema (Figure 2C). Gastroscopy revealed annular stenosis in the descending duodenum that did not allow the passage of an endoscope. The mucosa around the stenosis was edematous without signs of ulceration, hyperplasia or other abnormalities (Figure 3A). Endoscopic ultrasound revealed diffuse thickening of the mucosal layer but normal submucosal muscular layer (Figure 3B). Biopsy and histopathological examination of the swollen mucosa around the stenotic area revealed infiltration of nonspecific inflammatory cells. Chest CT revealed bilateral pulmonary infiltration (Figure 4A and B).
Malignancy was initially considered based on the mechanical duodenal obstruction with increased CA19-9 and dilated pancreatic duct. However, as no mass could be identified on radiological investigations, and histological examination of the swollen mucosa showed nonspecific changes, she was diagnosed with idiopathic duodenal obstruction and hospital-acquired pneumonia (HAP).
A nasogastrojejunal tube was placed and the patient received total enteral feeding. She also received ceftazidime for HAP. All her medication was carefully monitored by a pharmacological consultant. However, during the following 2 mo, she continued to have voluminous gastric drainage up to 2 L/d and recurrent fever. Intestinal obstruction remained unchanged, and she still had bilateral infiltration in her lungs. She became progressively sicker. After consultation with gastroenterologists and surgeons, exploratory laparotomy and gastrojejunostomy were scheduled.
However, just before surgery, the patient had a sudden high fever of 38.8°C, trembling, dyspnea, hypotension, and paroxysmal atrial fibrillation. Her white blood cell count spiked to 20 × 109/L. Septic shock was diagnosed and she received fluid resuscitation, broad-spectrum antibiotics, and noninvasive respiratory ventilation. The patient slowly stabilized with normal temperature and sinus rhythm. Two weeks later, her temperature rose to 38.5°C again. Repeat chest CT showed two thick-walled hollows with a crescent effusion in the left lung (Figure 4C and D). Sputum cultures only revealed carbapenem-resistant Pseudomonas aeruginosa. Stool cultures, serum 1,3-b-D-glucan test and galactomannan test were negative. Considering the patient’s immunosuppressive status following renal transplantation, culture results and imaging characteristics, both intravenous ceftazidime (1 g q8 h) and voriconazole (0.2 g q12 h) were administered.
Her temperature returned to normal 3 d later. One week later, which was nearly 3 mo after the occurrence of intestinal obstruction, she felt hungry and gastric drainage was significantly reduced to about 400 mL/d even after drinking. Upper GI radiography showed complete resolution of the duodenal stenosis with normal GI motility (Figure 1B). Two weeks later, the pulmonary lesions were significantly absorbed (Figure 4E and F). The patient started an oral fluid diet and then a semifluid diet, ceftazidime was stopped and antifungal regimen was switched to oral voriconazole 0.2 g twice daily.
After 2 wk of oral ingestion, she again developed immediate postprandial vomiting, increased gastric drainage of 1.5-2.0 L/d, abdominal bloating, regurgitation and constipation. Bowel sounds were decreased on auscultation. Axial CT revealed an intestinal air–fluid level and enlarged gastric volume (Figure 5A), with no signs of stenosis on CT or upper GI radiography (Figure 5B). At the same time, the patient also developed a nonproductive cough, wheezing and shortness of breath, which progressed to nonfluent speech. Stool and sputum cultures were all negative. Repeat chest CT showed further absorption of the previous hollows without new lesions (Figure 4G and H).
Multidisciplinary consultation with gastroenterologists, respiratory specialists, infectious disease specialists and nutritionists was conducted. Considering her complicated clinical condition involving both GI and respiratory systems, antacids, enemas, prokinetics, and bronchodilator nebulization were administered, and oral intake was discontinued. During the next week, her symptoms gradually improved and gastric drainage decreased to < 400 mL/d. She again tried a fluid diet and slowly progressed to a normal diet during the next month. She experienced several brief episodes of nausea, regurgitation and dyspnea afterwards, especially when her bowel movements decreased. Enemas and antacids eliminated the symptoms. The nasojejunal tube was finally removed after regaining full oral ingestion, which was 6 mo after her first symptoms of intestinal obstruction. Oral voriconazole was successfully discontinued after 3 mo of treatment. At follow-up of 6 mo and 1 year, the patient was free from either intestinal or pulmonary symptoms.
The present case was characterized by progression from slow intestinal motility after CAP to mechanical duodenal obstruction accompanied with HAP, followed by pseudo-intestinal obstruction complicated by airway hyperresponsiveness (AHR) (Figure 6).
During the first phase of intestinal obstruction, abdominal CT revealed asymmetric bowel wall thickening adjacent to a diverticulum with peridiverticular mesenteric fat stranding, suggesting diverticulitis[7]. Duodenal diverticulum, usually suggesting a defective local mucosal barrier[8], is mostly asymptomatic. However, it may become inflamed and lead to diverticulitis, bowel wall thickening, luminal obstruction and even perforation and hemorrhage in conditions of increased intra-abdominal pressure, inflammation, dysbiosis and dysmotility[9,10]. In the present case, the obstruction started after decreased transit mobility during pneumonia, thus, inspissated food could have caused the diverticular inflammation, which in turn, obstructed the intestinal lumen and probably accounted for elevated CA19-9 and pancreatic duct dilation[11]. Additionally, persistent pneumonia could have increased systemic release of inflammatory cytokines, including tumor necrosis factor-α, interleukin (IL)-6, IL-10, and C-reactive protein[12,13], which might also have exaggerated the bowel mucosal inflammation, leading to segmental strictures at places with defective mucosal barrier[14,15]. Control of systemic inflammation with multiple modalities, including antibiotics and antifungal regimens, finally led to resolution. Thus, although the exact etiology of the duodenal obstruction was uncertain, the imaging and clinical course was consistent with diverticulitis as there was no evidence of pancreatitis, neoplasm, or peptic ulcer disease on CT imaging or endoscopy.
In the second phase, the patient experienced another episode of ileus symptoms without radiological evidence of intestinal stricture, indicative of pseudo-obstruction. Although the patient was at risk of pseudo-obstruction due to her past medical history and medication, her renal function, electrolyte levels, and thyroid function remained normal during the entire disease course. The patient recovered from long-term pneumonia and severe septic shock, which can hamper intestinal motility and contribute to gastroesophageal reflux symptoms. Such reflux may directly induce reflux aspiration into the lungs and therefore stimulate acid-sensitive receptors in the bronchial tree, leading to her AHR symptoms in this disease phase[16,17]. In turn, AHR could increase the negative intrathoracic pleural pressure and diaphragmatic pressure, causing deterioration of regurgitation[18]. Thus, a vicious cycle formed involving GI dysmotility, gastroesophageal reflux, AHR, dysregulated inflammation and innervation, and ultimately impaired GI and lung function. Treatment to disrupt this vicious cycle starts with a proton pump inhibitor[19]. Enemase was used to relieve and prevent constipation, and therefore reduce intestinal pressure. Prokinetics helped to resume normal intestinal motility, and steroid nebulization helped to control the bronchial airway reactivity. With multidisciplinary treatment covering GI, pulmonary, nutritional, and infectious symptoms, the patient finally fully recovered.
There are several limitations in this case report. First, we only obtained local mucosal pathology around the stenotic area, as the exploratory surgery was postponed due to sudden septic shock at that time. Second, we failed to perform electrogastrography during the pseudo-obstruction phase, which would have provided better evaluation of the gut motility. However, the obstruction occurred 2 wk after she had resumed oral intake, and this was inconsistent with the commonly seen postobstructive gastroparesis.
This complicated case, involving idiopathic mechanical duodenal obstruction and pseudo-obstruction, emphasized the concurrent management of other organ dysfunction in addition to local intestinal disease, as well as evaluating diseases in a continuum. Thus, we suggest that clinicians should bear in mind that intestinal symptoms might also involve systemic abnormalities and should be managed comprehensively. Further investigations are required to understand the inter-relationship between intestinal dysfunction and general wellbeing.
We thank the patient and her family for their support.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Coskun A, Kozarek R, Taheri S S-Editor: Zhang L L-Editor: Webster JR P-Editor: Liu JH
| 1. | Millet I, Doyon FC, Pages E, Faget C, Zins M, Taourel P. CT of gastro-duodenal obstruction. Abdom Imaging. 2015;40:3265-3273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Khanna S, Gupta P, Khanna R, Dalela D. Distal Duodenal Obstruction: a Surgical Enigma. Indian J Surg. 2017;79:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Stanghellini V, Cogliandro RF, de Giorgio R, Barbara G, Salvioli B, Corinaldesi R. Chronic intestinal pseudo-obstruction: manifestations, natural history and management. Neurogastroenterol Motil. 2007;19:440-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Greenberg J, Greenberg J, Helmstetter N. Chronic intestinal pseudo-obstruction due to Strongyloides stercoralis. IDCases. 2018;13:e00425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Di Nardo G, Di Lorenzo C, Lauro A, Stanghellini V, Thapar N, Karunaratne TB, Volta U, De Giorgio R. Chronic intestinal pseudo-obstruction in children and adults: diagnosis and therapeutic options. Neurogastroenterol Motil. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 6. | Bernardi MP, Warrier S, Lynch AC, Heriot AG. Acute and chronic pseudo-obstruction: a current update. ANZ J Surg. 2015;85:709-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Transue DL, Hanna TN, Shekhani H, Rohatgi S, Khosa F, Johnson JO. Small bowel diverticulitis: an imaging review of an uncommon entity. Emerg Radiol. 2017;24:195-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Medsinge A, Remer EM, Winans CG. Duodenal diverticulitis followed by enterolith-associated small bowel obstruction. Emerg Radiol. 2012;19:261-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Khan K, Saeed S, Maria H, Sbeih M, Iqbal F, Ramcharan A, Donaldson B. Duodenal Diverticular Perforation after Small Bowel Obstruction: A Case Report. Case Rep Surg. 2018;2018:6197828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Ferreira-Aparicio FE, Gutiérrez-Vega R, Gálvez-Molina Y, Ontiveros-Nevares P, Athie-Gútierrez C, Montalvo-Javé EE. Diverticular disease of the small bowel. Case Rep Gastroenterol. 2012;6:668-676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Galli C, Basso D, Plebani M. CA 19-9: handle with care. Clin Chem Lab Med. 2013;51:1369-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | El Solh A, Pineda L, Bouquin P, Mankowski C. Determinants of short and long term functional recovery after hospitalization for community-acquired pneumonia in the elderly: role of inflammatory markers. BMC Geriatr. 2006;6:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Hedlund J. Community-acquired pneumonia requiring hospitalisation. Factors of importance for the short-and long term prognosis. Scand J Infect Dis Suppl. 1995;97:1-60. [PubMed] |
| 14. | Zhu P, Jiang H, Fu J, Chen W, Wang Z, Cui L. Cytokine levels in abdominal exudate predict prolonged postoperative ileus following surgery for colorectal carcinoma. Oncol Lett. 2013;6:835-839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Ramos GP, Papadakis KA. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin Proc. 2019;94:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 645] [Article Influence: 107.5] [Reference Citation Analysis (2)] |
| 16. | Stein MR. Possible mechanisms of influence of esophageal acid on airway hyperresponsiveness. Am J Med. 2003;115 Suppl 3A:55S-59S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Li Q, Kong L, Zhang S, Zhong Z, Liu X, Wang J, Kang J. A novel external esophageal perfusion model for reflux-associated respiratory symptoms. Pathobiology. 2010;77:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Bor S, Kitapcioglu G, Solak ZA, Ertilav M, Erdinc M. Prevalence of gastroesophageal reflux disease in patients with asthma and chronic obstructive pulmonary disease. J Gastroenterol Hepatol. 2010;25:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Naik RD, Vaezi MF. Extra-esophageal gastroesophageal reflux disease and asthma: understanding this interplay. Expert Rev Gastroenterol Hepatol. 2015;9:969-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |