Published online Sep 26, 2020. doi: 10.12998/wjcc.v8.i18.4234
Peer-review started: June 28, 2020
First decision: July 24, 2020
Revised: August 3, 2020
Accepted: August 22, 2020
Article in press: August 22, 2020
Published online: September 26, 2020
Processing time: 85 Days and 10.6 Hours
Encrusted cystitis (EC) is a chronic inflammation of the bladder associated with mucosal encrustations. Early diagnosis and optimal treatment are not well established. Here, we report a case of EC successfully treated with com-bination therapy.
A 27-year-old man presented with frequency, urgency, dysuria, gross hematuria and suprapubic pain for 2 mo. He was diagnosed with EC based on characteristic calcifications of the bladder wall (most of them were struvite), cystoscopy and histopathological examination. He was cured after combined therapy of elimination of encrustations, bladder instillation of hyaluronic acid and injection of botulinum-A neurotoxin into bladder submucosal tissue.
Bladder instillation of hyaluronic acid and injection of botulinum-A neurotoxin into the bladder submucosal tissue can be used for treatment of EC.
Core Tip: Encrusted cystitis is a chronic inflammation of the bladder associated with mucosal encrustations. Early diagnosis and optimal treatment are not well established. We report a case of encrusted cystitis successfully treated with combined therapies of elimination of encrustations, bladder instillation of hyaluronic acid and injection of botulinum-A neurotoxin into the bladder submucosal tissue. To our knowledge, we report the first description of encrusted cystitis treated with bladder instillation of hyaluronic acid and injection of botulinum-A neurotoxin into the bladder submucosal tissue.
- Citation: Fu JG, Xie KJ. Successful treatment of encrusted cystitis: A case report and review of literature. World J Clin Cases 2020; 8(18): 4234-4244
- URL: https://www.wjgnet.com/2307-8960/full/v8/i18/4234.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i18.4234
Encrusted cystitis (EC), first described in 1914, is characterized by localized ulcerative inflammation with deposits of magnesium ammonium phosphate and sodium hyaluronate on the bladder surface and on the walls of the ulcer. It is a rare form of chronic cystitis[1]. The etiology of this rare entity is presently controversial. Many factors are involved in the development of EC, including the presence of specific bacteria, most commonly Corynebacterium urealyticum (C. urealyticum)[2], a pre-existing urological procedure or a pre-existing lesion of the mucosa[1,3]. C. urealyticum grows slowly and is often missed in rapid urine cultures, especially if the bacterial load is low, and thus presents a potential diagnostic challenge[2]. The diagnosis should be based on sterile pyuria, alkaline urinary pH and characteristic calcifications of the bladder wall as observed by computed tomography and confirmed by prolonged culture, supplemented with DNA polymerase chain reaction sequencing if available[4,5]. Treatment of EC consists of three complementary elements: Treatment of infection; acidification of urine; and endoscopic removal of encrustations containing microorganisms[1,6,7]. Here, we report a case of EC successfully treated with combination therapy.
A 27-year-old man presented with frequency, urgency, dysuria, gross hematuria and suprapubic pain for 2 mo.
At 2 mo previous, the patient underwent ureteroscopic lithotripsy. At 3 d after the operation, he began to complain of frequency and urgency, which gradually aggravated. At 1 mo after lithotripsy, he experienced dysuria, gross hematuria, microlith expulsion, putrefaction expulsion and suprapubic pain. When he presented to our hospital, he needed micturition every 10 min, and his sleep quality was badly affected.
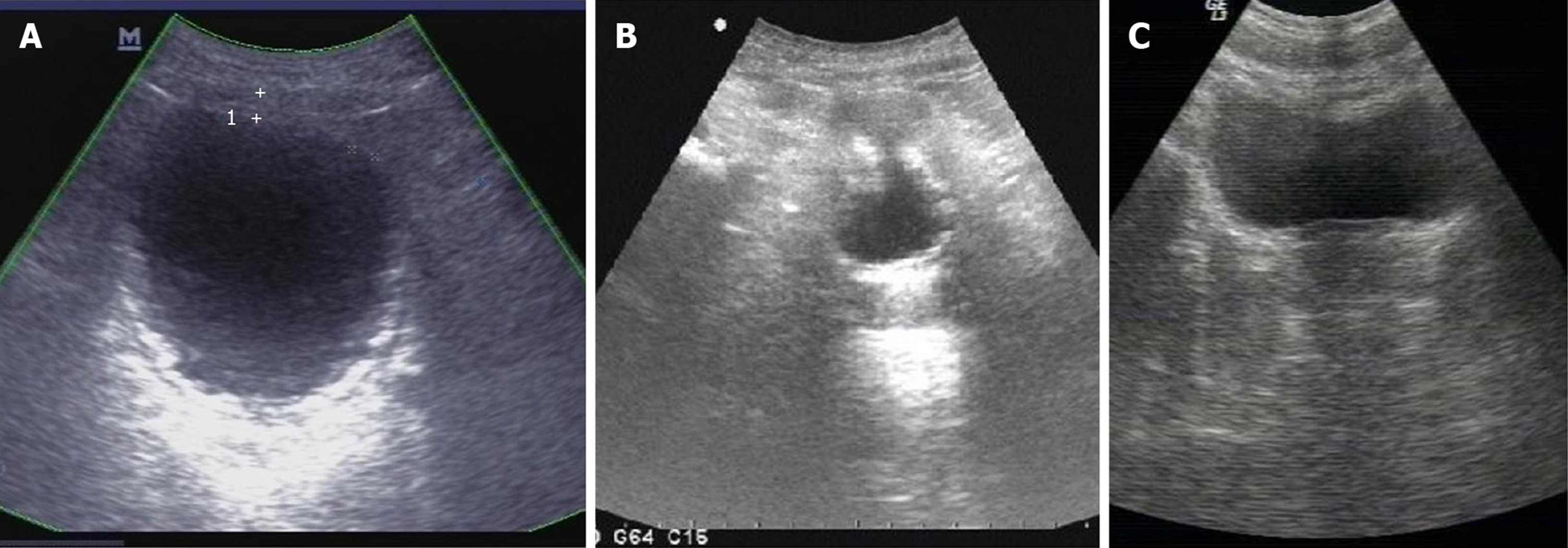
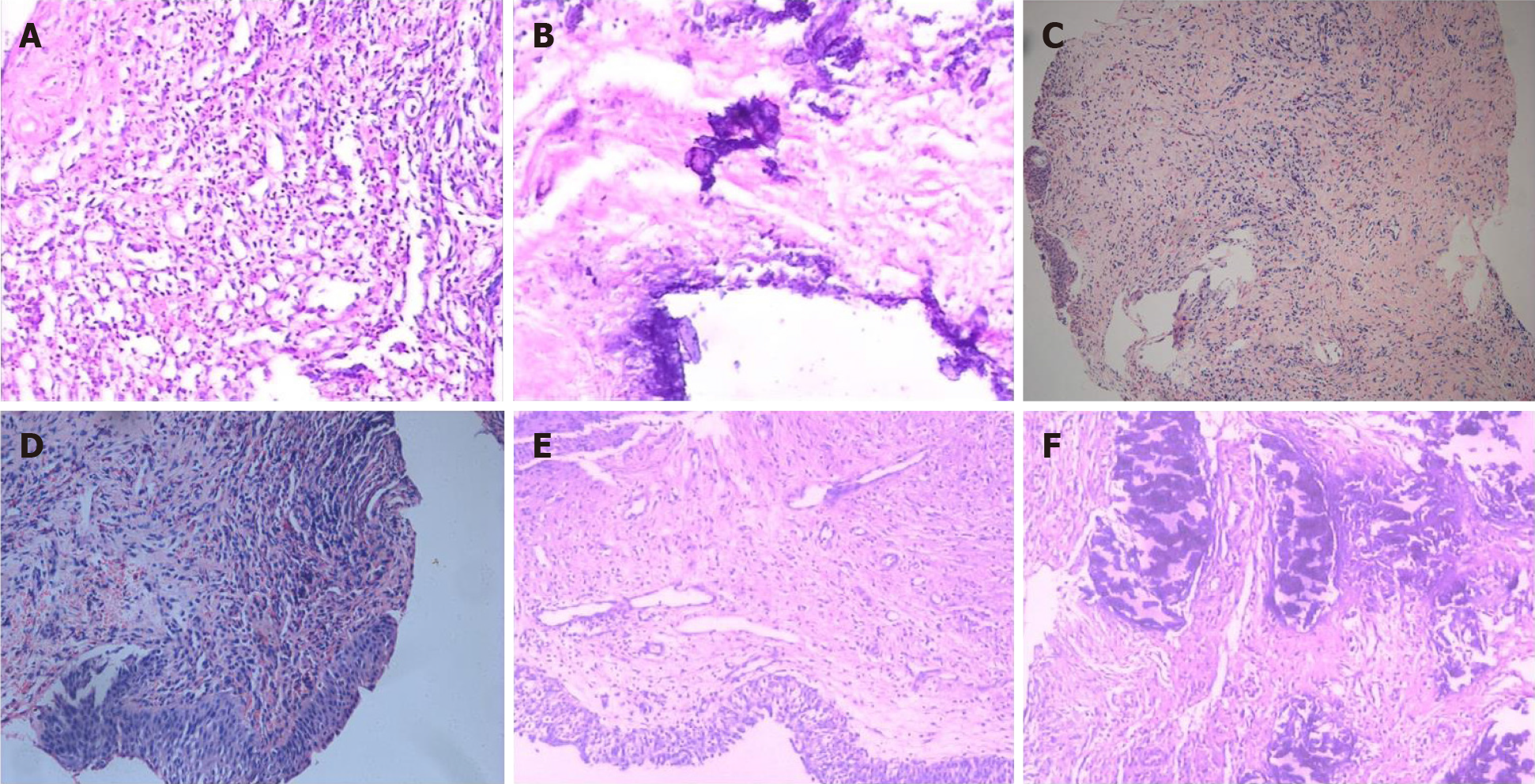
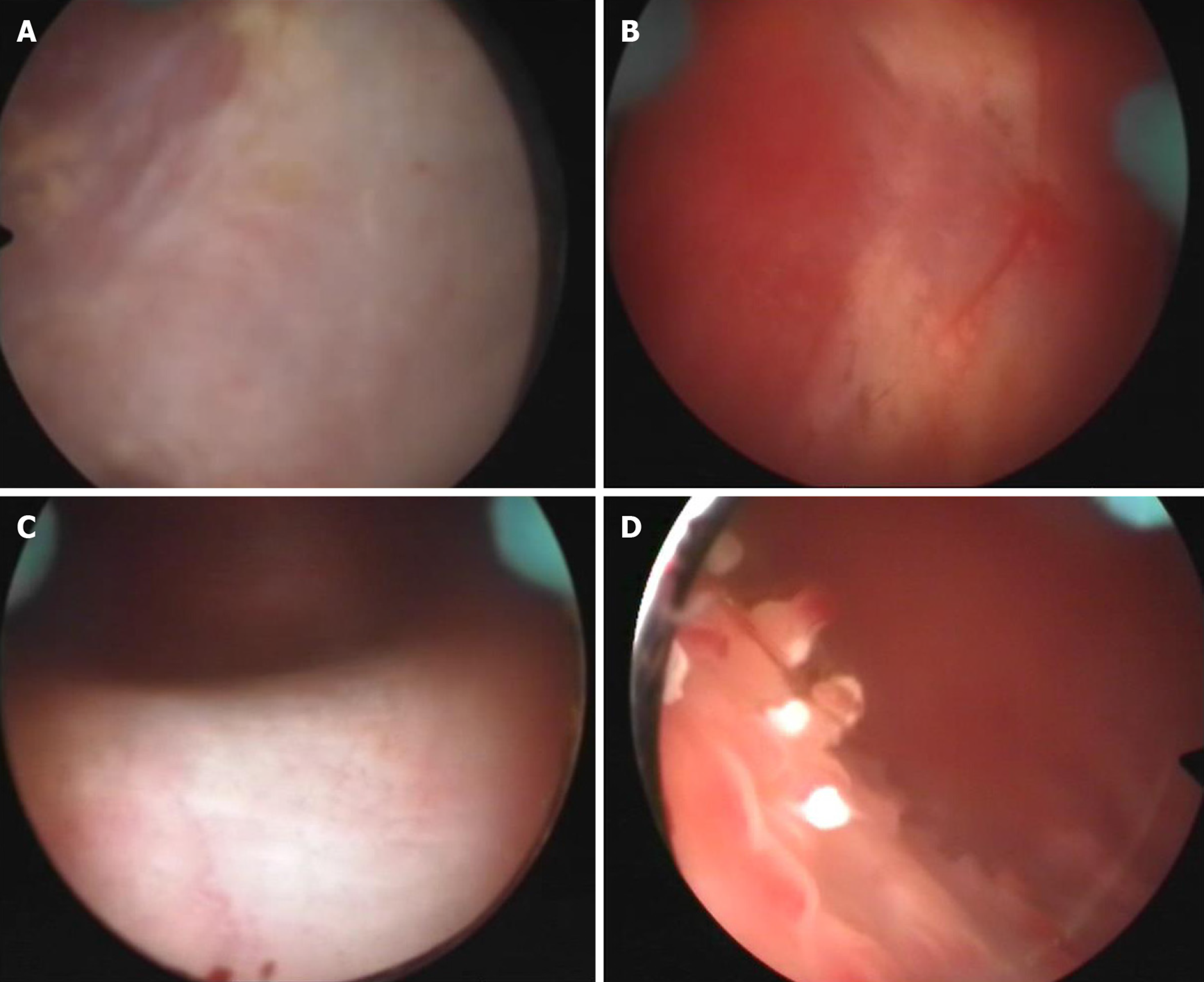
At disease onset, urinalysis showed pH 7.0, leukocyte count 500/mL, erythrocyte count 200/mL and nitrite (-). Urine bacterial and fungal culture, urine acid-fast bacillus, blood tuberculosis antibody and tuberculin tests were all negative. Chest radiography showed no abnormality. On day 22 after disease onset, ultrasonography showed that the bladder wall was thickened and rough with multiple strong echogenic spots attached to it (Figure 1A). The patient started on empirical antituberculosis treatment with combination of rifampin, isoniazid, ethambutol and pyrazinamide, which lasted for 11 d. However, the symptoms did not improve. On day 30 after disease onset, cystoscopy showed diffuse white necrotic tissue in the bladder wall and flocculent substance in the bladder cavity. Histopathological examination showed that there was inflammatory granulation tissue on the bladder wall with local necrosis and calcification (Figure 2A and B). On day 45 after disease onset, cystoscopy showed reduced bladder volume, swollen, congested and stiff bladder wall, multiple calcifications, and no typical miliary nodules. Pathological examination showed chronic inflammation and epithelial hyperplasia of the bladder mucosa and calcium deposition and proliferation of fibrous tissue. Infiltration of neutrophils, eosinophils and lymphocytes was observed in the lamina propria of the bladder mucosa (Figure 2C and D). Since the onset of EC, the patient had received a variety of antibiotics with poor efficacy (ceftriaxone sodium 2.0 g, bid, 3 d; levofloxacin 0.2 g, bid, 8 d; levofloxacin 0.2 g, bid, 3 d; amikacin 0.4 g, qd, 5 d; cefaclor 0.25 g, tid, 10 d; norfloxacin 0.4 g, bid, 10 d; latamoxef disodium 2.0 g, bid, 10 d).
The patient had no previous disease history.
Physical examination was normal except for suprapubic tenderness.
Urinalysis revealed hematuria, pyuria and pH 5.5. Conventional (not prolonged) urine culture was sterile. Urine acid-fast bacillus and blood tuberculosis antibody tests were negative.
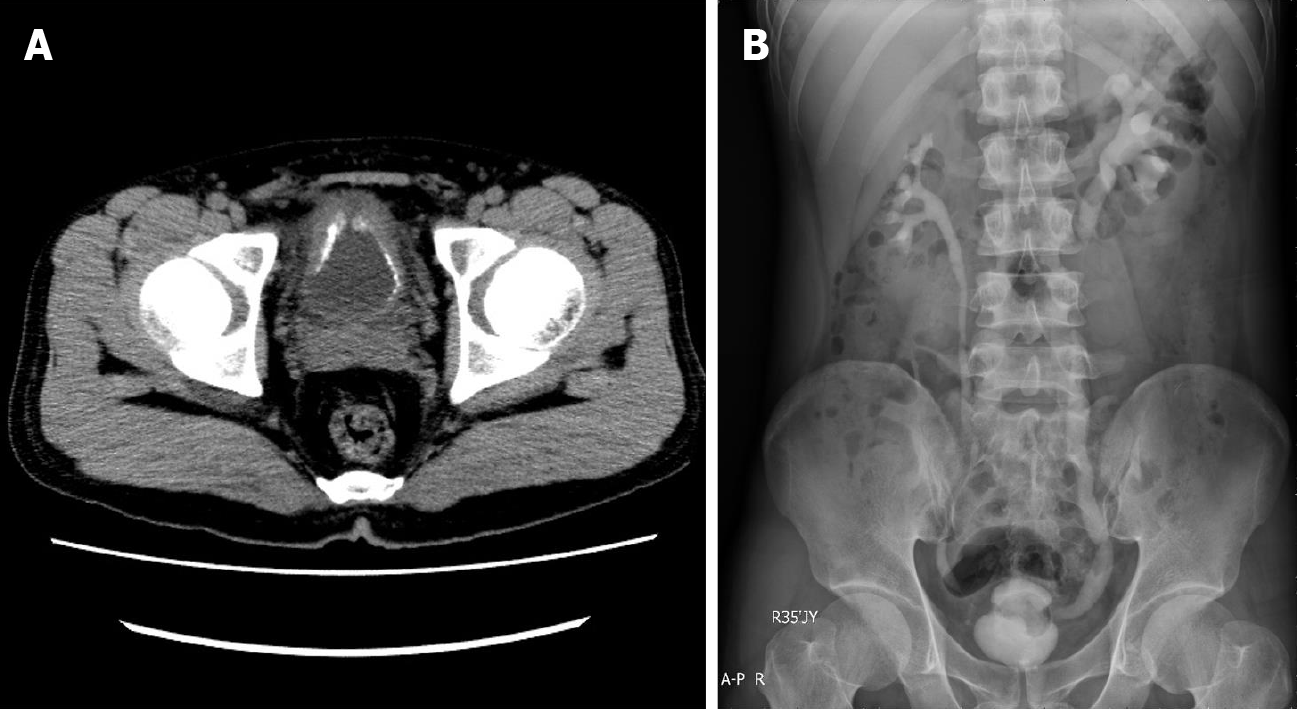
Ultrasonography performed on day 60 after disease onset showed that the bladder wall was thickened (the thickest part was about 9 mm) and rough with multiple strong echogenic spots attached to it (Figure 1B). Computed tomography showed that the bladder wall had extensive thickening and many calcifications (Figure 3A). Intravenous pyelography on day 60 after disease onset showed that the bladder was gourd-like with a rough margin, and hydronephrosis was found in the left upper urinary tract (Figure 3B).
Personal and family history was unremarkable.
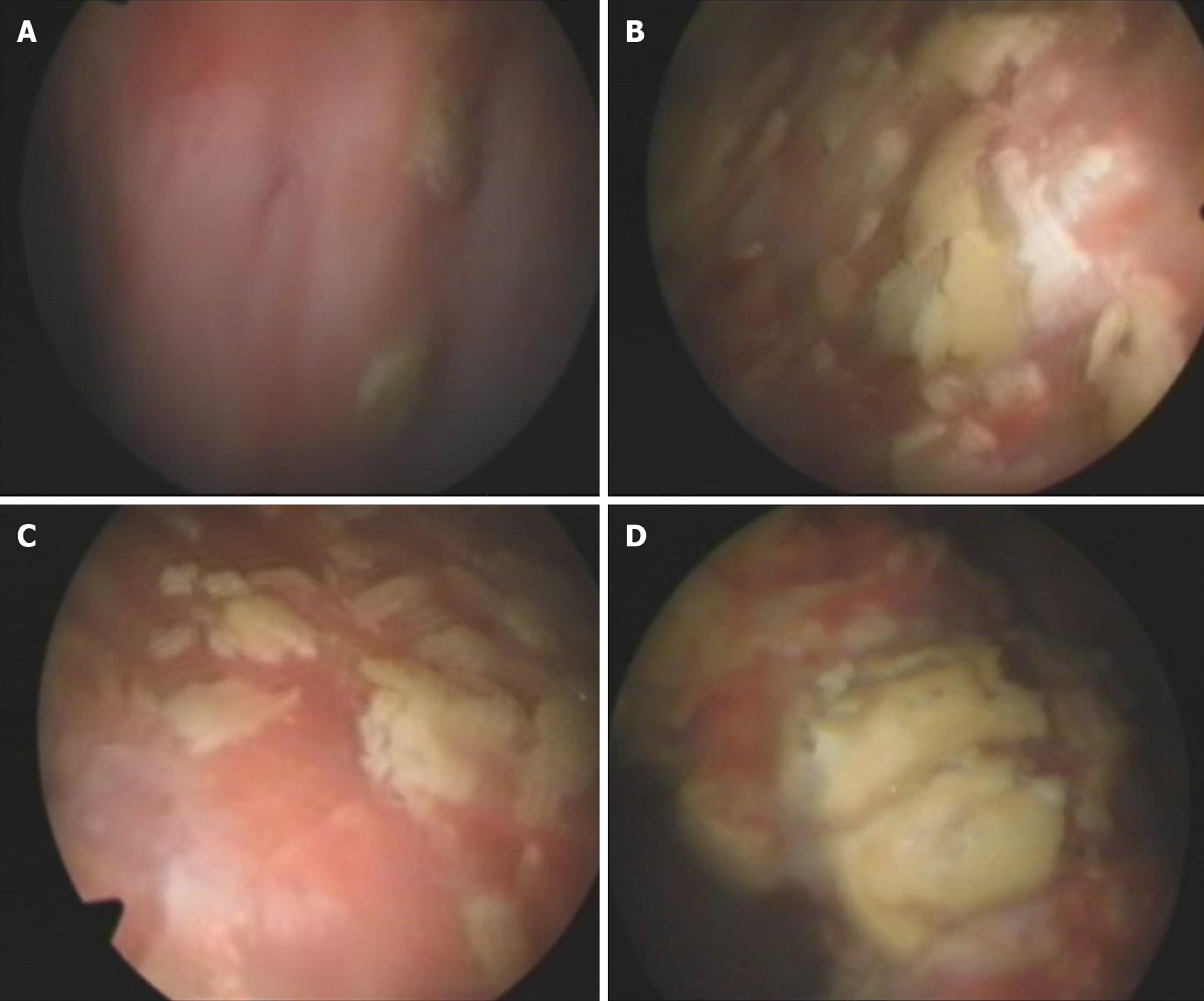
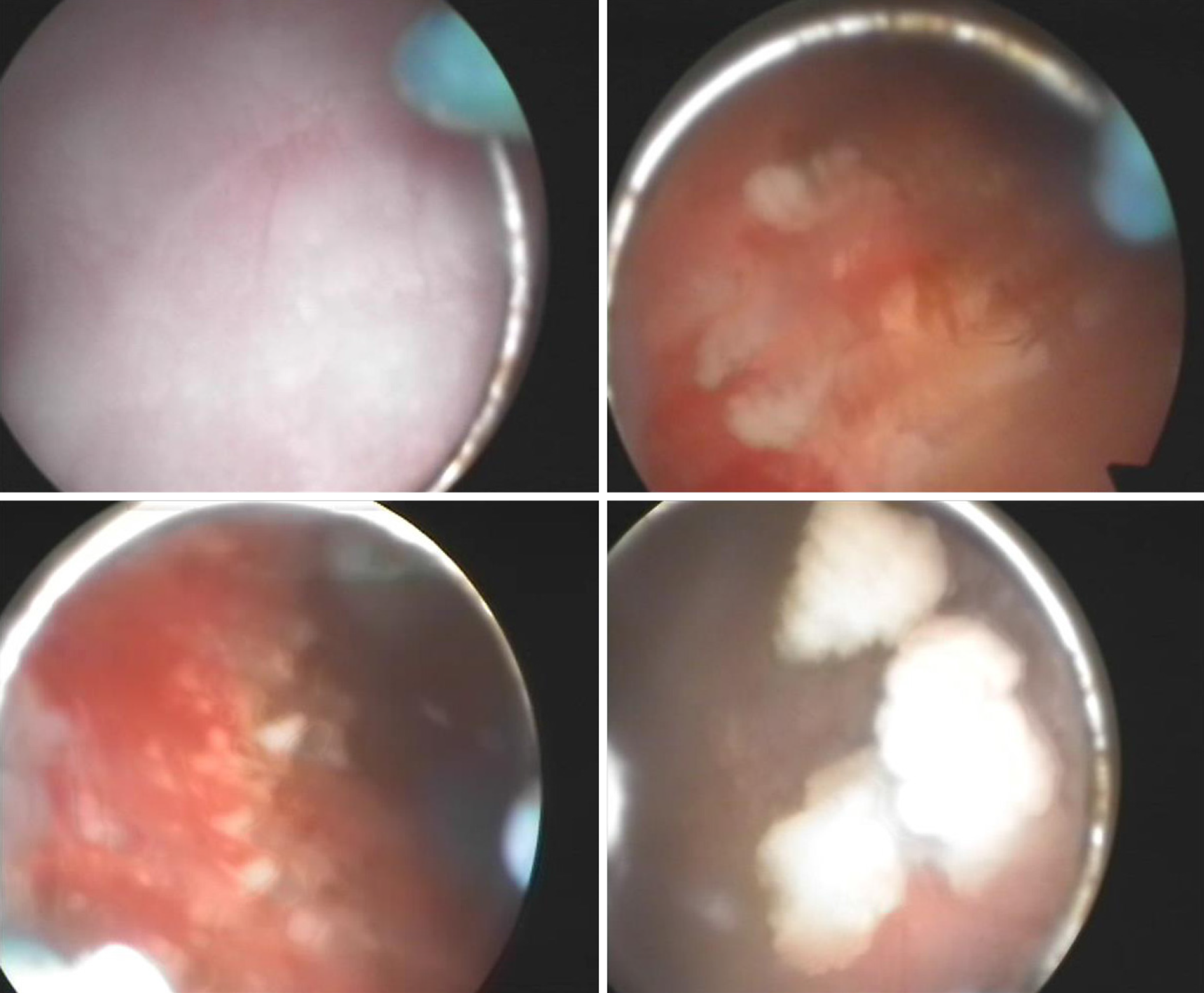
Cystoscopy on October 8, 2010 showed a marked inflammatory appearance of all parts of the bladder mucosa with ulcerations and whitish plaques corresponding to multiple ECs (Figure 4). Infrared spectrophotometry of the encrustations confirmed the presence of struvite. Histopathological examination showed that the bladder mucosa was edematous and necrotic with encrusted crystals and a polymorphonuclear infiltrate forming a thick conglomerate (Figure 2E and F).
EC.
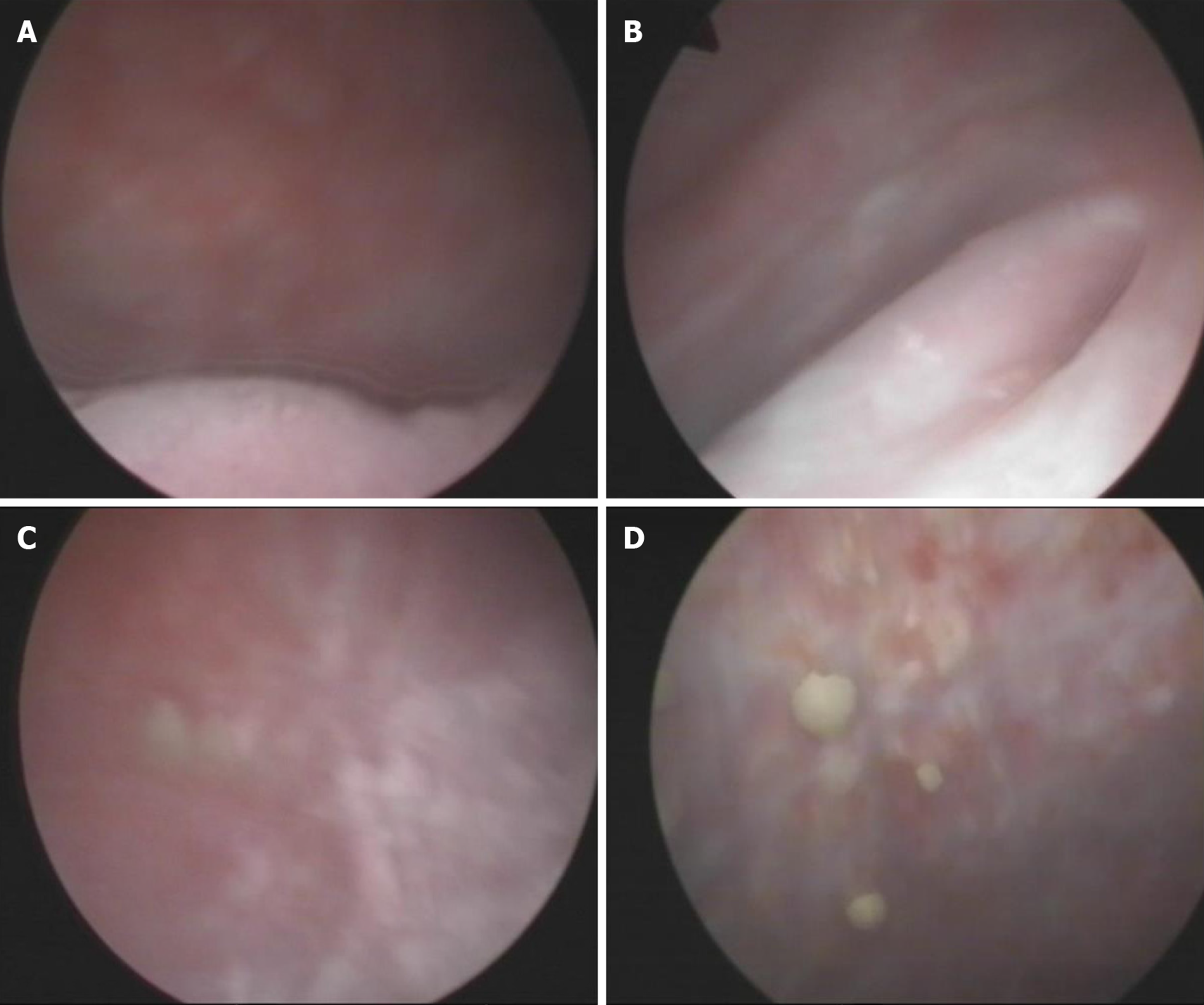
Endoscopic removal of encrustations was performed on October 8, 2010. The patient was prescribed sefamandole (2 g bid) for 3 d and solifenacin (5 mg qd) for 1 wk. However, the efficacy was poor. He was then prescribed hyaluronic acid (HA) bladder instillations (50 mL qw) for 1 mo. One month later, his symptoms were relieved slightly, and he needed to go for micturition every 30 min. Cystoscopy on November 10, 2010 showed that the apex and bilateral bladder wall mucosa still had hyperemia and edema with ulceration and multiple ECs (Figure 5). The second endoscopic removal of encrustations was performed. Ceftriaxone sodium (1g bid) was administered for 3 d, and solifenacin (5 mg qd) and HA (50 mL q2w) were administered for 1 mo. One month later, his symptoms were further relieved. Cystoscopy on December 8, 2010 showed that the ulcers on the top and bilateral bladder wall were healed, but there were still a few ECs on the mucosa (Figure 6). Because of the few ECs, a third endoscopic removal of encrustations was performed. Cefamandole (2 g bid) was administered for 5 d. Solifenacin (5 mg qd) and HA (50 mL q4w) were administered for 2 mo. Two months later, his dysuria, gross hematuria and suprapubic pain symptoms were relieved. He needed to go for micturition every 70 min, voiding 100-150 mL each time. However, he still complained of obvious frequency and urgency. Cystoscopy on February 16, 2011 showed no mucosal ulcers but still a few mucosal encrustations on the top and bilateral bladder wall (Figure 7). A fourth endoscopic removal of encrustations was performed. Cefamandole (2 g bid) was administered for 5 d. Because he still complained of frequency and urgency, he received botulinum-A neurotoxin (BoNT/A) therapy. BoNT/A was injected into the submucosal tissue of bladder through “COOK” bladder injection needle under cystoscopy. The total dose of BoNT/A was 200 U, which was randomly injected into the bladder wall at 40 points.
At 3 mo after the fourth operation, the symptoms of frequency, urgency, urodynia, gross hematuria and suprapubic pain disappeared. He needed to go for micturition six to seven times during the daytime and once during the night with a volume of 300-400 mL each time. Urinalysis was normal. Ultrasonography showed that there were still a few strong echogenic spots attached to the bladder wall. During follow-up on February 8, 2012, the patient reported no recurrence of frequency, urgency, urodynia, gross hematuria and suprapubic pain. He needed to go for micturition four to seven times during the daytime, with a volume of 300-500 mL each time, and zero times at night. Ultrasonography showed that the bladder wall was smooth without hyperechoic spots (Figure 1C). During the latest telephone follow-up on March 30, 2020, the patient reported no recurrence of lower urinary tract symptoms.
EC is a chronic inflammatory condition in the bladder characterized by mucosal inflammation with encrustations and ulcers. Most of the literature related to EC is case reports with about 160 cases being reported. EC is a nosocomial infectious disease. The risk factors for EC are: Corynebacterium parasiticum infection (such as long-term hospitalization); invasive urinary system procedures (such as open or endoscopic surgery, indwelling urinary catheter, nephrostomy tube, ureteral stent, etc.); previous urinary tract lesions (such as urinary tract tumor, infection, bladder perfusion chemotherapy, etc.); and immunosuppression (such as renal transplantation, long-term use of broad spectrum antibiotics, chronic diseases, drug abuse, AIDS, etc.)[1,3-19]. The interval from the operation to the onset of disease can be several days to 3 years[1,3,4,6,7,13,15,16,18,19].
It has been reported that there are more than 40 types of urease-producing bacteria that can cause EC, and C. urealyticum is the most common[1,3,7-9,11,16,20-22]. C. urealyticum is a common skin colonizer of hospitalized elderly individuals who are receiving broad spectrum antibiotics. It is an opportunistic pathogen and has strong urease activity and obvious predilection for indwelling urinary tract catheters and urothelial cells (especially when the mucosa has inflammation or trauma). C. urealyticum can infect the urinary tract through indwelling catheters, cystostomy tubes or nephrostomy tubes[2,23]. Due to its strong urease activity, C. urealyticum can decompose urea to produce ammonia after infection of the urinary tract, which results in alkaline urine. Alkaline urine causes magnesium ammonium phosphate, calcium phosphate and other salt substances in the urine to become supersaturated and to precipitate on the infected mucosa and form mucosal encrustations[1,2]. In addition, urease can also mediate the cytotoxic effect of ammonia on the urothelium[2,11]. However, Del Prete et al[11] found that bladder epithelial cells differentiate into osteoclasts after C. urealyticum infection, triggering the synthesis of typical bone-like protein. They suggested that the formation of mucosal calcification is a process of tissue injury and scar repair regulated by calmodulin. Jelic et al[24] proposed that the formation of mucosal calcification may be related to nanobacterial particles, which can promote the precipitation of free calcium and phosphate in urine under physiological conditions.
EC patients have no specific symptoms. The common symptoms of EC are frequency urgency, urodynia, dysuria, gross hematuria, suprapubic pain, elimination of gritty material or struvite stones with the urine and an ammonia-like odor of the urine[1,3-7,12-16,18,19]. A quarter to half of patients may have fever[1]. There are red blood cells, white blood cells, magnesium ammonium phosphate and calcium phosphate fragments in the urine of EC patients, and the urine pH of most patients is > 7[1,3,4,13].
C. urealyticum is a slow-growing, nonhemolytic, Gram-positive, nonsporulating, lipophilic and aerobic opportunistic pathogenic bacterium. It has the typical characteristics of Corynebacterium. The biggest difference between C. urealyticum and other Corynebacterium species is that it has strong urease activity and cannot acidify sugar substances[1,2]. The diagnosis of C. urealyticum requires culture for 48-72 h at 37°C on media enriched with 5% CO2 or sheep blood agar[1,2]. C. urealyticum can be cultured in urine, scraped calcified foci and blood of patients with bacteremia. The positive standard of culture is a bacterial count of ≥ 105 CFU/mL[1]. For patients with negative culture of C. urealyticum, polymerase chain reaction can also be used to detect the DNA of C. urealyticum[2,20].
Sonography, radiography, computed tomography and magnetic resonance imaging are useful diagnostic tools as they can distinguish calcification from bladder mucosa or the bladder cavity[1,4,6,7,11-13,15,19]. Cystoscopy shows erosion of bladder mucosa with calcification. Calcification can be as small as cashmere or as large as stone and embedded in the bladder wall. With the progression of the disease, bladder wall necrosis and calcification can lead to bilateral hydronephrosis and then to renal damage[1,13,17]. Necrosis and calcification of the bladder wall can also lead to decreased bladder compliance and eventually to bladder contracture and complete loss of bladder function[1,3,11]. The main components of calcification are magnesium ammonium phosphate (> 50%), some carbonaceous apatite (15%-35%) and a small amount of other components (such as protein)[1,4,19].
The final diagnosis must account for histological examination, which shows three differentiated layers: A superficial layer with necrosis and microcalcifications; an intermediate layer with inflammatory changes; and a third layer corresponding to the bladder muscularis[1,7,11]. Diagnosis of EC can be confirmed by combining the history, symptoms, signs and the above auxiliary examinations. However, sometimes the patients do not have alkaline urine and C. urealyticum is not detected in urine culture[25]. Therefore, early diagnosis is always delayed. The differential diagnoses of bladder mucosal encrustation includes tuberculosis, schistosomiasis, malakoplakia, necrotic urothelial carcinoma and urea-splitting bacterial infections[1,26]. Bladder mucosal encrustation can also occur after intravesical instillations of formaldehyde, cyclophosphamide or mitomycin C[1,26]. In our case, the urine pH was not alkaline and urine culture was sterile. He was not diagnosed with EC until comprehensive analysis of risk factors, symptoms, imaging findings, cystoscopic findings, infrared spectrophotometry of the encrustations and histopathological data.
Treatment of EC consists of three complementary elements: antimicrobial therapy; acidification of urine and chemolysis; and elimination of encrustations that contain microorganisms. Resistance to most antibiotics is a significant characteristic of C. urealyticum[1,2,23]. Therefore, the application of antibiotics should be based on the results of urine culture and drug sensitivity tests. All Corynebacterium are stably sensitive to glycopeptides, especially vancomycin and teicoplanin[1,2,23]. The antibacterial effect of glycopeptides has been proven in experimental and clinical studies, and its efficacy does not depend on urine pH[1]. The course of treatment of glycopeptides is about 14 d to 2 mo[2]. In general, C. urealyticum is highly resistant to β-lactams and amino-glycosides, and its sensitivity to fluoroquinolones, macrolides, keto esters, rifampicin and tetracycline is unstable[2,23,27].
Urine acidification therapy includes oral administration and bladder perfusion. The most commonly used oral drug is acetohydroxamic acid[1,28], which has a chemical structure similar to that of urea. It can competitively inhibit the activity of urease, reduce the decomposition of urea and reduce the concentration of urine ammonia and pH[28]. Its urinary excretion rate ranges from 35% to 65%, and the recommended dose is 15 mg/kg/d. It can effectively reduce the urine pH and ammonia concentration[1,28]. The most widely used acid solution is Suby’s G solution (citric acid 32.3 g, sodium carbonate 4.4 g, magnesium oxide 3.8 g, distilled water 1000 mL) and Thomas C24 solution (sodium gluconate 27 g, citric acid 27 g, malic acid 27 g, distilled water 1000 mL)[1]. Because of their ability to form calcium citrate complexes, they can acidify urine and kill bacteria[1]. Antimicrobial therapy is usually combined with urine acidification therapy[1,2]. The combined therapy of anti-infection and urine acidification should last until the lesion mucosa is completely recovered and repeated urine culture is negative. The general course is 4 wk[1].
As the calcification contains a large number of bacteria and limits the role of antibiotics, local treatment with cystoscopic or surgical removal of the mucosal encrustations is required (especially when the calcification is thick and cannot be dissolved by urine acidification therapy)[29]. Because the calcification is attached to the mucosa, it may be difficult to completely scrape out at one time. So, repeated operations are required and may lead to massive bleeding of the bladder mucosa[1]. Because of the delayed diagnosis and drug resistance of C. urealyticum, treatment of EC is difficult. If the diagnosis can be established as soon as possible and the correct treatment is given, EC can be completely cured. On the contrary, delayed diagnosis and treatment may lead to bladder contracture and renal insufficiency, which may eventually lead to bladder resection.
In our case, because bacterial culture was negative and urine pH was not alkaline, we did not use glycopeptides and urine acidification therapy. Because of recurrence of calcification on the bladder wall, we performed transurethral removal of mucosal encrustations four times.
Severe urinary tract infections (such as EC) can induce a primary defect in the glycosaminoglycan layer of the mucosa, which result in bladder mucosal edema and erosion. The literature reports bladder instillation of HA is effective for mucosal rehabilitation in patients with recurrent urinary tract infections[30,31]. HA is a major mucopolysaccharide found widely in the connective, epithelial and neural tissues. HA in the urothelium constitutes a protective barrier[32]. In our case, bladder mucosal erosion was also significantly improved after bladder instillation of HA. However, after calcification of the bladder mucosa was removed and the mucosa was repaired, the patient still complained of obvious urgency and frequency. It is known that the urothelium plays a significant role as a pressure-sensing organ with a complex series of transduction mechanisms that modulate the micturition reflex and afferent feedback from the lower urinary tract[33]. BoNT/A can inhibit the release of acetylcholine, ATP and substance P and reduce axonal expression of the capsaicin and purinergic receptors as a primary peripheral effect. This may be followed by central desensitization through a decrease in central uptake of substance P and neurotrophic factors. The summation of these effects is a profound and long-lasting inhibition of the afferent and efferent mechanisms that are thought to be the pathophysiological basis for urgency and frequency[34]. In our case, the urgency and frequency were both relieved after injection of BoNT/A into the bladder submucosal tissue.
In addition to antibiotic therapy, urinary acidification and elimination of mucosal encrustations, there are other therapeutic methods for EC, such as bladder instillation of HA and injection of BoNT/A into the bladder submucosal tissue.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Brisinda G, Okajima H S-Editor: Yan JP L-Editor: Filipodia P-Editor: Liu JH
| 1. | Meria P, Desgrippes A, Arfi C, Le Duc A. Encrusted cystitis and pyelitis. J Urol. 1998;160:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Soriano F, Tauch A. Microbiological and clinical features of Corynebacterium urealyticum: urinary tract stones and genomics as the Rosetta Stone. Clin Microbiol Infect. 2008;14:632-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Ozkan TA, Yalcin MS, Dillioglugil O, Cevik I. Encrusted cystitis caused by corynebacterium urealyticum: a case report with novel treatment strategy of intravesical dimethyl sulfoxide. Int Braz J Urol. 2018;44:1252-1255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Zheng J, Wang G, He W, Jiang N, Jiang H. Imaging characteristics of alkaline-encrusted cystitis. Urol Int. 2010;85:364-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Favre G, García-Marchiñena P, Bergero M, Dourado L, González MI, Tejerizo J, Damia O. [Diagnosis and treatment of the encrusted cystitis]. Actas Urol Esp. 2010;34:477-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Johnson MH, Strope SA. Encrusted cystitis. Urology. 2012;79:e31-e32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Curry CR, Saluja K, Das S, Thakral B, Dangle P, Keeler TC, Watkin WG. Encrusted Cystitis Secondary to Corynebacterium glucuronolyticum in a 57-Year-Old Man Without Predisposing Factors. Lab Med. 2015;46:136-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Pierciaccante A, Pompeo ME, Fabi F, Venditti M. Successful treatment of Corynebacterium urealyticum encrusted cystitis: a case report and literature review. Infez Med. 2007;15:56-58. [PubMed] |
| 9. | El Sayegh H, Elouardani M, Iken A, Nouini Y, Lachkar A, Benslimane L, Belahnech Z, Faik M. [Encrusted cystitis due to Corynebacterium urealyticum]. Rev Med Interne. 2008;29:328-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Namsupak J, Headley T, Morabito RA, Zaslau S, Kandzari SJ. Encrusted cystitis managed with multimodal therapy. Can J Urol. 2008;15:3917-3919. [PubMed] |
| 11. | Del Prete D, Polverino B, Ceol M, Vianello D, Mezzabotta F, Tiralongo E, Iafrate M, De Canale E, Mengoli C, Valente M, Anglani F, D'Angelo A. Encrusted cystitis by Corynebacterium urealyticum: a case report with novel insights into bladder lesions. Nephrol Dial Transplant. 2008;23:2685-2687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Anagnostou N, Siddins M, Gordon DL. Encrusted cystitis and pyelitis. Intern Med J. 2012;42:596-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Tanaka T, Yamashita S, Mitsuzuka K, Yamada S, Kaiho Y, Nakagawa H, Arai Y. Encrusted cystitis causing postrenal failure. J Infect Chemother. 2013;19:1193-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | González Guerrero JL, Mohedano Molano JM, García Martín L, Fernández Martín MA. [Conservative treatment in a case of encrusted cystitis]. Rev Esp Geriatr Gerontol. 2016;51:296-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Perrucci E, Lancellotta V, Benedetto MD, Palumbo I, Matrone F, Chiodi M, Lombi R, Marcantonini M, Mariucci C, Aristei C. Encrusted cystitis after definitive radiotherapy for cervical cancer: a case report. J Contemp Brachytherapy. 2016;8:541-543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Lansalot-Matras P, Dubourdieu B, Bosc R, Crenn G, Berthod N, Loriette M, Marchou B. [Encrusted cystitis by Corynebacterium urealyticum]. Med Mal Infect. 2017;47:167-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Duffy M, Gallagher A. Encrusted Cystitis with Suspected Ureteral Obstruction Following Cystoscopic-Guided Laser Ablation of Ectopic Ureters in a Dog. J Am Anim Hosp Assoc. 2018;54:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Bonacina M, Albano D, Durmo R, Barbera F, Bertagna F, Giubbini R. Encrusted cystitis detected by 18F-FDG PET/CT. Rev Esp Med Nucl Imagen Mol. 2019;38:250-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Pagnoux C, Bérezné A, Damade R, Paillot J, Aouizerate J, Le Guern V, Salmon D, Guillevin L. Encrusting cystitis due to Corynebacterium urealyticum in a patient with ANCA-associated vasculitis: case report and review of the literature. Semin Arthritis Rheum. 2011;41:297-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Guimarães LC, Soares SC, Albersmeier A, Blom J, Jaenicke S, Azevedo V, Soriano F, Tauch A, Trost E. Complete Genome Sequence of Corynebacterium urealyticum Strain DSM 7111, Isolated from a 9-Year-Old Patient with Alkaline-Encrusted Cystitis. Genome Announc. 2013;1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Khallouk A, Wallerand H, Kleinclauss F, Bittard H, Bernardini S. [Conservative management of Corynebacterium urealyticum encrusted cystitis]. Prog Urol. 2006;16:496-498. [PubMed] |
| 22. | Penta M, Fioriti D, Chinazzi A, Pietropaolo V, Conte MP, Schippa S, Tecca M, Gentile V, De Dominicis C, Chiarini F. Encrusted cystitis in an immunocompromised patient: possible coinfection by Corynebacterium urealyticum and E. coli. Int J Immunopathol Pharmacol. 2006;19:241-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Tauch A, Trost E, Tilker A, Ludewig U, Schneiker S, Goesmann A, Arnold W, Bekel T, Brinkrolf K, Brune I, Götker S, Kalinowski J, Kamp PB, Lobo FP, Viehoever P, Weisshaar B, Soriano F, Dröge M, Pühler A. The lifestyle of Corynebacterium urealyticum derived from its complete genome sequence established by pyrosequencing. J Biotechnol. 2008;136:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Jelic TM, Roque R, Yasar U, Tomchin SB, Serrato JM, Deem SG, Tierney JP, Chang HH. Calcifying nanoparticles associated encrusted urinary bladder cystitis. Int J Nanomedicine. 2008;3:385-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Huguet Pérez J, Salvador Bayarri J, Vicente Rodríguez J. [Encrusted cystitis. Is it always alkaline?]. Arch Esp Urol. 1999;52:157-164. [PubMed] |
| 26. | Pollack HM, Banner MP, Martinez LO, Hodson CJ. Diagnostic considerations in urinary bladder wall calcification. AJR Am J Roentgenol. 1981;136:791-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | López-Medrano F, García-Bravo M, Morales JM, Andrés A, San Juan R, Lizasoain M, Aguado JM. Urinary tract infection due to Corynebacterium urealyticum in kidney transplant recipients: an underdiagnosed etiology for obstructive uropathy and graft dysfunction-results of a prospective cohort study. Clin Infect Dis. 2008;46:825-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Kosikowska P, Berlicki Å. Urease inhibitors as potential drugs for gastric and urinary tract infections: a patent review. Expert Opin Ther Pat. 2011;21:945-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 29. | Soriano F, Rodriguez-Tudela JL, Castilla C, Avilés P. Treatment of encrusted cystitis caused by Corynebacterium group D2 with norfloxacin, ciprofloxacin, and teicoplanin in an experimental model in rats. Antimicrob Agents Chemother. 1991;35:2587-2590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | De Vita D, Antell H, Giordano S. Effectiveness of intravesical hyaluronic acid with or without chondroitin sulfate for recurrent bacterial cystitis in adult women: a meta-analysis. Int Urogynecol J. 2013;24:545-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Constantinides C, Manousakas T, Nikolopoulos P, Stanitsas A, Haritopoulos K, Giannopoulos A. Prevention of recurrent bacterial cystitis by intravesical administration of hyaluronic acid: a pilot study. BJU Int. 2004;93:1262-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Miodosky M, Abdul-Hai A, Tsirigotis P, Or R, Bitan M, Resnick IB, Gesundheit B, Zilberman I, Ioffe L, Leubovic A, Slavin S, Shapira MY. Treatment of post-hematopoietic stem cell transplantation hemorrhagic cystitis with intravesicular sodium hyaluronate. Bone Marrow Transplant. 2006;38:507-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Kumar V, Cross RL, Chess-Williams R, Chapple CR. Recent advances in basic science for overactive bladder. Curr Opin Urol. 2005;15:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Apostolidis A, Dasgupta P, Fowler CJ. Proposed mechanism for the efficacy of injected botulinum toxin in the treatment of human detrusor overactivity. Eur Urol. 2006;49:644-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 262] [Article Influence: 13.8] [Reference Citation Analysis (0)] |