Published online Sep 26, 2020. doi: 10.12998/wjcc.v8.i18.4151
Peer-review started: February 27, 2020
First decision: April 8, 2020
Revised: May 16, 2020
Accepted: August 25, 2020
Article in press: August 25, 2020
Published online: September 26, 2020
Processing time: 207 Days and 22.2 Hours
Celiac disease (CD) is a systemic, chronic immune-mediated disease triggered by gluten ingestion in genetically-susceptible individuals, with a prevalence of 1% worldwide. Sjogren's syndrome (SS) is also a systemic autoimmune disease, mainly characterized by ocular and oral sicca symptoms and signs. Sharing a common genetic background, CD and SS are known associated autoimmune diseases, but currently available guidelines are not reporting it.
We report the case of a 39-year-old woman, who was in the care of her rheumatologist for 2 years with SS. On routine follow-up she was found to have iron deficiency, without anemia. She had no gastrointestinal complaints and denied any obvious source of blood loss. IgA tissue transglutaminase antibodies were positive and endoscopy with duodenal biopsies revealed crypt hyperplasia and villous atrophy. A diagnosis of CD was set and gluten-free diet was recommended.
We present a review of existing data in the literature regarding the association of the two diseases, summarizing prevalence studies of CD in SS patients and the other way around. Screening recommendations and future research perspectives are also discussed, highlighting clinically relevant unanswered questions with respect to the association of CD with SS.
Core Tip: Celiac disease (CD) and Sjögren's syndrome (SS) are associated autoimmune diseases, sharing a common genetic background. Although there is some evidence supporting the association of the two diseases, currently available guidelines are not sufficiently reporting it. We herein report a case of a patient with SS who was diagnosed with CD and summarize existing data in the literature regarding the association of the two diseases and discuss future research topics that remain to be answered with regard to this association. To the best of our knowledge this is the first literature review regarding these two pathologies concomitance.
- Citation: Balaban DV, Mihai A, Dima A, Popp A, Jinga M, Jurcut C. Celiac disease and Sjögren’s syndrome: A case report and review of literature. World J Clin Cases 2020; 8(18): 4151-4161
- URL: https://www.wjgnet.com/2307-8960/full/v8/i18/4151.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i18.4151
Within the circle of related-autoimmune diseases, celiac disease (CD) and Sjögren’s syndrome (SS) come together with type 1 diabetes mellitus, autoimmune thyroid disease, and primary biliary cholangitis[1]. In fact, a major stake when diagnosing one of the above autoimmune diseases is screening for the other ones, especially in the presence of a suggestive clinical scenario.
SS is an autoimmune disease mainly characterized by ocular and oral sicca symptoms and signs, positivity for certain autoantibodies (anti-SS-A/Ro and anti-SS-B/La), and suggestive minor salivary gland biopsies. All these items were integrated in classifications criteria, the most recent being the 2016 American College of Rheumatology/European League Against Rheumatism SS’s Classification Criteria[2]. In the same paper, the importance of extraglandular manifestations was highlighted by increasing the target population to which the classification criteria can be applied.
Meanwhile, CD is a gluten-dependent, autoimmune enteropathy, affecting 1% of the population with a continuously rising incidence, mainly because of an increase of correct positive diagnosis[3]. In the last few decades the paradigm of CD’s clinical spectrum dramatically changed from the classical forms with diarrhea to atypical forms. Moreover, the importance of extraintestinal manifestations and of clinical associated conditions has been increasingly recognized[4-7]; hence, CD is now regarded as a chronic, systemic disease triggered by the ingestion of gluten in genetically susceptible individuals. Since the 1960s[8], several publications have been addressing the co-association between SS and CD – first with case reports[9-11], then small series and cohorts.
However, awareness among internal medicine specialists, rheumatologists, and gastroenterologists remains unsatisfactory and currently available guidelines are not reporting this association[2,12].
We herein present the case of a patient with SS who was diagnosed with CD, and summarize the existing data about the association of the two autoimmune disorders, with a focus on clinical application. To the best of our knowledge, this is the first literature review analyzing the existing data related to these two pathologies concomitance.
We report the case of a 39-year-old woman who was in the care of her rheumatologist after being diagnosed with primary SS. Her illness began two years before with bilateral knee arthralgia, debilitating fatigue, xerophthalmia and xerostomia. With positive antinuclear antibodies, anti-Ro [> 200 UI/mL (normal value < 15)] and anti-La [57.5 UI/mL (normal value < 15)] antibodies, positive Shirmer's test, positive Rose Bengal coloration, and salivary gland biopsy demonstrating focal lymphocytic sialadenitis with a focus score of 4, she was diagnosed with SS. She was prescribed hydroxychloroquine and topical treatment, with improvement of sicca and articular symptoms.
Two years later she came into the Rheumatology Department for a routine follow-up visit. She reported good symptom control with therapy without adverse reactions, and her physical examination was unremarkable.
However, routine blood work however revealed lymphopenia-1100/mL (normal range 1170-3170), iron deficiency without anemia [serum iron–23 ug/dL (70-180), ferritin–18 μg/L (20-250), hemoglobin–12.9 g/dL (12-16)] and low vitamin D levels–10 ng/mL (20-160). Except regular menses, the patient's history did not reveal any obvious sources of blood loss. Upon further history taking, she did not report any digestive symptoms.
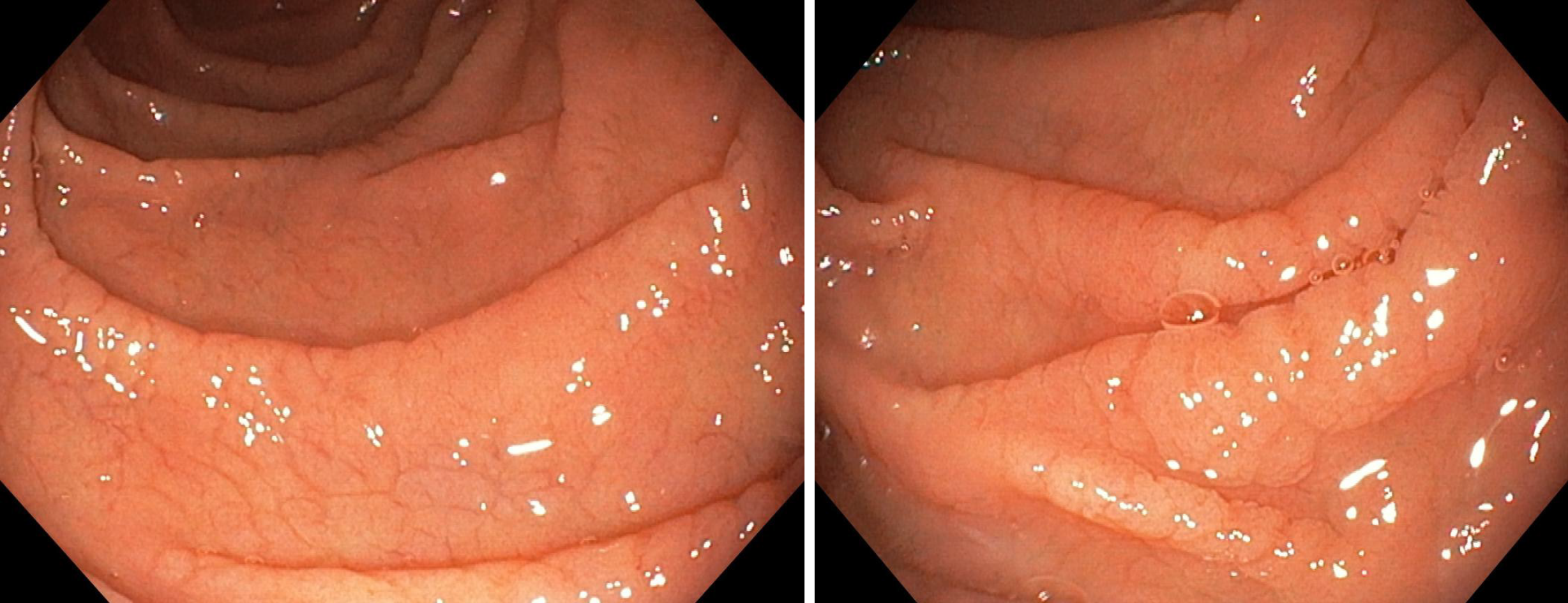
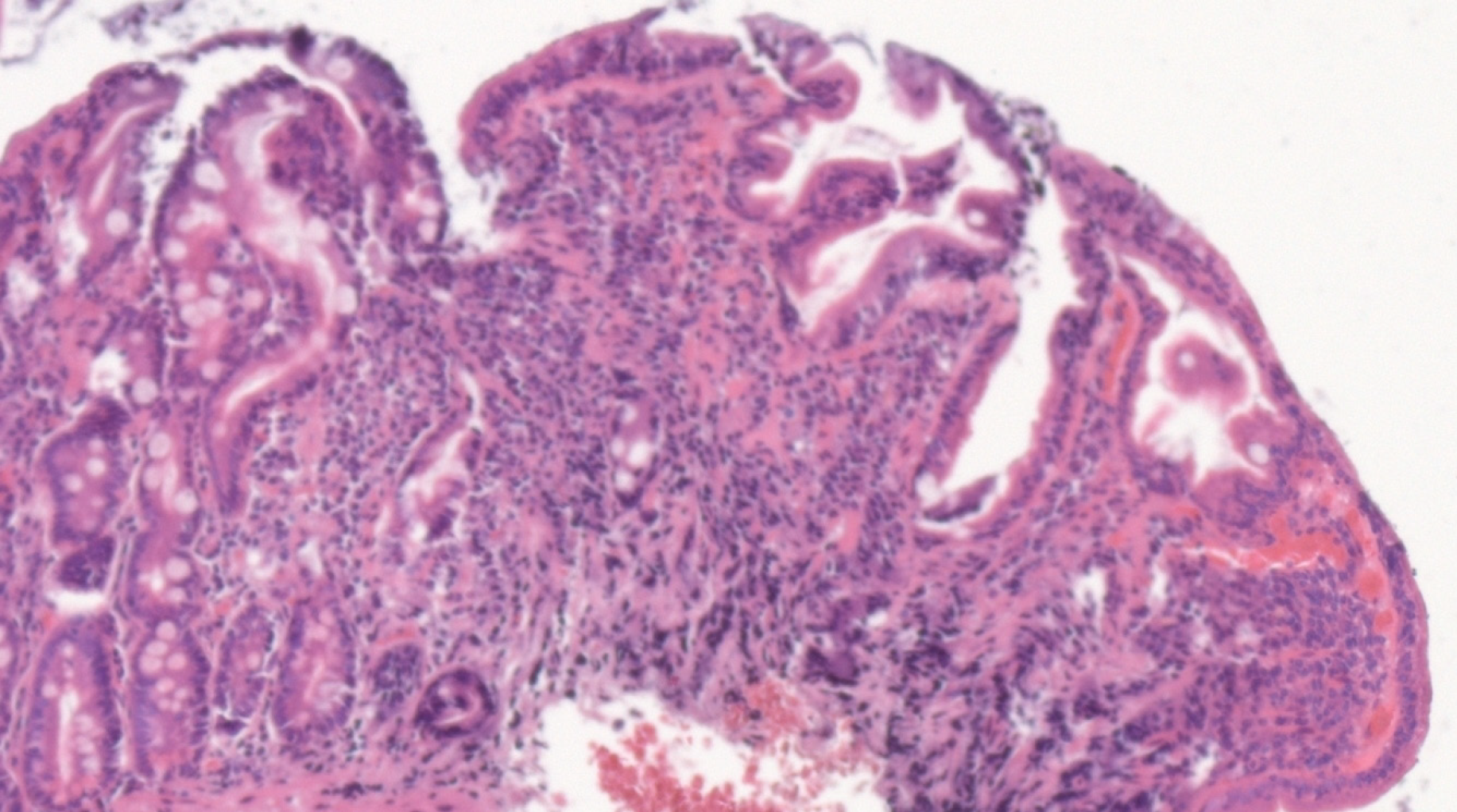
Considering the autoimmune background and the suspicion of associated malabsorption syndrome, screening for CD was recommended. Blood was drawn for serum IgA and IgA tissue transglutaminase (tTG) antibodies. The diagnosis approach was completed with upper gastrointestinal endoscopy which showed mucosal fissures and scalloping in the distal duodenum, with multiple biopsies being taken both from the bulb and distal duodenum (Figure 1 A and B). The histopathology report revealed marked villous atrophy with crypt hyperplasia and intraepithelial lymphocytosis, corresponding to Marsh 3c classification (Figure 2). IgA tissue transglutaminase antibody levels were over 200U (Quanta Lite® h-tTG IgA/Inova, cut-off positivity 20 U).
Thus, a diagnosis of CD was made.
Nutritional therapy, consisting of a gluten-free diet, was recommended.
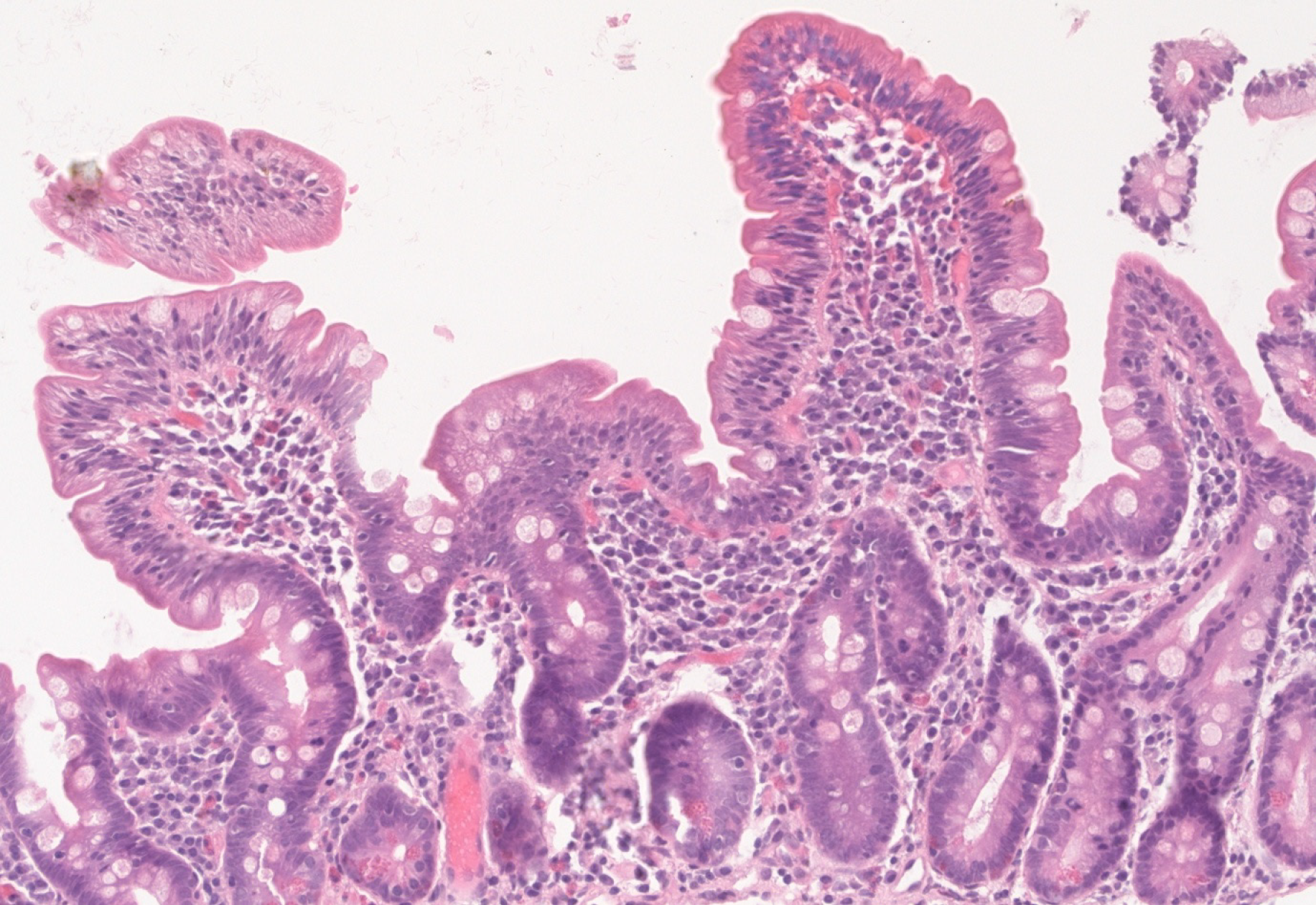
Upon follow-up, serum iron and ferritin levels normalized, IgA tTG gradually decreased to negative titers and control biopsy revealed restoring of normal villous architecture but with persisting intraepithelial lymphocytosis (Figure 3).
In our case presentation, we report a diagnosis of CD in a patient with SS, without any gastrointestinal complaints, starting from mild laboratory changes during a routine follow-up visit. Notably, the subtle alterations in the blood work-up along with a high index of suspicion of the clinician triggered CD testing.
Although the association of CD and SS is well recognized, awareness among practitioners of related medical specialties is far from being satisfactory[13], so opportunities to diagnose CD/SS are often missed. Both disorders are known to be significantly underdiagnosed and this exposes patients to complications and impaired quality of life[14,15]. In this setting, searching for CD in SS patients and vice versa could represent an important case-finding tool.
Several papers have looked at the frequency of CD in SS patients. The prevalence of CD among patients with SS ranges from 1% to 14.7% (Iltanen et al[16]) – as noted in Table 1[16-22], while the prevalence of CD in general population is about 1%. However, existing data in the literature has important limitations – the studies performed in cohorts of patients with SS included a limited number of patients and the diagnostic criteria used were not similar. The Finnish series by Iltanen et al[16] comprised a small number of patients (34 SS) and reported a high prevalence of 14.7% CD in patients with SS. All five CD patients had atrophic mucosal injury in the biopsy samples, with recovery on a gluten-free diet, while on serology, they were positive for either IgA-antiendomysial antibodies (EMA) or the old generation IgA antigliadin antibodies (AGA); the authors acknowledged the low specificity of IgA-AGA as serological screening in SS. As in our case, the clinical picture of CD patients diagnosed by screening was not prominent, and two of them had nutritional deficiencies (iron and folic acid deficiency, respectively). Of note, four SS non-CD patients had normal villous architecture with an increased density of intraepithelial γδ T cells, while two of them were also positive for HLA DQ2, which could represent CD latency. A rather high seroprevalence (12%) was also reported in a small cohort of SS patients (n = 50) in the study by Luft et al[20], five out of the six positive being biopsy-confirmed CD (10%); the authors also found that prevalence of tTG was higher in SS as compared with other rheumatic diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis or systemic sclerosis. On the other hand, Fasano et al[22] reported a low prevalence, of 2%, in a cohort of 98 SS patients, and so did Bizzaro et al[19], who found a seroprevalence similar to the one in the general population, of 1%: The one patient in a cohort of 100 SS was IgG tTG positive, but IgA and IgG EMA were negative, as was HLA typing, and according to study protocol, was not biopsied. Other studies such as the one by Caio et al[21] also reported on serology only, lacking histological confirmation; in their study, there was also a SS case with isolated, low titer, IgG anti-deamidated gliadin peptide antibodies (DGP) positivity, which could raise the CD prevalence to 7.7% if histologically-confirmed. In the largest cohort published, Bartoloni et al[18] reported a prevalence of 7.1% biopsy- proven CD, with 24 out of the 25 confirmed cases being previously diagnosed with CD; screening detected only one additional subclinical CD. The major issue of the aforementioned studies reporting on prevalence of CD in SS is the heterogeneity of criteria used for CD diagnosis, which partly explains the wide range of prevalence rates. However, when summarizing available data, there seems to be a higher prevalence of CD in SS patients than in general population. Although other larger studies have reported strong associations of SS with other CD-related autoimmune diseases such as ATD or PBC, CD was not looked for in these cohorts[23,24]. We hypothesize that overlooking CD in a patient with SS might be due to the gastrointestinal involvement secondary to SS itself, which would be labelled as a disease manifestation and would not trigger testing for CD.
| Ref. | SS (n) | Country | CD prevalence (SS + CD) | Serological criteria | Histological criteria |
| Iltanen et al[16] | 34 SS+, 28 controls | Finland | 14.7% (n = 5) | SS+ CD; 3/5 IgA EMA+; 4/5 IgA AGA+; 5/5 IgA EMA or AGA +; SS – CD; 0/28 IgA EMA +; 9/28 IgA AGA + | All 5 seropositive - Marsh 3 |
| Szodoray et al[17] | 111 SS+ | Hungary | 4.5% (n =5 ) | 6/111 tTG and/or AGA and/or EMA + | 5/6 CD+, one with jejunitis |
| Bartoloni et al[18] | 354 SS+, 14298 controls | Italy | 7.1% (n = 25), 6.8% (n = 24) previously diagnosed CD, 1 latent CD | 24 previously diagnosed (2013 WGO guideline criteria), 1 IgA tTG+ | All 25 |
| Bizzaro et al[19] | 100 SS+ | Italy, Israel | 1% (n = 1) | 1 IgG tTG + (IgA tTG-) | Histology not done |
| Luft et al[20] | 50 SS+, 50 controls 40 disease-controls (CD+) | Canada | 12% (n = 6) | 6 IgA tTG + | 5/6 biopsy-proven CD |
| Caio et al[21] | 52 SS+ | Italy | 5.8% (n = 3) | 3/42 IgA tTG+, IgG DGP + and IgA EMA+ | Histology not used |
Conversely, other authors have also looked at the SS occurrence in CD patients, results of which are summarized in Table 2[25-29]. Using the California criteria for SS, Collin et al[28] reported a prevalence of 3.3% for SS in patients with CD, a significant association in contrast with other connective tissue disease[28], while Caglar et al[27] reported a prevalence of 6.5% (SS-A/SS-B antibody)[27]. Ayar et al[29] found a high frequency of dry eye/mouth in CD patients, with approximately one in four being symptomatic, but only few had a biopsy-based diagnosis of SS; the frequency of SS classified according to the 2012 ACR or to American-European Consensus Group criteria was 4.9%[29]. Similarly, in a small cohort of 82 CD patients, Erbasan et al[25] reported a high prevalence of dry eye (29.3%) and dry mouth (24.4%) symptoms; in this group, the diagnosis of SS was proven by biopsy in 1.2% of CD patients[25]. Moreover, four patients (4.9%) were diagnosed with undifferentiated connective tissue disease, two of them being positive for anti-SS-A/La antibodies, but having though negative biopsies. As for the studies analyzing CD prevalence in SS, the ones looking at SS prevalence in patients with CD are also very heterogeneous with respect to diagnostic criteria.
| Ref. | CD (n) | Country | SS prevalence (SS + CD) | Diagnostic criteria |
| Erbasan et al[25] | 82 | Turkey | 1.2% | 29.3% dry eye, 24.4% dry mouth; 14.6% ANA+ |
| Bibbò et al[26] | 255 | Italy | 2.4% | |
| Caglar et al[27] | 31 | Turkey | 6.5% | 12.9% ANA+; 6.5% SS-A/Ro+ and/or SS-B/La+ |
| Collin et al[28] | 335 | Finland | 3.3% | |
| Ayar et al[29] | 81 | Turkey | 4.9% | 23.5% dry eye, 27.2% dry mouth |
Even if symptomatology like dry mouth or eye is frequently reported in CD patients, biopsy in search for SS is only rarely assessed. Therefore, the available data are incomplete. Even so, the concomitance of SS and CD seems not to be random.
In summary, there is scarce data on SS prevalence in CD, and from the available publications, there is a lack of uniformity with respect to specific classification criteria, study design, and population examined.
The antibody spectrum in CD and SS has been looked at in a few studies. On one hand, there is data supporting a high prevalence of antinuclear antibodies in patients with CD, ranging from 8.9% (da Rosa Utiyama et al[30]) to 14.6% (Erbasan et al[25]). Additionally, the anti-SS-A/Ro and anti-SS-B/La antibodies were reported to have a prevalence of 6.5% in 31 patients with CD[27]. On the other hand, earlier studies using gliadin-based serology have shown significant positivity of CD-related antibodies in SS: Teppo et al[31] found higher values of gliadin, gluten and reticulin antibodies in SS patients compared to rheumatoid arthritis or SLE. Significant false positivity of native AGA has also been reported also in other autoimmune diseases, such as SLE[32,33]. A study searching for immune and autoimmune gluten-related phenomenon in SLE patients found higher prevalence for tTG IgA and DGP (3.4% and 4.7%, respectively), but similar to general population prevalence for EMA (0.8%). Moreover, the tTG-IgA were found to be correlated with leucocytes, lymphocytes as well as with the complement levels[33].
To overcome the limitation of gliadin-based serology, clinicians should remember that EMA has the highest specificity for diagnosing CD, and IgA-tTG remains the best test for initial screening with very high sensitivity and specificity[34,35].
The association between CD and SS-related autoantibodies might be based on common genetic background of these two pathologies, related to DR3-DQ2 heterodimer coded by the DQA1*0501 and DQB1*0201 alleles[36,37]. Patients possessing these genetic markers had extractable nuclear antigen antibodies, and especially SS-B/La antibodies more frequent than patients negative for these alleles[38]. Moreover, it is well recognized that CD is associated with specific HLA types in virtually all populations that have been tested, and this very high negative predictive value of HLA-DQ2/DQ8 genotyping can be used to effectively rule out the disease in selected clinical situations[22,39]. For SS, the most relevant HLA genes involved in susceptibility are DR3–DQ2, with alleles DRB1*03:01, DQA1*05:01, and DQB1*02:01 being risk factors while, DQA1*03:01, DQA1*05:01 and DQB1*05:01 alleles were found to be protective factors[40].
Besides this common immunogenetic background, the link between SS and CD is also supported by studies researching gluten-challenge and gluten-free diet in SS patients. Therefore, the study by Patinen at al[41] showed improvement in terms of the autoimmune inflammatory process in the salivary glands of SS patients who were on a gluten-free diet because they had associated CD, while Lidén et al[42] revealed rectal mucosal inflammation in response to gluten challenge in SS patients, thus proving gluten-sensitivity of these patients. Not least, dermatitis herpetiformis, as a cutaneous equivalent of CD, has also been reported in SS[43].
Additionally, the histopathologic exam shows lymphocytes infiltrates in both SS and CD, namely a focal lymphocytic sialadenitis, with a focus score of ≥ 1 foci/4 mm2 in SS accordingly to the 2016 ACR/ EULAR SS’s classification criteria[2], and intraepithelial lymphocytic infiltration, more than 25 lymphocytes/100 epithelial cell, with associated villous and crypt architectural changes, in CD[12].
Moreover, both SS and CD pathologies are associated with an increased risk of lymphoma occurrence. Initially considered a T cell dysregulation, we recognized that the major derangement and dysregulation of the B lymphocytes in both peripheral circulation and glandular tissues of the SS patients plays a central part in the pathogenic processes[44]. The lymphoma risk is increased in SS patients especially for the marginal zone lymphoma, namely the parotid gland mucosa associated lymphoid tissue lymphoma, and the diffuse large B-cell lymphoma[45]. In CD, particularly patients with refractory CD, defined as persistent clinical and histological changes after at least 12 months of sustained gluten-free diet, especially in cases with monoclonal rearrangement of T cell receptor (type 2), occurrence of intestinal lymphoma might complicate the clinical course. The risk of both B and T cell lymphoma is increased in CD, and is the highest for the non-Hodgkin T cell intestinal lymphoma (six to nine times higher than in general population)[46].
Despite the strong association between the two conditions, currently available guidelines fail to recommend screening for SS in CD patients and vice versa[2,12], except the 2009 National Institute for Health and Clinical Excellence guideline that recommended that patients with SS may be considered for CD testing[47]. However, the updated and most recent NICE guideline on CD does not recommend screening for CD in patients with SS[48]. Similarly, the 2017 United States Preventive Services Task Force does not include the SS in the target group for the CD screening[49].
Although data in the literature is not as large as for other associated autoimmune diseases such as type 1 DM or ATD disease[6,50], testing for CD should be thought of in a symptomatic SS patient or upon detection of isolated iron deficiency, alterations of liver functions tests or pancreatic enzymes. However, there are some authors who recommend routine CD screening in patients with SS[51]; this would be readily available by IgA tTG testing, which has become increasingly accessible and improved significantly over the last years in terms of standardization of the kits used[52]. It is important to remember that IgA deficiency is more frequently found in CD patients, therefore the serum IgA level should also be tested, and IgG-based serology should be done in cases with IgA deficiency[53]. In this population of IgA-deficient individuals, DGP testing has been proven to be an accurate tool to identify CD[54]. Nevertheless, one should keep in mind the possibility of seronegative CD, meaning villous atrophy (VA) with negative CD-serology, when diagnosis can be supported by celiac-type mucosal injury on histopathology, exclusion of other causes of VA, along with HLA typing and response to gluten-free diet[55-57].
Thus, there are some clinical scenarios needing the search of CD in SS patients or SS in CD patients in Table 3[23]. Further studies should focus on indications, screening methods, timing of screening and frequency of testing for SS in CD patients and vice versa in Table 4.
| When to consider CD in SS patients? | When to consider SS in CD patients? |
| Suggestive gastrointestinal symptoms; Suspected malabsorption; Hypogammaglobulinemia and low levels of total protein; High levels of transaminases without evident cause; Anemia of unknown cause; Herpetiform dermatitis; Autoimmune thyroiditis (frequent in SS | Dry eyes and dry mouth symptoms; Enlarged parotid glands; Hypergammaglobulinemia and high levels of total protein; Polyneuropathy; Arthritis (arthralgias?); Purpura |
| Describing the prevalence of SS in patients with CD using the new classification criteria for SS |
| Describing the prevalence of CD in SS patients using newer generation serology tests |
| Prospective studies in patients with both conditions regarding evolution and prognosis, especially regarding the possible additive risk of lymphoma |
| Describing the role of gluten free diet regarding SS symptoms in patients associating both diseases |
| Developing clear recommendations for screening for SS in CD and for CD in SS |
Moreover, another important question that is yet to be answered is about the role of gluten-free diet in non-CD, SS patients. Several papers have reported benefits of the gluten free diet in various non-celiac autoimmune diseases, but results remain controversial[58,59]. Considering all the drawbacks of a gluten-free diet in the absence of gluten-intolerance, the role of gluten-free diet in SS remains to be studied and cannot be recommended yet on the basis of current evidence.
Sharing also a common genetic background, CD and SS are found to be associated, but evidence to quantify the magnitude of this association is lacking. This concomitance of these two diseases is however easily overlooked in clinical practice and currently available guidelines fail to recognize it. Further research should look at indications for screening, testing methods, as well as the role of the gluten-free diet in non-CD SS patients.
We would like to thank Dr. Vasilescu Florina for her support with the histology slides.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): E
P-Reviewer: Iovino P, Kansu A, Kaya M, Ribaldone DG, Vasudevan A S-Editor: Zhang L L-Editor: A P-Editor: Wang LL
| 1. | Lauret E, Rodrigo L. Celiac disease and autoimmune-associated conditions. Biomed Res Int. 2013;2013:127589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 2. | Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, Rasmussen A, Scofield H, Vitali C, Bowman SJ, Mariette X; International Sjögren's Syndrome Criteria Working Group. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren's Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017;69:35-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 1105] [Article Influence: 138.1] [Reference Citation Analysis (0)] |
| 3. | Ludvigsson JF, Murray JA. Epidemiology of Celiac Disease. Gastroenterol Clin North Am. 2019;48:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 4. | Ribaldone DG, Astegiano M, Fagoonee S, Rizzetto M, Pellicano R. Epilepsy and celiac disease: review of literature. Panminerva Med. 2011;53:213-216. [PubMed] |
| 5. | Ciaccio EJ, Lewis SK, Biviano AB, Iyer V, Garan H, Green PH. Cardiovascular involvement in celiac disease. World J Cardiol. 2017;9:652-666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Freeman HJ. Endocrine manifestations in celiac disease. World J Gastroenterol. 2016;22:8472-8479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Dima A, Jurcut C, Jinga M. Rheumatologic manifestations in celiac disease: what should we remember? Rom J Intern Med. 2019;57:3-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Pittman FE, Holub DA. Sjoegren's syndrome and adult celiac disease. Gastroenterology. 1965;48:869-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Peña AS. Systemic lupus erythematosus, Sjögren's syndrome, and purpura in a patient with coeliac disease. Neth J Med. 1987;31:305-307. [PubMed] |
| 10. | Maclaurin BP, Matthews N, Kilpatrick JA. Coeliac disease associated with auto-immune thyroiditis, Sjogren's syndrome, and a lymphocytotoxic serum factor. Aust N Z J Med. 1972;2:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Lancaster-Smith MJ, Strickland ID. Autoantibodies in adult coeliac disease. Lancet. 1971;1:1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Al-Toma A, Volta U, Auricchio R, Castillejo G, Sanders DS, Cellier C, Mulder CJ, Lundin KEA. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J. 2019;7:583-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 598] [Article Influence: 99.7] [Reference Citation Analysis (1)] |
| 13. | Jinga M, Popp A, Balaban DV, Dima A, Jurcut C. Physicians' attitude and perception regarding celiac disease: A questionnaire-based study. Turk J Gastroenterol. 2018;29:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Laurikka P, Nurminen S, Kivelä L, Kurppa K. Extraintestinal Manifestations of Celiac Disease: Early Detection for Better Long-Term Outcomes. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 15. | Violato M, Gray A. The impact of diagnosis on health-related quality of life in people with coeliac disease: a UK population-based longitudinal perspective. BMC Gastroenterol. 2019;19:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Iltanen S, Collin P, Korpela M, Holm K, Partanen J, Polvi A, Mäki M. Celiac disease and markers of celiac disease latency in patients with primary Sjögren's syndrome. Am J Gastroenterol. 1999;94:1042-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Szodoray P, Barta Z, Lakos G, Szakáll S, Zeher M. Coeliac disease in Sjögren's syndrome--a study of 111 Hungarian patients. Rheumatol Int. 2004;24:278-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Bartoloni E, Bistoni O, Alunno A, Cavagna L, Nalotto L, Baldini C, Priori R, Fischetti C, Fredi M, Quartuccio L, Carubbi F, Montecucco C, Doria A, Mosca M, Valesini G, Franceschini F, De Vita S, Giacomelli R, Mirabelli G, Bini V, Gabrielli A, Catassi C, Gerli R. Celiac Disease Prevalence is Increased in Primary Sjögren's Syndrome and Diffuse Systemic Sclerosis: Lessons from a Large Multi-Center Study. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 19. | Bizzaro N, Villalta D, Tonutti E, Doria A, Tampoia M, Bassetti D, Tozzoli R. IgA and IgG tissue transglutaminase antibody prevalence and clinical significance in connective tissue diseases, inflammatory bowel disease, and primary biliary cirrhosis. Dig Dis Sci. 2003;48:2360-2365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Luft LM, Barr SG, Martin LO, Chan EK, Fritzler MJ. Autoantibodies to tissue transglutaminase in Sjögren's syndrome and related rheumatic diseases. J Rheumatol. 2003;30:2613-2619. [PubMed] |
| 21. | Caio G, De Giorgio R, Ursini F, Fanaro S, Volta U. Prevalence of celiac disease serological markers in a cohort of Italian rheumatological patients. Gastroenterol Hepatol Bed Bench. 2018;11:244-249. [PubMed] |
| 22. | Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, Elitsur Y, Green PH, Guandalini S, Hill ID, Pietzak M, Ventura A, Thorpe M, Kryszak D, Fornaroli F, Wasserman SS, Murray JA, Horvath K. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1183] [Cited by in RCA: 1139] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 23. | Baldini C, Ferro F, Mosca M, Fallahi P, Antonelli A. The Association of Sjögren Syndrome and Autoimmune Thyroid Disorders. Front Endocrinol (Lausanne). 2018;9:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Kassan SS, Moutsopoulos HM. Clinical manifestations and early diagnosis of Sjögren syndrome. Arch Intern Med. 2004;164:1275-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 452] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 25. | Erbasan F, Çoban DT, Karasu U, Çekin Y, Yeşil B, Çekin AH, Süren D, Terzioğlu ME. Primary Sjögren's syndrome in patients with celiac disease. Turk J Med Sci. 2017;47:430-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Bibbò S, Pes GM, Usai-Satta P, Salis R, Soro S, Quarta Colosso BM, Dore MP. Chronic autoimmune disorders are increased in coeliac disease: A case-control study. Medicine (Baltimore). 2017;96:e8562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Caglar E, Ugurlu S, Ozenoglu A, Can G, Kadioglu P, Dobrucali A. Autoantibody frequency in celiac disease. Clinics (Sao Paulo). 2009;64:1195-1200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Collin P, Reunala T, Pukkala E, Laippala P, Keyriläinen O, Pasternack A. Coeliac disease--associated disorders and survival. Gut. 1994;35:1215-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 318] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 29. | Ayar K, Tunз R, Pekel H, Kьзьk A, Esen HH, Çifзi S, Ataseven H. The frequency of Sjogren’s syndrome in celiac patients; a cross-sectional controlled clinical trial. Ann Rheum Dis. 2015;74:1094–1095. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | da Rosa Utiyama SR, da Silva Kotze LM, Nisihara RM, Carvalho RF, de Carvalho EG, de Sena MG, de Messias Reason IJ. Spectrum of autoantibodies in celiac patients and relatives. Dig Dis Sci. 2001;46:2624-2630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Teppo AM, Maury CP. Antibodies to gliadin, gluten and reticulin glycoprotein in rheumatic diseases: elevated levels in Sjögren's syndrome. Clin Exp Immunol. 1984;57:73-78. [PubMed] |
| 32. | Rensch MJ, Szyjkowski R, Shaffer RT, Fink S, Kopecky C, Grissmer L, Enzenhauer R, Kadakia S. The prevalence of celiac disease autoantibodies in patients with systemic lupus erythematosus. Am J Gastroenterol. 2001;96:1113-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Dima A, Jurcut C, Balanescu E, Badea C, Lacatus N, Popp A. Immune and autoimmune gluten-related phenomenon in systemic lupus erythematosus. Lupus. 2017;26:1235-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Schyum AC, Rumessen JJ. Serological testing for celiac disease in adults. United European Gastroenterol J. 2013;1:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Chou R, Bougatsos C, Blazina I, Mackey K, Grusing S, Selph S. Screening for Celiac Disease: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2017;317:1258-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 36. | Sollid LM, Thorsby E. HLA susceptibility genes in celiac disease: genetic mapping and role in pathogenesis. Gastroenterology. 1993;105:910-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 402] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 37. | Kang HI, Fei HM, Saito I, Sawada S, Chen SL, Yi D, Chan E, Peebles C, Bugawan TL, Erlich HA. Comparison of HLA class II genes in Caucasoid, Chinese, and Japanese patients with primary Sjögren's syndrome. J Immunol. 1993;150:3615-3623. [PubMed] |
| 38. | D'Onofrio F, Miele L, Diaco M, Santoro L, De Socio G, Montalto M, Grieco A, Gasbarrini G, Manna R. Sjogren's syndrome in a celiac patient: searching for environmental triggers. Int J Immunopathol Pharmacol. 2006;19:445-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Ludvigsson JF, Bai JC, Biagi F, Card TR, Ciacci C, Ciclitira PJ, Green PH, Hadjivassiliou M, Holdoway A, van Heel DA, Kaukinen K, Leffler DA, Leonard JN, Lundin KE, McGough N, Davidson M, Murray JA, Swift GL, Walker MM, Zingone F, Sanders DS; BSG Coeliac Disease Guidelines Development Group; British Society of Gastroenterology. Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut. 2014;63:1210-1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 689] [Cited by in RCA: 783] [Article Influence: 71.2] [Reference Citation Analysis (2)] |
| 40. | Cruz-Tapias P, Rojas-Villarraga A, Maier-Moore S, Anaya JM. HLA and Sjögren's syndrome susceptibility. A meta-analysis of worldwide studies. Autoimmun Rev. 2012;11:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 41. | Patinen P, Aine L, Collin P, Hietanen J, Korpela M, Enckell G, Kautiainen H, Konttinen YT, Reunala T. Oral findings in coeliac disease and Sjögren's syndrome. Oral Dis. 2004;10:330-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Lidén M, Kristjánsson G, Valtýsdóttir S, Hällgren R. Gluten sensitivity in patients with primary Sjögren's syndrome. Scand J Gastroenterol. 2007;42:962-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Fraser NG, Rennie AG, Donald D. Dermatitis herpetiformis and Sjögren's syndrome. Br J Dermatol. 1979;100:213-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Ibrahem HM. B cell dysregulation in primary Sjögren's syndrome: A review. Jpn Dent Sci Rev. 2019;55:139-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 45. | Gorodetskiy VR, Probatova NA, Radenska-Lopovok SG, Ryzhikova NV, Sidorova YV, Sudarikov AB. Clonal relationship of marginal zone lymphoma and diffuse large B-cell lymphoma in Sjogren's syndrome patients: case series study and review of the literature. Rheumatol Int. 2020;40:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 46. | Caio G, Volta U, Sapone A, Leffler DA, De Giorgio R, Catassi C, Fasano A. Celiac disease: a comprehensive current review. BMC Med. 2019;17:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 564] [Cited by in RCA: 563] [Article Influence: 93.8] [Reference Citation Analysis (0)] |
| 47. | Centre for Clinical Practice at NICE (UK). Coeliac Disease: Recognition and Assessment of Coeliac Disease [Internet]. London: National Institute for Health and Clinical Excellence (UK); 2009. [PubMed] |
| 48. | Internal Clinical Guidelines Team (UK). Coeliac Disease: Recognition, Assessment and Management. London: National Institute for Health and Care Excellence (UK); 2015. [PubMed] |
| 49. | US Preventive Services Task Force. Bibbins-Domingo K, Grossman DC, Curry SJ, Barry MJ, Davidson KW, Doubeni CA, Ebell M, Epling JW Jr, Herzstein J, Kemper AR, Krist AH, Kurth AE, Landefeld CS, Mangione CM, Phipps MG, Silverstein M, Simon MA, Tseng CW. Screening for Celiac Disease: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;317:1252-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 50. | Akirov A, Pinhas-Hamiel O. Co-occurrence of type 1 diabetes mellitus and celiac disease. World J Diabetes. 2015;6:707-714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 51. | Roblin X, Helluwaert F, Bonaz B. Celiac disease must be evaluated in patients with Sjögren syndrome. Arch Intern Med. 2004;164:2387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Stern M; Working Group on Serologic Screening for Celiac Disease. Comparative evaluation of serologic tests for celiac disease: a European initiative toward standardization. J Pediatr Gastroenterol Nutr. 2000;31:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 53. | Balaban DV, Popp A, Ionita Radu F, Jinga M. Hematologic Manifestations in Celiac Disease-A Practical Review. Medicina (Kaunas). 2019;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 54. | Leffler DA, Schuppan D. Update on serologic testing in celiac disease. Am J Gastroenterol. 2010;105:2520-2524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 237] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 55. | Aziz I, Peerally MF, Barnes JH, Kandasamy V, Whiteley JC, Partridge D, Vergani P, Cross SS, Green PH, Sanders DS. The clinical and phenotypical assessment of seronegative villous atrophy; a prospective UK centre experience evaluating 200 adult cases over a 15-year period (2000-2015). Gut. 2017;66:1563-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 56. | Volta U, Caio G, Boschetti E, Giancola F, Rhoden KJ, Ruggeri E, Paterini P, De Giorgio R. Seronegative celiac disease: Shedding light on an obscure clinical entity. Dig Liver Dis. 2016;48:1018-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 57. | DeGaetani M, Tennyson CA, Lebwohl B, Lewis SK, Abu Daya H, Arguelles-Grande C, Bhagat G, Green PH. Villous atrophy and negative celiac serology: a diagnostic and therapeutic dilemma. Am J Gastroenterol. 2013;108:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 58. | Lerner A, Ramesh A, Matthias T. Going gluten free in non-celiac autoimmune diseases: the missing ingredient. Expert Rev Clin Immunol. 2018;14:873-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 59. | Lerner A, Ramesh A, Matthias T. Are Non-Celiac Autoimmune Diseases Responsive to Gluten-Free Diet? Int J Celiac Dis. 2017;5:164-167. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |