Published online Sep 26, 2020. doi: 10.12998/wjcc.v8.i18.4122
Peer-review started: April 19, 2020
First decision: July 25, 2020
Revised: August 2, 2020
Accepted: August 29, 2020
Article in press: August 29, 2020
Published online: September 26, 2020
Processing time: 155 Days and 5.9 Hours
Sweet’s syndrome, also known as acute febrile neutrophilic dermatosis, is a rare skin disorder that may be associated with cancer.
A 58-year-old female presented with a cholestatic syndrome and significant weight loss three months before admission. Five months earlier, she had abruptly developed skin lesions with erythematous papules that evolved to erythematous blisters. Clinical evaluation and laboratory tests confirmed hepatic cholangiocarcinoma. Skin lesions histopathological findings showed neutrophilic dermatosis, massive edema, fibrin, necrosis, and elastosis. These results, in association with the macroscopic aspects of the findings, led to the diagnosis of paraneoplastic Sweet’s syndrome due to cholangiocarcinoma. As staging was consistent with an advanced tumor without a cure perspective, we opted to perform percutaneous biliary drainage, and subsequently, palliative care. Eventually, after a few weeks, the patient died.
In conclusion, the diagnosis of the underlying disease-causing Sweet’s syndrome must be accurate, and patients need to be followed-up, as neoplasia such as cholangiocarcinoma may be a later manifestation.
Core Tip: Sweet's syndrome preceding solid neoplasia, especially cholangiocarcinoma, is a rare condition. Only two cases of Sweet's syndrome associated with cholangiocarcinoma have been reported. Bullous skin lesions should be evaluated with a broad differential diagnosis, including the possibility of preceding the diagnosis of neoplasia. The evaluation and follow-up of patients with this condition is essential, seeking diagnosis and treatment in early stages of the neoplastic lesion.
- Citation: Lemaire CC, Portilho ALC, Pinheiro LV, Vivas RA, Britto M, Montenegro M, Rodrigues LFF, Arruda S, Lyra AC, Cavalcante LN. Sweet syndrome as a paraneoplastic manifestation of cholangiocarcinoma: A case report. World J Clin Cases 2020; 8(18): 4122-4127
- URL: https://www.wjgnet.com/2307-8960/full/v8/i18/4122.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i18.4122
Sweet’s syndrome, also known as acute febrile neutrophilic dermatosis, is a rare inflammatory disorder characterized by the abrupt appearance of painful, edematous, and erythematous papules, plaques, or nodules on the skin. Fever and leukocytosis frequently accompany the cutaneous lesions. It may be clinically divided into three types: Classical or idiopathic, malignancy-associated, and drug-induced[1]. The malignancy-associated form was described in approximately 20% of Sweet’s syndrome cases[2]. Within these cases, the most common reported association has been with hematopoietic neoplasia (nearly in 85%), while fewer cases were associated with solid tumors, as was our described case[3]. It is interesting to note that there is a scarcity of case reports about the association between Sweet’s syndrome and cholangiocarcinoma. Therefore, we describe the case of a patient who was diagnosed with the paraneoplastic Sweet’s syndrome and had this malignant biliary tumor, to remind physicians about this possible association.
A 58-year-old female presented at our service on March 21st of 2018, with a three-month history of cholestasis symptoms, as well as significant weight loss (about 20 kg). She reported jaundice, choluria, acholia, and itching.
Two months before the development of the gastrointestinal symptoms, she had suddenly progressed with skin lesions (erythematous papules) that evolved to erythematous blisters.
She denied other comorbidities, fever, and diarrhea.
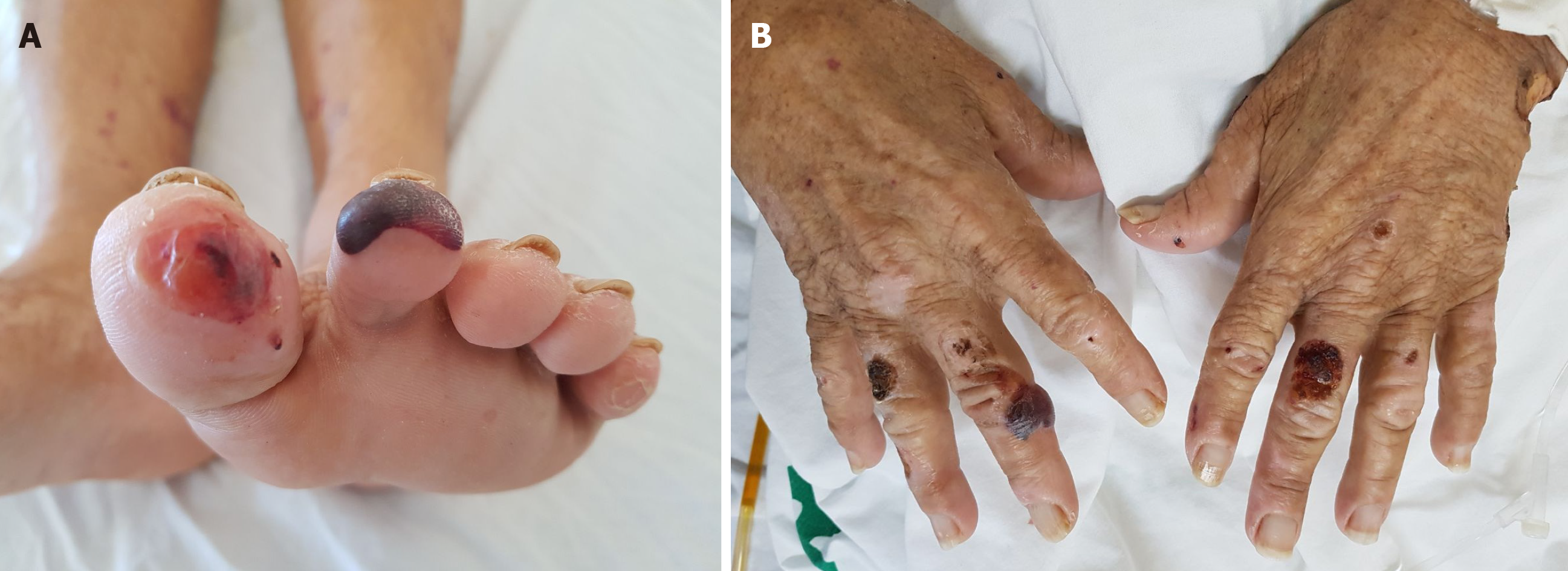
On physical examination, she was malnourished, pale, afebrile, and presented significant jaundice. The abdominal exam was remarkable for a mild diffuse abdominal tenderness to deep palpation, as well as for an enlarged nontender, firm, and irregular liver. There were also blistering and crusted skin lesions, simulating pemphigus, throughout the scalp, hands, and feet (Figure 1).
Tumor markers analysis showed a CA 19.9 of 1281.5 U/mL (upper limit of normal, 37.0 U/mL), alpha-fetoprotein of 2.13 ng/mL (upper limit of normal, 10.0 ng/mL), and CEA of 14.8 ng/mL (upper limit of normal, 4.9 ng/mL). The remaining laboratory tests showed leukocytes count of 8.900/m3 (80%, neutrophils), C-reactive protein of 23 mg/L (upper limit of normal, 10.0 mg/L), marked increased cholestatic liver enzymes, and bilirubin levels. Aminotransferases were mild to moderately elevated.
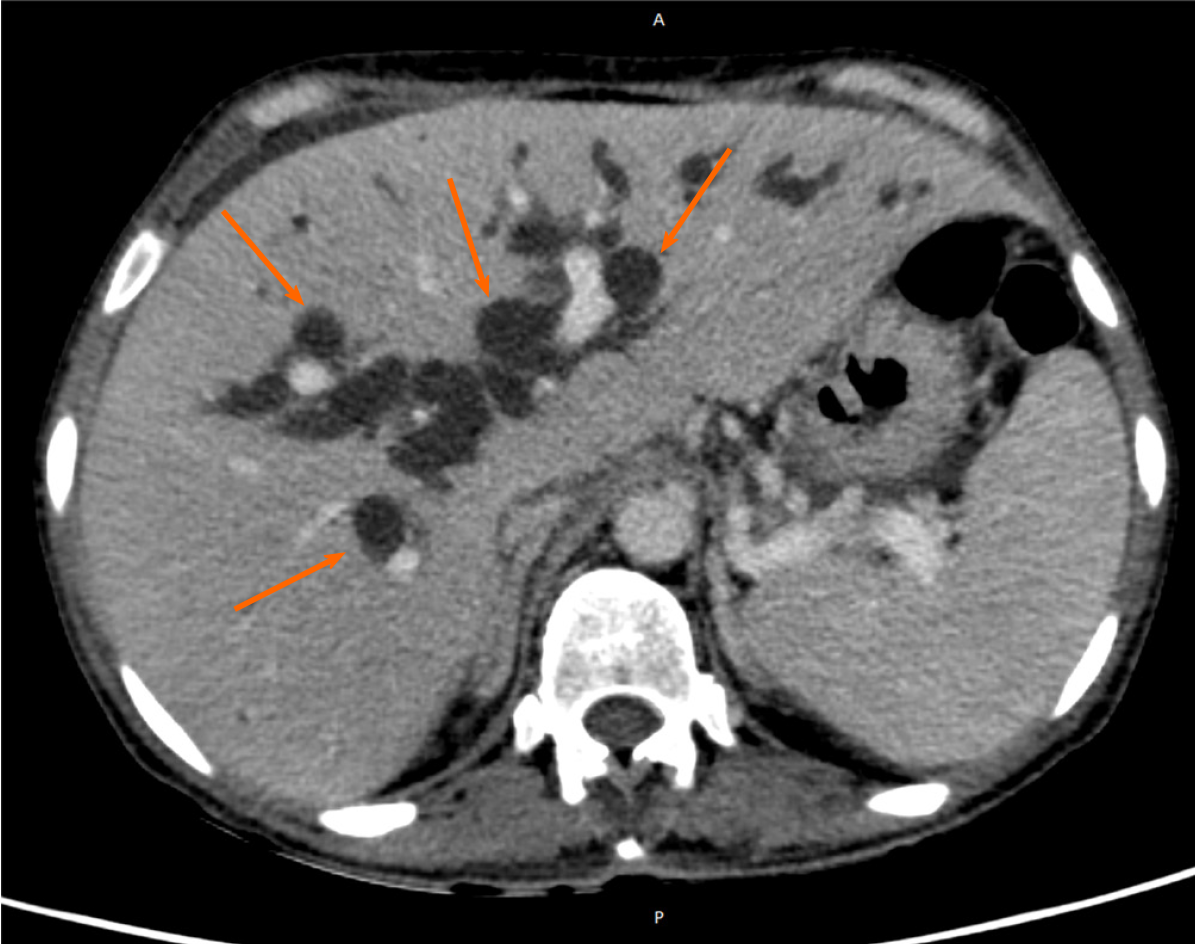
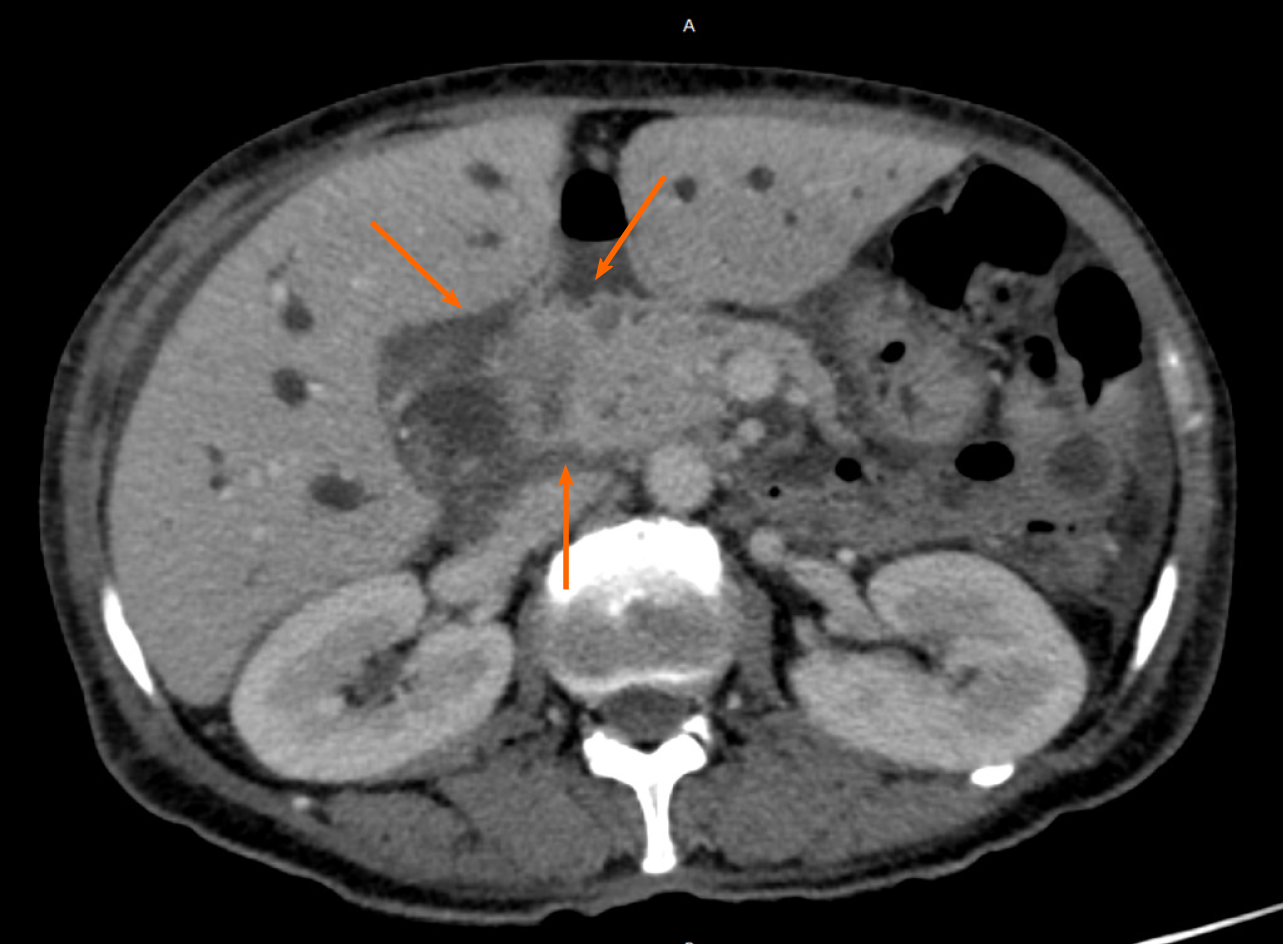
The patient underwent an abdomen computed tomography that revealed an infiltrative mass causing dilatation of the bile ducts, as well as increased mesenteric lymph nodes, and ascites (Figures 2 and 3). Further investigation with a magnetic resonance of the upper abdomen with cholangiopancreatography, confirmed stricture of the common bile and hepatic ducts due to probable neoplasia, and upstream dilatation of the biliary tree.
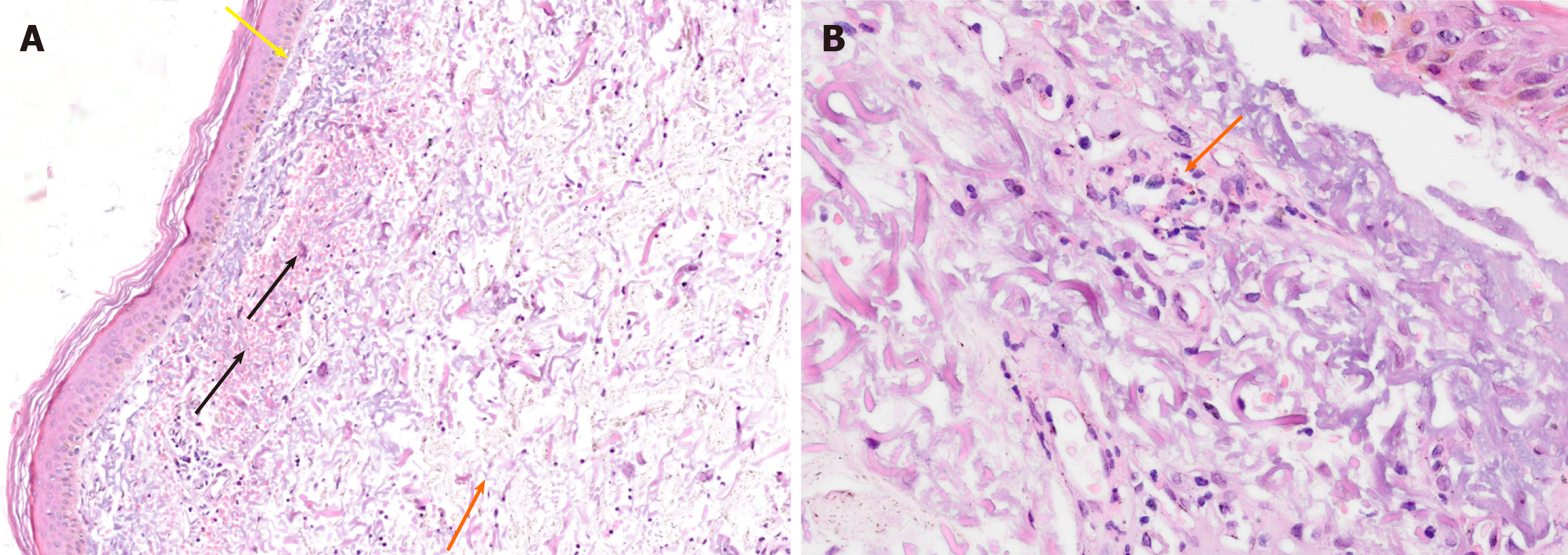
The patient underwent an echo-endoscopy with fine-needle aspiration biopsy of the tumor that confirmed the diagnosis of cholangiocarcinoma. Biopsies of the skin blistering and crusted lesions simulating pemphigus throughout the scalp, hands, and feet, were performed. Microscopy analysis showed neutrophilic dermatosis with massive edema, fibrin, necrosis, and elastosis (Figure 4).
The aforementioned results, in association with the macroscopic aspects of the lesions, led to the diagnosis of paraneoplastic Sweet’s syndrome. The patient was started on steroids and antihistaminies to treat the skin lesions without success.
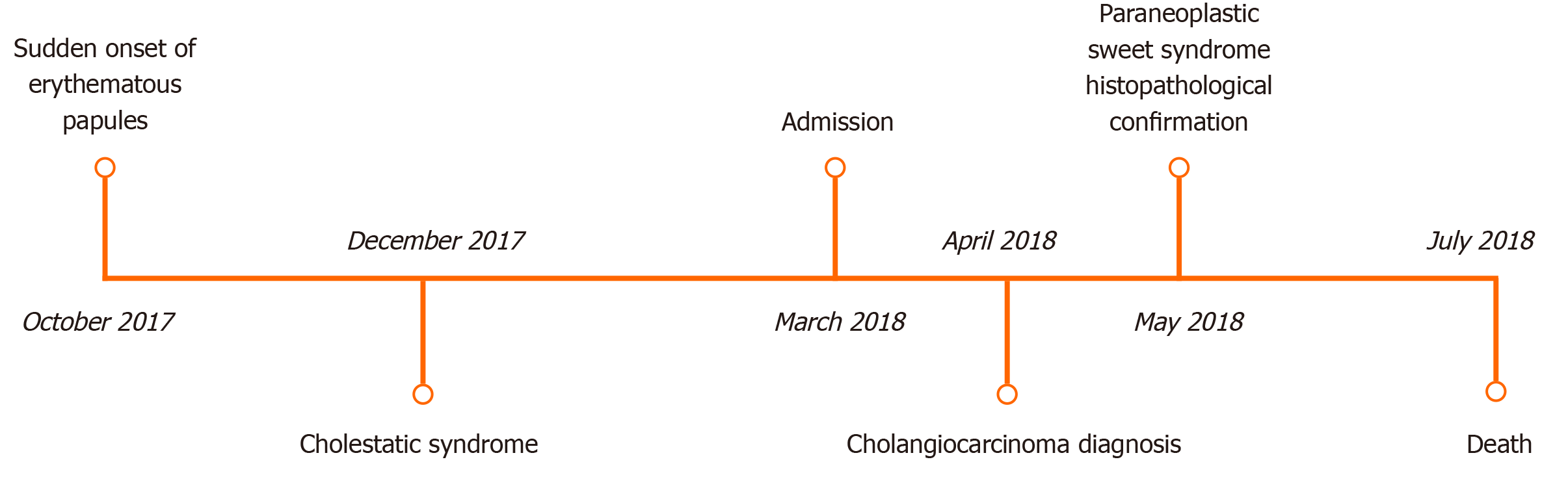
Due to the advanced stage of the tumor, with no cure perspective, we opted to perform percutaneous biliary drainage for symptomatic relief and subsequent palliative care. Figure 5 describes the chronology of the events.
We reported the case of a patient diagnosed with Sweet’s syndrome as a paraneoplastic manifestation of a cholangiocarcinoma, in which the primary symptoms were cholestatic syndrome and weight loss. The clinical evolution, laboratory tests, and skin lesions biopsy results made it possible to confirm the diagnosis of Sweet’s syndrome based on Su and Liu’s criteria[4] (1896) modified by von den Driesch[5]. Sweet’s syndrome is characterized by the sudden appearance of erythematous papules and/or plaques, which can contain vesicular surfaces and usually affect thorax, back, face, neck, and upper extremities[5]. Our patient presented with abrupt onset of erythematous papules, histopathological evidence of dense neutrophilic infiltrate with no signs of leukocytoclastic vasculitis and had an underlying visceral malignancy. Laboratory values at presentation showed > 8000 leukocytes as well > 70% neutrophils.
There are three possible clinical categorizations for this syndrome based upon etiology: Classical or idiopathic, malignancy-associated, and drug-induced[1]. Malignancy-associated Sweet’s syndrome corresponds to approximately 20% of the cases[2]. It is most associated with hematological neoplasia (in 85% of the cases), and less frequently, with solid tumors, as was the case of our patient[3]. The main form of presentation in these cases associated with malignancy is as a paraneoplastic syndrome. The appearance of the skin lesions can precede the primary illness’ diagnosis in months to years[6]. The histopathological and laboratory test results favored the patient’s diagnosis of a paraneoplastic Sweet’s syndrome in our case.
Gold standard treatment is to administer systemic steroid therapy, which may lead to a fast remission of the symptomatology. Treatment of the primary underlying disease is also related to the chances of patient recovery[3]. Nevertheless, in our case, corticosteroid and antihistamine therapy were unsuccessful. Another therapeutic approach that we adopted was biliary drainage to proportionate symptomatic relief. There are no current well-established guidelines to treat malignancy-associated Sweet's syndrome[3].
To our knowledge, there are only two reported cases of Sweet’s syndrome in association with cholangiocarcinoma. The first case ever describing this association, was published in 2006. In this case of a 68-year-old woman, skin lesions preceded the diagnosis of the malignant tumor, as it occurred with our patient[7]. The other case report described a 72-year-old man with cholangiocarcinoma in which Sweet’s syndrome manifested 3 years after the initial diagnosis, five months after chemotherapy interruption due to a semi-urgent surgery[8].
In conclusion, the diagnosis of the underlying disease-causing Sweet’s syndrome must be accurate, and patients need to be followed-up, as neoplasia such as cholangiocarcinoma may be a later manifestation.
We thank the Digestive Endoscopy, Radiology, and the Surgery teams at the Hospital Geral Roberto Santos for their support in the clinical management of the case. We also thank Fiocruz-Bahia for kindly providing the images shown on this case report.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fava G, Zhang X S-Editor: Huang P L-Editor: A P-Editor: Li JH
| 1. | Cohen PR. Sweet's syndrome--a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 525] [Cited by in RCA: 529] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 2. | Cohen PR, Kurzrock R. Sweet's syndrome and cancer. Clin Dermatol. 1993;11:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 151] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Raza S, Kirkland RS, Patel AA, Shortridge JR, Freter C. Insight into Sweet's syndrome and associated-malignancy: a review of the current literature. Int J Oncol. 2013;42:1516-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | von den Driesch P. Sweet's syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31:535-556; quiz 557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 471] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 6. | Chung VQ, Moschella SL, Zembowicz A, Liu V. Clinical and pathologic findings of paraneoplastic dermatoses. J Am Acad Dermatol. 2006;54:745-762; quiz 763-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Shinojima Y, Toma Y, Terui T. Sweet syndrome associated with intrahepatic cholangiocarcinoma producing granulocyte colony-stimulating factor. B. r J Dermatol. 2006;155:1103-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Opneja A, Mahajan S, Kapoor S, Marur S, Yang SH, Manno R. Unusual Paraneoplastic Presentation of Cholangiocarcinoma. Case Rep Med. 2015;2015:806835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |