Published online Sep 26, 2020. doi: 10.12998/wjcc.v8.i18.4100
Peer-review started: May 20, 2020
First decision: June 19, 2020
Revised: June 30, 2020
Accepted: August 22, 2020
Article in press: August 22, 2020
Published online: September 26, 2020
Processing time: 122 Days and 0.5 Hours
Pembrolizumab is an anti-programmed death receptor 1 (PD-1) that was shown to have a tolerable safety profile with 17% of grade 3-4 drug-related adverse events, notable response rate of 16% with median duration of response of 8 mo, and median overall survival of 8 mo. Severe mucositis is a very rare complication with only two cases of grade 4 mucositis reported, and both cases had good response to intravenous methylprednisolone and subsequent oral prednisone tapering. We report the first case of pembrolizumab-induced severe mucositis that was refractory to steroid treatment.
An 80-year-old woman with a past medical history of recurrent right cheek nodular melanoma status post resection and new right lung metastatic melanoma on immunotherapy presented with dysphagia and odynophagia for 2 mo. She initially received 2 doses of ipilimumab 1 year ago with good outcome, but treatment was discontinued after developing severe diarrhea and rash. Pembrolizumab was then initiated 4 mo after disease progression. Significant improvement was noted after 3 doses. However, after 6 cycles of pembrolizumab, patient developed odynophagia and malnutrition. Improvement of symptoms was noted after discontinuation of pembrolizumab and initiation of steroids. 3 mo later, patient developed pharyngeal swelling with hoarseness and new oxygen requirement due to impending airway obstruction while being on prednisone tapering regimen, finally ended up with intubation and tracheostomy. Histologic analysis of left laryngeal and epiglottis tissue showed granulation tissue with acute on chronic inflammation, negative for malignancy and infection. Patient achieved marked improvement after 2 doses of infliximab of 5 mg/kg every 2 wk while continuing on prednisone tapering course.
We report the first case of pembrolizumab-induced grade 4 mucositis that had limited recovery with prolonged steroid course but had rapid response with addition of infliximab. The patient had recurrent mucositis symptoms whenever steroids was tapered but achieved complete response after receiving two doses of infliximab while continuing to be on tapering steroids. The success of infliximab in this patient with pembrolizumab-induced severe mucositis presents a potentially safe approach to reduce prolonged steroid course and accelerate recovery in managing this rare complication.
Core Tip: Grade 3-4 mucositis is a rare adverse effect of the immune checkpoint inhibitor with 2 cases reported that both responded well to steroid tapering course. We report a case of pembrolizumab-induced severe mucositis that was refractory to steroids at first but had significant improvement after administration of infliximab while continuing to be on steroid tapering course.
- Citation: Dang H, Sun J, Wang G, Renner G, Layfield L, Hilli J. Management of pembrolizumab-induced steroid refractory mucositis with infliximab: A case report. World J Clin Cases 2020; 8(18): 4100-4108
- URL: https://www.wjgnet.com/2307-8960/full/v8/i18/4100.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i18.4100
Melanoma is the most aggressive and deadliest form of skin cancer with a 3-year survival rate of 15% for stage IV melanoma[1-3]. Surgery remains the primary treatment for stage I-IIIB melanoma[4,5]. For advanced melanoma, chemotherapy was the earliest treatment, however there is no improvement in overall survival rate with Dacarbazine and Temozolomide with complete response occurring in less than 5% and 5-year survival rate of 2%-6%[6-8]. Since the association between cancer and the immune system was proposed in the 19th century, several immunotherapies have been developed and approved[2,9]. Pembrolizumab, an anti-programmed death receptor (PD-1), was approved in 2015 by the Food and Drug Administration (FDA) for the treatment of advanced melanomas, and it was shown to have a tolerable safety profile with 17% of patients developing grade 3-4 drug-related adverse events in the phase IB study and similar safety profile with 15% of patients with grade 3-4 events in the subsequent phase II study[2,10,11]. Although being concluded to be better tolerated than chemotherapy, multiple side effects of PD-1/PD-L1 inhibitors have been studied in a systematic review and meta-analysis including all-grade rash, colitis, hypothyroidism, transaminitis, and pneumonitis, with grade 3-4 mucositis remaining as a rare adverse effect of the immune checkpoint inhibitor[12,13]. To date, only 2 cases of pembrolizumab-induced severe mucositis have been reported, with patients achieved adequate recovery after steroid treatment[14,15]. Here, we report a first case of pembrolizumab-induced severe mucositis refractory to steroid therapy, but responded well to addition of infliximab to the steroid tapering course.
An 80-year-old woman with a past medical history of recurrent right cheek nodular melanoma and new lung metastasis previously on immunotherapy presented for worsening dysphagia and odynophagia for one month.
The patient initially developed dysphagia, odynophagia and associated malnutrition after 6 cycles of pembrolizumab. Immunotherapy was discontinued, and patient was started on prednisone slow taper with initial improvement of symptoms. However, after 2 mo, the patient developed throat swelling with hoarseness and new oxygen requirement.
The patient initially had a right cheek skin pigmented lesion diagnosed as nodular melanoma in July 2015. She subsequently underwent tumor resection, and sentinel lymph node biopsy showed 0/4 positive lymph nodes. She was then started on active surveillance. In December 2016, the patient noticed a lump near the right parotid gland, and positron emission tomography/computed tomography (PET/CT) showed 1 cm fluorodeoxyglucose (FDG) avid nodule in the subcutaneous tissue of her right lower cheek consistent with malignancy and a new 1 cm FDG avid right upper lobe lung nodule with a standardized uptake value (SUV) max of 4.5. Fine needle aspiration (FNA) of right lower cheek subcutaneous nodule revealed metastatic melanoma. The patient underwent right superficial parotidectomy, modified radical neck dissection, and excision of the right subcutaneous peri-mandibular facial mass in January 2017. In February, local radiation therapy and adjuvant Ipilimumab immunotherapy were started. However, the patient developed severe diarrhea and rash after 2 doses of ipilimumab, so ipilimumab was discontinued, and she resumed active surveillance again. In March 2018, PET/CT showed an increased right upper lobe lung mass of 3.5 cm × 3 cm with SUV max of 17.3, pembrolizumab and radiation therapy to right lung were initiated. Near resolution of right lung metastatic lesion was noted after 3 doses.
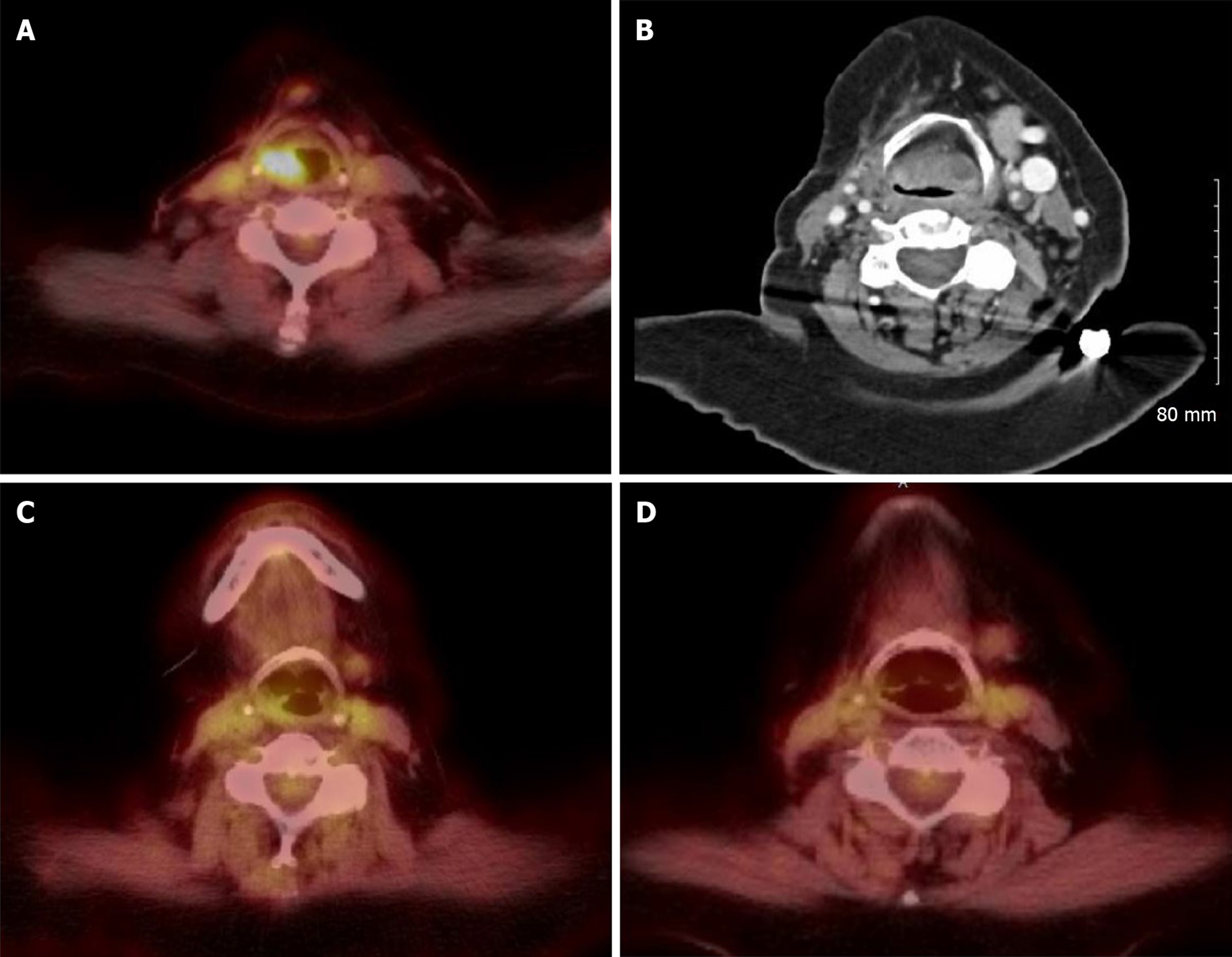
In October 2018, PET scan revealed metabolic activity in the right supraglottic area (Figure 1A). The patient was hospitalized and underwent direct laryngoscopy with biopsy which showed a developed posterior supraglottic mass with inflamed mucosa encroached over posterior supraglottic airway and generalized inflamed mucosa throughout lower pharynx. Biopsy showed friable purulent debris with focal multinucleated giant cells without any evidence of fungal, bacterial, acid fast organisms or melanoma cells, indicating a diagnosis of immunotherapy associated mucositis. Pembrolizumab was held and patient was started on prednisone 40 mg oral daily with improvement of symptoms, and she was discharged home with prednisone slow taper.
Past medical history was significant for depression, diabetes mellitus type 2, hypertension.
Height: 5’4.9” (165 cm); Weight 199.3 Lbs (90.4 kg); Body Mass Index 33.2 kg/m2. Physical examination detected copious amount of thick mucus in the oropharynx.
CT neck showed near complete occlusion of the airway due to tissue thickening of epiglottis and larynx (Figure 1B).
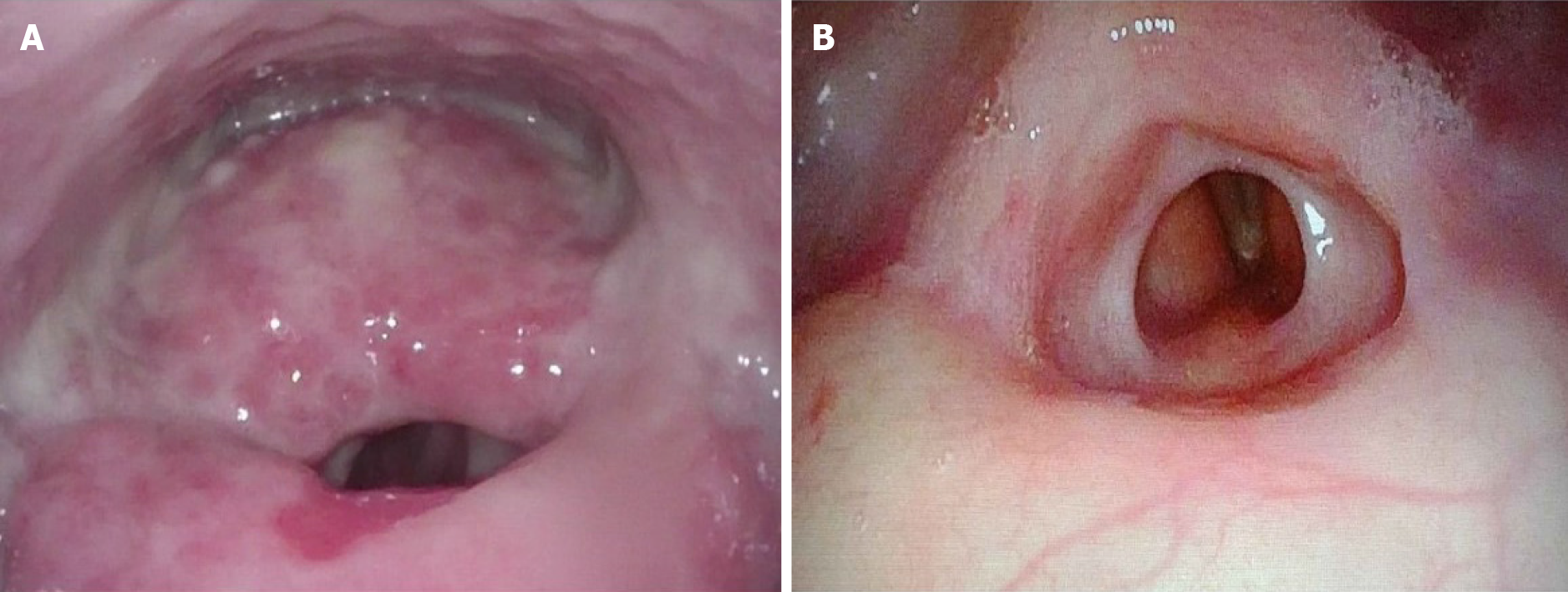
Direct laryngopharyngoscopy with biopsies of pharyngeal, supraglottic, and glottic mucosa showed impending airway obstruction with massive swelling of posterior supraglottic larynx and diffuse inflamed granular mucosa involving majority of supraglottic larynx and lower larynx.
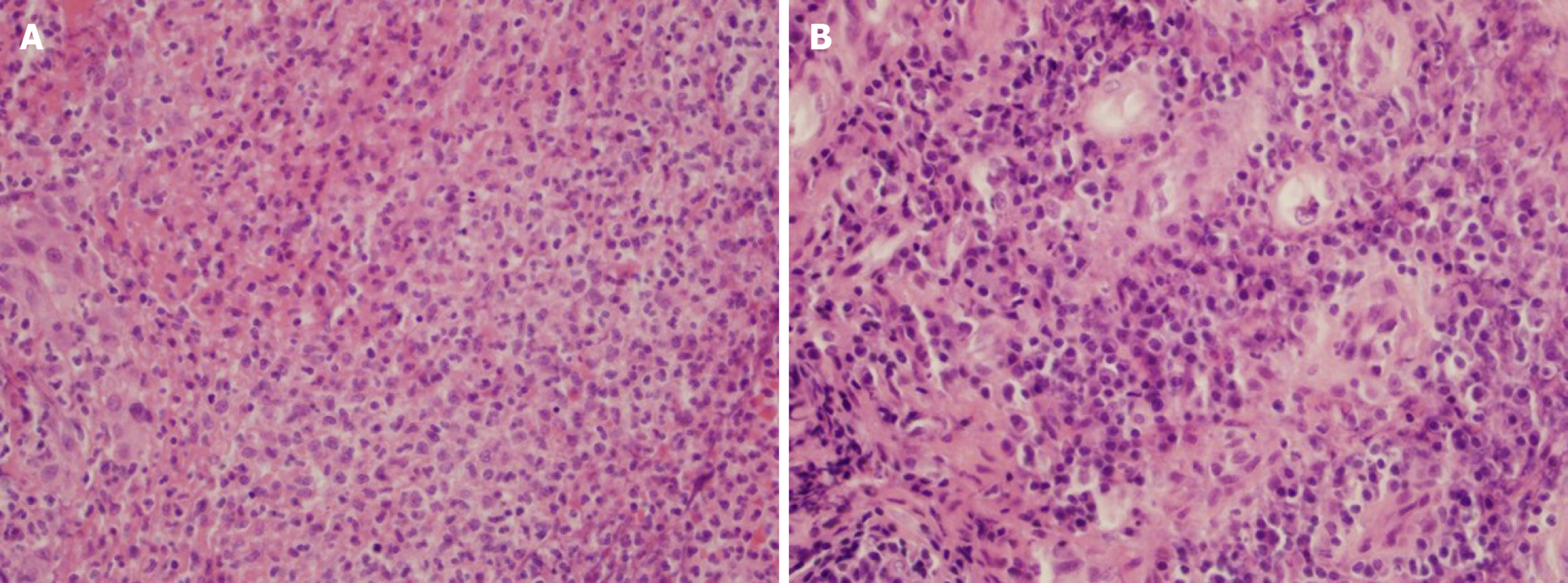
Histologic analysis of tissue biopsies demonstrated fibrinopurulent exudate and granulation tissue with acute and chronic inflammation in left and right arytenoid tissues of the larynx and also epiglottic tissue (Figure 2).
Tissue culture with Periodic acid-Schiff (PAS), Grocott-Gomori’s methenamine silver (GMS) stain, gram stain, Fite, Kinyoun, and Wright-Giemsa stains were negative for typical and atypical bacteria, acid-fast bacteria (AFB), and fungi.
Direct laryngoscopy shows threatened airway with massive swelling of posterior supraglottic larynx obliterating normal posterior supraglottic landmarks and diffuse inflamed granular mucosa with weeping character involving nearly all of supraglottic larynx and much of lower pharynx. Open tracheostomy is required because of impending airway obstruction, and direct laryngopharyngoscopy with biopsies of pharyngeal, supraglottic, and glottic mucosa is recommended for further evaluation of the nature of the supraglottic mass.
Due to lack of evidence for infection, starting empiric antibiotic is not indicated. Subsequent tissue culture with PAS, GMS stain, gram stain, Fite, Kinyoun, and Wright-Giemsa stains were negative for typical and atypical bacteria, AFB, and fungi. However, Pneumocystic Jiroveci Pneumonia prophylaxis with Bactrim is recommended until the patient is on less than 20 mg of prednisone per day.
Grade 4 pembrolizumab-induced mucositis in light of lack of evidence of malignancy or infectious etiologies.
Due to slow improvement with steroids and adverse effects from prolonged steroids use, the patient was started on infliximab 5 mg/kg intravenous (IV) at 0 and 2 wk with concurrent prednisone 40 mg oral daily. She improved remarkably shortly after the first dose of infliximab with improvement of supraglottic soft tissue on PET/CT (Figure 1C). The patient continued to be on prednisone tapering regimen with 5 mg taper every 3 wk.
She had significant improvement of supraglottic soft tissue after 2 doses of infliximab on PET/CT (Figure 1D) and repeat direct laryngoscopy (Figure 3), and no suspicious FDG avid metastasis was observed in PET/CT in June 2019. Follow-up flexible fiberoptic laryngopharyngoscopy showed clear nasal cavities, clear nasopharynx with some improvement of mucosa of lower pharynx and upper larynx. In addition, there was reduction in size of posterior supraglottic mass, however there was appearance of new contraction of tissues that narrows patient’s posterior supraglottic lumen. Both true vocal cords were visualized and maintained surprisingly good mobility and voice. The patient remained stable and has no evidence of odynophagia, dysphagia, or recurrent melanoma by the time of this paper submission.
PD-1 and PD-L1 inhibitors augment immune system’s capability to eradicate cancer cell[16-18], but multiple adverse effects have been reported, including neurologic syndromes, endocrine disorders, dermatologic symptoms, hepatitis, pneumonitis, myocarditis, and mucositis[2,14,17]. However severe mucositis remains as a very rare complication. During the randomized, open-label, section phase 3 trial of PD-1 inhibitor nivolumab, grade 3-4 mucositis was a very rare complication with only 1.3% of patients developing grade 1-2 mucosal inflammation and none developed grade 3-4 mucosal inflammation, compared with 14% all grade and 1.8% grade 3-4 mucosal inflammation in standard chemotherapy[12].
Symptoms and mechanisms of oropharyngeal mucositis are important for work up and differential diagnoses. Symptoms include odynophagia and dysphagia due to painful oropharyngeal ulcers with diffuse erythema, absence of thrush, and presence of patchy areas of mucolytic changes involving the supraglottic mucosa and true vocal folds with no evidence of neoplasm. Mechanism of pembrolizumab-induced mucositis is due to PD-1 blockage-induced changes in genes implicated in cytolysis and NK cell function, and frequency of T cells was significantly increased with CD8(+) memory T cells being the most prominent phenotype[19,20]. Biopsy in our patient shows tissue fragments containing epithelioid and multinucleated histiocytes arranged in central areas of necrosis. The inflammation is centered around vessels in some areas, consistent of T-cell infiltration of the mucosal layer. In general, immune-checkpoint inhibitor-induced adverse effects remain as an exclusion diagnosis, so differential diagnosis of infectious etiologies and immune-mediated etiologies should be considered first. In fact, workup usually involves imaging studies, direct laryngoscopy and biopsy as well as bacterial and fungal culture even in the absence of visible thrush or leukocytosis. Direct visualization and biopsy typically show ulcerated mucositis with granulation tissue and absence of infectious features and malignancy[14,15].
Treatment for pembrolizumab-related adverse effects is still very limited. Steroids remains the mainstream treatment for pembrolizumab-related adverse effects[21]. To date, only 2 cases of severe mucositis have been reported[14,15]. The first case of pembrolizumab-induced grade 4 immune mucositis and esophagitis was reported in 2018, and the adverse event resolved with discontinuation of pembrolizumab and initiation of intravenous methylprednisolone and subsequent oral prednisone tapering, although one recurrence of mucositis occurred during tapering period[14]. In a recent case report, the patient developed pembrolizumab-induced grade 4 mucositis after 1 year of treatment with 18 cycles of therapy with symptoms rapidly progressing, and the patient also developed recurrence of mucositis during the prednisone tapering period; therefore, prednisone regimen was adjusted to a prolonged and slow prednisone taper with the patient slowly achieving complete recovery from immunotherapy-mediated mucositis[15].
Our case presents an interesting scenario where the patient had limited improvement with long course of steroids treatments but responded well to infliximab. Due to the patient having refractory symptoms with steroid tapering and adverse effects with prolonged steroid use including agitation and impaired fasting glucose, we started a trial of infliximab 5 mg/kg IV at 0 and 2 wk with concurrent steroid taper starting at 40 mg oral daily. Infliximab is a genetically engineered IgG1 murine-human chimeric monoclonal antibody that binds TNF-α with high affinity[22]. TNF-α is a tightly regulated pro-inflammatory cytokine that binds TNFR1 (tumor necrosis factor receptor 1) and TNFR2 (tumor necrosis factor receptor 2) and affects two distinct signaling pathways, nuclear factor kappa-B1 (NF-kB1) pathway or the caspase-3-dependent apoptosis pathway[23,24]. Upon binding of TNF-α to TNFR1, NF-kB1 antagonizes programmed cell death (PCD) by promoting expression of antiapoptotic proteins[25]. On the other hand, pembrolizumab binds PD-1 receptor and downregulates apoptosis pathway by inhibiting Bcl-xL of Bcl-receptor family[26-28]. Therefore, blocking of TNF-α by infliximab may play a key role in upregulating apoptosis and potentially revert antiapoptotic effect of PD-1 inhibitor on immunologic cell lines. In fact, administration of TNF-α antagonist was shown to rapidly reduce cellularity at the site of inflammation within 48 h that includes macrophages, plasma cells, and T cells[29,30]. Our patient achieved complete response with 2 doses of infliximab while being on concurrent prednisone regimen. Repeat PET/CT 4 days after first dose of infliximab showed resolving right supraglottic soft tissue fullness and FDG avidity. Subsequent PET/CT taken 3 mo after 2 doses of infliximab showed complete resolution of right supraglottic soft tissue fullness and FDG avidity. It is evident that infliximab has augmented the recovery and thus prevented prolonged steroids use which has led to multiple adverse effects in this patient. Improvement of the patient may be from both steroid and infliximab, and which one plays a more important role remains to be investigated. However, addition of infliximab to the traditional steroid course provides a safe new approach to treat pembrolizumab-induced severe mucositis as patient had rapid recovery without any significant adverse effects. Patient had no relapse by the time of this paper submission.
The use of infliximab in pembrolizumab-induced adverse effects has also been reported in pembrolizumab-induced pneumonitis and Crohn’s disease. In fact, pneumonitis is also an uncommon toxicity of immune-checkpoint inhibitors, with 1.4% developed grade 3-4 pneumonitis[22]. In two recent case reports in 2018 and 2019, both patients developed severe pneumonitis following pembrolizumab treatment and were refractory to steroids treatment, one patient achieved complete response with administration of infliximab while continuing on oral prednisone[31], and the other patient rapidly improved after administration of one single dose of infliximab[32]. Similarly, in Crohn’s disease, faster symptom resolution was observed with treatment with infliximab[33] since immune-mediated mucosal inflammation is theorized secondary to recruitment of macrophages and formation of granulomas[34], which was comparable to our patient’s biopsy result that showed fibrinopurulent exudate and granulation tissue with acute and chronic inflammation. All together these findings suggest a potential role of infliximab in treating immune-related severe mucositis, pneumonitis, and potentially other adverse effects.
One of our concern is whether discontinuation of immunotherapy due to adverse events will lead to tumor recurrence and poor efficacy outcome. In a retrospective analysis of randomized phase II and III trials, similar efficacy outcomes including median progression-free survival duration were observed in melanoma patients who discontinued treatment[35]. Our patient did not have any evidence of cancer recurrence evidenced by PET/CT in June 2019, which was 8 mo after discontinuation of pembrolizumab and 3 mo after administration of infliximab with concurrent steroid taper. Another concern is if a patient has recurrent melanoma, how do we treat the patient again. In other researches, it has been showed that adverse event rate and severity are correlated with both dosing and number of immune checkpoint inhibitors[36,37], so a potential strategy would be restarting pembrolizumab at a lower dose for a longer period of time, or changing to other targeted therapies.
Our case is the first example of pembrolizumab-induced severe pharyngeal mucositis with impending airway obstruction that had limited recovery to prolonged steroids use and required intubation finally, but had rapid improvement and near complete recovery with infliximab while continuing to be on tapering steroids course. Pembrolizumab-induced severe pharyngeal mucositis is rare. The pathogenesis involves dysregulation of NK and T cell function, and mucosal cytolysis. Diagnosis remains an exclusion diagnosis, so infectious etiologies and tumor recurrence should be ruled out. Discontinuation of immunotherapy has not been shown to decrease progression-free survival duration. If melanoma relapses, a potential strategy is to decrease dose and frequency or use other targeted therapies.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American College of Physician, No. 03400848.
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Anschau F S-Editor: Ma YJ L-Editor: A P-Editor: Liu JH
| 1. | Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC, Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3360] [Cited by in RCA: 3393] [Article Influence: 212.1] [Reference Citation Analysis (0)] |
| 2. | Domingues B, Lopes JM, Soares P, Pópulo H. Melanoma treatment in review. Immunotargets Ther. 2018;7:35-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 453] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 3. | Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1007] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 4. | Batus M, Waheed S, Ruby C, Petersen L, Bines SD, Kaufman HL. Optimal management of metastatic melanoma: current strategies and future directions. Am J Clin Dermatol. 2013;14:179-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3134] [Cited by in RCA: 3537] [Article Influence: 393.0] [Reference Citation Analysis (0)] |
| 6. | Kim C, Lee CW, Kovacic L, Shah A, Klasa R, Savage KJ. Long-term survival in patients with metastatic melanoma treated with DTIC or temozolomide. Oncologist. 2010;15:765-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, Seiter S, Gore M, Aamdal S, Cebon J, Coates A, Dreno B, Henz M, Schadendorf D, Kapp A, Weiss J, Fraass U, Statkevich P, Muller M, Thatcher N. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 879] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 8. | Wilson MA, Schuchter LM. Chemotherapy for Melanoma. Cancer Treat Res. 2016;167:209-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 9. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5751] [Article Influence: 239.6] [Reference Citation Analysis (0)] |
| 10. | Bauml J, Seiwert TY, Pfister DG, Worden F, Liu SV, Gilbert J, Saba NF, Weiss J, Wirth L, Sukari A, Kang H, Gibson MK, Massarelli E, Powell S, Meister A, Shu X, Cheng JD, Haddad R. Pembrolizumab for Platinum- and Cetuximab-Refractory Head and Neck Cancer: Results From a Single-Arm, Phase II Study. J Clin Oncol. 2017;35:1542-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 514] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 11. | Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, Heath K, McClanahan T, Lunceford J, Gause C, Cheng JD, Chow LQ. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1025] [Cited by in RCA: 1310] [Article Influence: 145.6] [Reference Citation Analysis (0)] |
| 12. | Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375:1856-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3044] [Cited by in RCA: 3673] [Article Influence: 408.1] [Reference Citation Analysis (0)] |
| 13. | Nishijima TF, Shachar SS, Nyrop KA, Muss HB. Safety and Tolerability of PD-1/PD-L1 Inhibitors Compared with Chemotherapy in Patients with Advanced Cancer: A Meta-Analysis. Oncologist. 2017;22:470-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 14. | Acero Brand FZ, Suter N, Adam JP, Faulques B, Maietta A, Soulières D, Blais N. Severe immune mucositis and esophagitis in metastatic squamous carcinoma of the larynx associated with pembrolizumab. J Immunother Cancer. 2018;6:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Pelster MS, Mott F, Lewin J. Pembrolizumab-induced mucositis in a patient with recurrent hypopharynx squamous cell cancer. Laryngoscope. 2020;130:E140-E143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Bajwa R, Cheema A, Khan T, Amirpour A, Paul A, Chaughtai S, Patel S, Patel T, Bramson J, Gupta V, Levitt M, Asif A, Hossain MA. Adverse Effects of Immune Checkpoint Inhibitors (Programmed Death-1 Inhibitors and Cytotoxic T-Lymphocyte-Associated Protein-4 Inhibitors): Results of a Retrospective Study. J Clin Med Res. 2019;11:225-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 17. | Yang S, Yu KH, Palmer N, Fox K, Kou SC, Kohane IS. Autoimmune Effects of Lung Cancer Immunotherapy Revealed by Data-Driven Analysis on a Nationwide Cohort. Clin Pharmacol Ther. 2020;107:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Gill A, Perez MA, Perrone CM, Bae CJ, Pruitt AA, Lancaster E. A case series of PD-1 inhibitor-associated paraneoplastic neurologic syndromes. J Neuroimmunol. 2019;334:576980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 19. | Das R, Verma R, Sznol M, Boddupalli CS, Gettinger SN, Kluger H, Callahan M, Wolchok JD, Halaban R, Dhodapkar MV, Dhodapkar KM. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol. 2015;194:950-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 367] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 20. | Ribas A, Shin DS, Zaretsky J, Frederiksen J, Cornish A, Avramis E, Seja E, Kivork C, Siebert J, Kaplan-Lefko P, Wang X, Chmielowski B, Glaspy JA, Tumeh PC, Chodon T, Pe'er D, Comin-Anduix B. PD-1 Blockade Expands Intratumoral Memory T Cells. Cancer Immunol Res. 2016;4:194-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 314] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 21. | So AC, Board RE. Real-world experience with pembrolizumab toxicities in advanced melanoma patients: a single-center experience in the UK. Melanoma Manag. 2018;5:MMT05. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, Chaft JE, Segal NH, Callahan MK, Lesokhin AM, Rosenberg J, Voss MH, Rudin CM, Rizvi H, Hou X, Rodriguez K, Albano M, Gordon RA, Leduc C, Rekhtman N, Harris B, Menzies AM, Guminski AD, Carlino MS, Kong BY, Wolchok JD, Postow MA, Long GV, Hellmann MD. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol. 2017;35:709-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 819] [Article Influence: 91.0] [Reference Citation Analysis (1)] |
| 23. | Mitoma H, Horiuchi T, Tsukamoto H, Ueda N. Molecular mechanisms of action of anti-TNF-α agents - Comparison among therapeutic TNF-α antagonists. Cytokine. 2018;101:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 211] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 24. | Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 1144] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 25. | Fan Y, Dutta J, Gupta N, Fan G, Gélinas C. Regulation of programmed cell death by NF-kappaB and its role in tumorigenesis and therapy. Adv Exp Med Biol. 2008;615:223-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Barbee MS, Ogunniyi A, Horvat TZ, Dang TO. Current status and future directions of the immune checkpoint inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology. Ann Pharmacother. 2015;49:907-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 27. | de Almagro MC, Vucic D. The inhibitor of apoptosis (IAP) proteins are critical regulators of signaling pathways and targets for anti-cancer therapy. Exp Oncol. 2012;34:200-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Clem RJ, Cheng EH, Karp CL, Kirsch DG, Ueno K, Takahashi A, Kastan MB, Griffin DE, Earnshaw WC, Veliuona MA, Hardwick JM. Modulation of cell death by Bcl-XL through caspase interaction. Proc Natl Acad Sci USA. 1998;95:554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 410] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 29. | Catrina AI, Trollmo C, af Klint E, Engstrom M, Lampa J, Hermansson Y, Klareskog L, Ulfgren AK. Evidence that anti-tumor necrosis factor therapy with both etanercept and infliximab induces apoptosis in macrophages, but not lymphocytes, in rheumatoid arthritis joints: extended report. Arthritis Rheum. 2005;52:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 194] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 30. | Smeets TJ, Kraan MC, van Loon ME, Tak PP. Tumor necrosis factor alpha blockade reduces the synovial cell infiltrate early after initiation of treatment, but apparently not by induction of apoptosis in synovial tissue. Arthritis Rheum. 2003;48:2155-2162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 167] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 31. | Andruska N, Mahapatra L, Hebbard C, Patel P, Paul V. Severe pneumonitis refractory to steroids following anti-PD-1 immunotherapy. BMJ Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Sawai Y, Katsuya Y, Shinozaki-Ushiku A, Iwasaki A, Fukayama M, Watanabe K, Nagase T. Rapid temporal improvement of pembrolizumab-induced pneumonitis using the anti-TNF-α antibody infliximab. Drug Discov Ther. 2019;13:164-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Johnson DH, Zobniw CM, Trinh VA, Ma J, Bassett RL, Abdel-Wahab N, Anderson J, Davis JE, Joseph J, Uemura M, Noman A, Abu-Sbeih H, Yee C, Amaria R, Patel S, Tawbi H, Glitza IC, Davies MA, Wong MK, Woodman S, Hwu WJ, Hwu P, Wang Y, Diab A. Infliximab associated with faster symptom resolution compared with corticosteroids alone for the management of immune-related enterocolitis. J Immunother Cancer. 2018;6:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 34. | Qin X. Why is damage limited to the mucosa in ulcerative colitis but transmural in Crohn's disease? World J Gastrointest Pathophysiol. 2013;4:63-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Schadendorf D, Wolchok JD, Hodi FS, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Chesney J, Robert C, Grossmann K, McDermott D, Walker D, Bhore R, Larkin J, Postow MA. Efficacy and Safety Outcomes in Patients With Advanced Melanoma Who Discontinued Treatment With Nivolumab and Ipilimumab Because of Adverse Events: A Pooled Analysis of Randomized Phase II and III Trials. J Clin Oncol. 2017;35:3807-3814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 346] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 36. | Shoushtari AN, Friedman CF, Navid-Azarbaijani P, Postow MA, Callahan MK, Momtaz P, Panageas KS, Wolchok JD, Chapman PB. Measuring Toxic Effects and Time to Treatment Failure for Nivolumab Plus Ipilimumab in Melanoma. JAMA Oncol. 2018;4:98-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 37. | Sznol M, Ferrucci PF, Hogg D, Atkins MB, Wolter P, Guidoboni M, Lebbé C, Kirkwood JM, Schachter J, Daniels GA, Hassel J, Cebon J, Gerritsen W, Atkinson V, Thomas L, McCaffrey J, Power D, Walker D, Bhore R, Jiang J, Hodi FS, Wolchok JD. Pooled Analysis Safety Profile of Nivolumab and Ipilimumab Combination Therapy in Patients With Advanced Melanoma. J Clin Oncol. 2017;35:3815-3822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 236] [Article Influence: 29.5] [Reference Citation Analysis (1)] |