Published online Sep 26, 2020. doi: 10.12998/wjcc.v8.i18.3956
Peer-review started: April 27, 2020
First decision: June 8, 2020
Revised: June 10, 2020
Accepted: August 25, 2020
Article in press: August 25, 2020
Published online: September 26, 2020
Processing time: 147 Days and 13.7 Hours
Since the appearance of the novel coronavirus (severe acute respiratory syndrome-coronavirus-2) and related coronavirus disease 2019 (COVID-19) in China in December 2019, a very high number of small and large patient series have been published in literature from around the world. Even though the classical presentation of COVID-19 is one with respiratory symptoms with or without pneumonia that can be self-limiting or evolve into severe respiratory distress syndrome with multiple organ failure, and secondary bacterial sepsis, a large body of evidence suggests a plethora of other types of clinical presentation. In this exhaustive review, we reviewed all of the published literature on COVID-19 to identify different types of clinical presentations affecting various organ systems, to provide an in-depth analysis that may prove useful for clinicians and health-workers on the frontline, battling the severe pandemic.
Core Tip: The novel severe acute respiratory syndrome-coronavirus-2 virus causing coronavirus disease 2019 (COVID-19) pandemic can present with typical or atypical symptoms and signs. Classical clinical presentation includes fever, cough, and sore throat with or without other associated symptoms such as anorexia, nausea, and lethargy in the presence or absence of pneumonia, which is commonly noted as ground-glass opacities on chest imaging. Gastrointestinal and hepatic involvement is very non-specific, but diarrhoea is known to predominate in some patients in the absence of typical symptoms and signs. Other systems involvement include electrographic abnormalities associated with clinical events such as syncope, viral myocarditis-related cardiac failure, meningoencephalitis, and acute inflammatory demyelinating polyneuropathy, a dermatological presentation with diffuse urticaria or viral exanthem, smell and taste dysfunction and local and systemic venous thromboembolism. Other rare presentations include keratoconjunctivitis and otitis media. Physicians caring for, and battling on the frontlines against COVID-19 should be aware of the “many faces” that this singular disease can present with, for timely diagnosis and prompt initiation of best treatment options.
- Citation: Philips CA, Mohan N, Ahamed R, Kumbar S, Rajesh S, George T, Mohanan M, Augustine P. One disease, many faces-typical and atypical presentations of SARS-CoV-2 infection-related COVID-19 disease. World J Clin Cases 2020; 8(18): 3956-3970
- URL: https://www.wjgnet.com/2307-8960/full/v8/i18/3956.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i18.3956
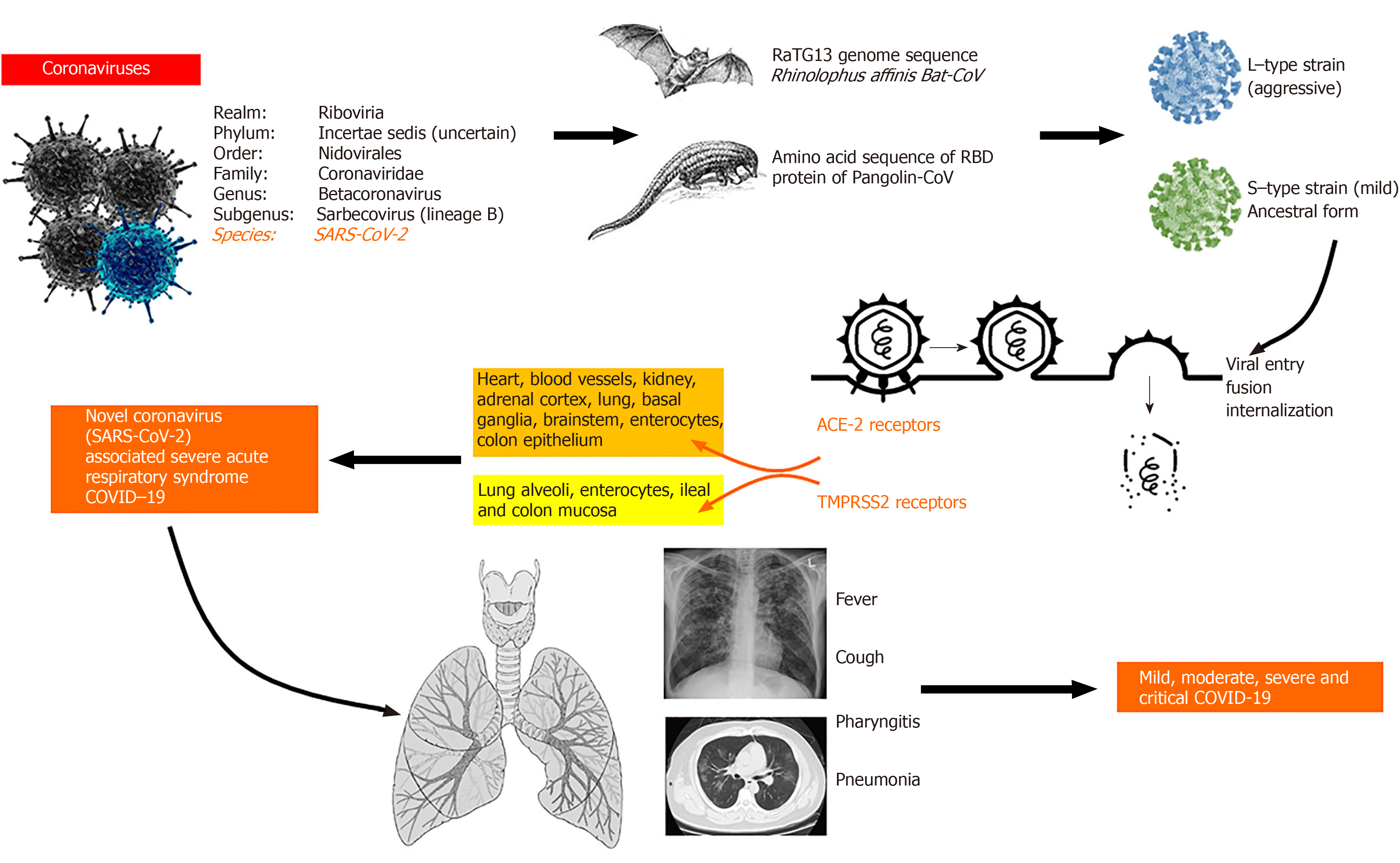
In December 2019, health authorities in Wuhan, the capital of central China’s Hubei province, reported cases of “pneumonia of unknown origin”. The etiological agent was identified as a novel coronavirus (nCoV) by the Chinese Center for Disease Control on January 7, 2020. The novel coronavirus was named severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) by the International Committee on Taxonomy of Viruses because its clinical presentation was similar to that of the SARS virus of 2003. In February 2020, the World Health Organization (WHO) renamed the disease as coronavirus disease 2019 (COVID-19), an acronym of coronavirus disease 2019. In the early weeks of March 2020, the WHO declared COVID-19 a pandemic[1,2]. At the time of this manuscript preparation, a total of 2422278 cases and 165924 deaths due to COVID-19 were reported worldwide (data extracted on 20/4/2020 from http://www.worldometers.info/coronavirus/). The nCoV belongs to the Nidovirales order and is a single-stranded virus with a crown-like appearance due to its spiked glycoprotein envelope. The sub-family Orthocoronavirinae comprises four genera namely the α-coronavirus, β-coronavirus, δ-coronavirus and γ-coronavirus. β-coronaviruses are divided into five lineages. The A-lineage of β-coronaviruses and α-coronaviruses cause self-limiting upper respiratory tract infections, while the B-lineage (currently designated Sarbecovirus) is associated with SARS and COVID-19 pandemics. The C-lineage was responsible for the middle-east respiratory syndrome (MERS) (camel flu, caused by the MERS-CoV) of 2012. To date, including the novel SARS-CoV-2, seven coronaviruses have been identified to cause infections in humans[1-3]. The nCoV is sensitive to heat and ultraviolet radiation, inactivated by lipid solvents such as chloroform and ether, and is resistant to chlorhexidine. A plausible explanation for the origin of SARS-CoV-2 is either natural selection in an animal host before the zoonotic transfer or natural selection in humans following the zoonotic transfer. Even though the first cases of COVID-19 were linked to the Huanan market in Wuhan and epidemiologists contemplate that an animal source of spread was likely present at this location this theory remains unproven. The nCoV is very similar to the bat SARS-CoV-like virus, and the likelihood of bats being reservoirs of the current pandemic is high. It was shown that the RaTG13 genome sequence isolated from Rhinolophus affinis bat species was approximately 96% identical to the SARS-CoV-2 genome. Nonetheless, the receptor-binding domain of the SARS-CoV-like virus spike protein in the bat species demonstrated divergence, which made is less efficient in binding to the human angiotensin-converting enzyme-2 (ACE-2) receptor[2-4]. The mechanism of nCoV infection includes requisite binding of the virus to the membrane-bound form of ACE-2 receptors and internalization of the complex within the host cell. In humans, the tissues that express ACE-2 receptors include that of the lung, heart, kidney, brain, and the gut. Amid discrepancy with purely bat-related mutational selection and transmission, researchers discovered genomic and evolutionary evidence on the occurrence of a SARS-CoV-2-like coronavirus in dead Malayan pangolins (called Pangolin-CoV). The discovered sequences were 91.02% and 90.55% identical to SARS-CoV-2 and Bat-CoV RaTG13, respectively, at the whole genome level. Furthermore, six key amino acid residues of the receptor-binding domain protein, involved in the interaction with human ACE-2 receptors were fully consistent between Pangolin-CoV and SARS-CoV-2. Nonetheless, four amino acid mutations were found in the bat RaTG13 genome sequence, implicating pangolins as possible natural reservoirs and intermediate mammalian hosts of the nCoV. Hence, an interplay between natural selection or mutations between bat and pangolin reservoirs and the subsequent transfer to humans may plausibly explain the emergence of the COVID-19 virus[3-6]. In a population genetic analysis of 103 SARS-CoV-2 genomes, two prevalent evolutionary types of SARS-CoV-2–the L type (approximately 70%) and the S type (30%) were identified. The strains in L type, thought to be derived from the S type (a milder ancestral form), were considered evolutionarily more aggressive and contagious. Nonetheless, this study and its findings came under severe scrutiny for the lackadaisical methodology and conclusions made based on “statistical artifacts”. Experts have cautioned against the portrayal of the two-strain COVID-19 virus theory[7,8]. It is believed, pending further confirmatory evidence, that human intervention might have caused severe selective pressure on the L type. Aggressive control of such host environments and activities could result in a selective increment in the S type, leading to a less aggressive disease course in subsequent infections. This could explain the phenomenon of variable death rates noted in some heavily infected populations[9] (Figure 1).
The average incubation period of SARS-CoV-2 is between 2 to 7 d, extending up to 24 d in some studies. The major route of human to human transmission is through respiratory droplets, and COVID-19 patients, including asymptomatic cases, remain the largest source for the spread of infection[10]. The basic reproduction number or R0, which is the expected number of cases directly generated by one case in a population where all individuals are susceptible to infection in the absence of prior infection and active or passive immunization for SARS-CoV-2 is 2.2. Transmission of the virus from contaminated articles has been described, and the possibility of faecal-oral transmission exists. The latter is contemplated given detection of the SARS-CoV-2 nucleic acid in faeces and urine of infected patients[11,12]. Caution regarding the possibility of transmission of the virus through conjunctival secretions and tears was put forth by certain authors but simultaneously refuted by others pending further confirmation in well-controlled large cohort studies[13]. Approximately 95% of patients develop symptoms within 12.5 d of contact while others remain asymptomatic, but continue to transmit the virus and could ultimately develop symptoms after a longer duration after initial exposure[1,3,14,15]. Most affected individuals are middle-aged (49 to 59 years) males, more than half of whom have an underlying chronic comorbid condition such as diabetes mellitus, systemic hypertension, chronic kidney disease, malignancy, or chronic lung disease. The case fatality rate of COVID-19 in patients with comorbid illnesses was demonstrated to be higher. The disease spectrum ranges from an asymptomatic carrier to severe respiratory failure leading to multiple organ dysfunction. However, a multitude of clinical presentations has been described. Common symptoms include fever, loss of appetite, nausea, myalgia, and arthralgia. Some authors have divided COVID-19 clinically into mild, moderate, severe, and critical stages. In the moderate stage, symptoms and signs of respiratory involvement predominate, worsening to respiratory failure in severe COVID and full-blown acute respiratory distress syndrome (ARDS) requiring mechanical ventilation and multiple organ failure requiring additional salvage support with a high likelihood of death in the critical stage[16,17]. Two phases of immune responses decide the outcome of patients with COVID-19[16-18]. Early in the disease, during incubation and the mild symptomatic stage, adaptive immune responses predominate. In hosts with good general health and favourable genetic predisposition (HLA status that remains undefined in SARS-CoV-2), antiviral immunity through adaptive responses promotes early recovery. In overwhelming viral replication, when adaptive immunity fails, tissue destruction in organ systems expressing ACE-2 receptors occurs. This tissue damage results in innate immunity-mediated inflammation arbitrated by macrophages and granulocytes, largely concentrated in the cardiopulmonary system that leads to life-threatening events. This cytokine release syndrome and associated poor prognosis are notable in COVID-19 patients with severe lymphocytopenia, manifested by high interleukin–6 levels. A drastic reduction in the numbers of CD4+ and CD8+ T cells and natural killer cells; a lower percentage of circulating functional monocytes, eosinophils, and basophils along with neutrophilia and an increase in neutrophil to lymphocyte ratio is seen in the innate immunity-mediated inflammatory stage of COVID-19. In patients who become refractory to aggressive medical and supportive care, upregulation of markers of immune cell exhaustion (for example, the NKG2A, on cytotoxic lymphocytes) has been demonstrated[19-21]. Indeed, it has been proposed that the use of extracorporeal membrane oxygenation (ECMO) in patients with severe lymphocytopenia in the immune exhaustion phase could be deleterious since ECMO itself has been associated with a reduction in the circulating lymphocyte population[22]. The diagnosis of COVID-19 requires serology confirmation, along with classic symptoms and signs. Serology can be performed on nasopharyngeal swabs or blood samples. An enzyme-linked immunosorbent assay (ELISA) or immuno-chromatography (card test) could be utilized. The ELISA, based on the Rp3 nucleoprotein detection of immunoglobulin M (active infection) or G (prior infection), has higher sensitivity than the card test. The latter has a more rapid turnover, but both have 100% specificity. Additionally, ELISA has high false-positive rates due to the cross-reactivity to viral nucleocapsid of other SARS viruses in the presence of shared sequence homology. Hence, ELISA specific to SARS-CoV-2 virus should ideally be towards S-protein, which is the transmembrane glycoprotein spike. The gold standard for the COVID-19 confirmatory test is the reverse-transcriptase polymerase chain reaction (RT-PCR) which has a sensitivity of approximately 90% on day one to three of infection, 80% on day 4 to 6 and < 50% after two weeks of the onset of symptoms. The best sample for a good viral load yield is the bronchoalveolar lavage fluid, but getting results may take a few days compared to a few hours of turn-around-time of serology-based tests. It was shown that pharyngeal virus shedding was very high during the first week of symptoms, peaking at 7.11 × 108 viral RNA copies per throat swab on the fourth day[5,23]. COVID-19 can present with non-specific and sometimes surprising symptoms and signs, and hence epidemiological factors such as exposure and close contact history remain the most important tools for detection. In the following sections, we review all typical and atypical presentations of COVID-19 that are useful for clinicians caring for patients on the pandemic battle frontline.
Patients with COVID-19 typically present with pharyngitis (sore throat) and dry cough along with the prodrome. Respiratory involvement can be in the form of mild upper tract symptoms and signs in the absence of pneumonia, mild pneumonia, severe pneumonia necessitating high dependency care or critical illness pneumonia requiring ventilatory support that can progress to multiple organ failure with or without superadded bacterial sepsis[24,25]. Patients with mild disease have only fever and associated prodrome without respiratory symptoms even though chest signs could still be present. In moderate COVID-19 pneumonia, respiratory symptoms and signs accompany early radiological features. Progression to severe pneumonia is underscored by the occurrence of respiratory distress with a respiratory rate above 30/min, peripheral capillary oxygen saturation (SpO2) < 93% at rest, the ratio of arterial oxygen partial pressure (PaO2 in mmHg) to fractional inspired oxygen (FiO2) ≤ 300 mmHg and rapid radiological disease progression (> 50% from admission). In patients who have all of the above features along with respiratory failure needing mechanical ventilation, shock, or extrapulmonary organ failure, critical COVID-19 is diagnosed[17,18,26,27]. Pneumonia in COVID-19 patients with pre-existing chronic lung disease and smokers can be catastrophic, leading to severe cardiopulmonary dysfunction and a high mortality rate. Patients can also be COVID-19 positive with detectable viral nucleic acids on RT-PCR assay but without symptoms. On the other hand, radiological changes in PCR-negative SARS-CoV-2 in the presence of virus-specific immunoglobulins G and M have been described. A small proportion of COVID-19 patients can also present with silent hypoxemia. These patients do not present with overt clinical symptoms but can develop symptoms necessitating urgent mechanical ventilation on follow-up. In this regard, some authors advise blanket oxygen saturation screening at presentation in suspected or confirmed COVID-19 cases to facilitate diagnosis and prognosis, respectively[28,29]. High resolution computed tomography (CT) of the chest plays a vital role in early disease detection and evaluation. The typical findings on CT include peripherally, and sub-pleural distributed multifocal ground-glass opacities (GGOs) with patchy consolidation with a predilection for involvement of the posterior part and lower lobes of the lungs. Some patients develop bilateral multifocal consolidation that represents “crazy-pavement” appearance that, along with pleural effusion, when extensive, can demonstrate “white drowned lung”. Patients rarely present with isolated consolidation, air-bronchogram sign, reverse “halo-sign”, vascular enlargement sign, or fine reticulations on high-resolution CT. According to current literature, the most common radiological presentations include GGOs, followed by GGOs with consolidation and isolated consolidation. Findings that differentiate COVID-19 pneumonia from other viral causes of pneumonia include a predominance of peripheral findings, GGOs, and, when present, reticulations with vasodilatation. Nonetheless, RT-PCR must be performed for confirmation of coronavirus pneumonia. The classical findings on chest CT imaging can also occur in asymptomatic patients. Nevertheless, cavitary lesions, calcifications, and lymphadenopathy are strikingly absent in COVID-19 lung disease[30-32]. Xu et al[33] described the lung pathology in a patient who died of severe COVID-19 pneumonia. The pathological features resembled SARS and MERS infection revealing diffuse alveolar damage with cellular fibromyxoid exudates, pneumocyte desquamation, and hyaline membrane formation. The pulmonary inflammation was predominantly interstitial and dominated by lymphocytes and multinucleated syncytial cells within the intra-alveolar spaces[33]. Apart from the typical lung symptoms and signs, Wang et al[34] described a PCR positive COVID-19 patient who developed spontaneous pneumomediastinum with subcutaneous emphysema that resolved uneventfully after aggressive medical management[34].
In COVID-19, ARDS can secondarily lead to a wide range of arrhythmias, shock, and heart failure. Nonetheless, primary cardiac involvement in COVID-19 has been described recently[35]. A report from Italy described the case of an otherwise healthy 53-year-old woman with PCR positive COVID-19 who presented with fever and cough. She was diagnosed with acute myopericarditis with systolic dysfunction that was confirmed on cardiac magnetic resonance (MR) imaging in the absence of respiratory involvement[36]. The patient was hypotensive, and electrocardiography (ECG) showed diffuse ST-segment elevation, had increased high-sensitivity troponin T and NT-pro-BNP (N-terminal pro-brain natriuretic peptide) along with biventricular edema on MR imaging. Even though the patient improved symptomatically, the outcome was not reported. Chinese authors from Chengdu described a COVID-19 patient with fulminant myocarditis associated with pneumonia, pleural effusion, and ECG features suggestive of acute myocardial infarction. Early initiation of systemic corticosteroids along with intravenous immunoglobulins resulted in complete recovery[37]. Two COVID-19 patients with ECG features of S1T3Q3 pattern with reversible nearly complete atrioventricular block and ST-segment elevation associated with multifocal ventricular tachycardia, respectively, were described by He et al[38]. While the former patient improved and was discharged to home, the second patient died in hospital. The authors proposed ACE-2 receptor-related signaling, hypoxemia, and the presence of severe systemic inflammation as important factors associated with ECG involvement in their COVID-19 patients. Sustained ventricular tachycardia or ventricular fibrillation was reported in 6% of patients at hospitalization in a study by Guo et al[39]. Chang et al[40] described a 49-year-old man who presented with syncope after a day of fever. At admission, an ST-segment elevation myocardial infarction was contemplated, but echocardiogram and coronary angiogram were normal. Serial ECGs were suggestive of type 2 Brugada pattern, and, in the presence of related clinical symptoms, the patient underwent coronary care unit management leading to complete recovery[40]. Large series on COVID-19 patients have demonstrated evidence of acute myocardial injury (increased high-sensitive cardiac troponin I above the 99th percentile upper reference limit), shock and non-specific and classical arrhythmias in the presence of severe lung disease, the majority of whom required intensive care management and poor clinical outcome. A patient with COVID-19 primarily presenting with cardiogenic shock due to acute cardiac injury requiring mechanical ventilation and venous-arterial ECMO who eventually died of secondary bacterial pneumonia was described by Tavazzi et al[41]. This was the first reported case of COVID-19 causing direct cardiac injury, underscored by the fact that endomyocardial biopsy showed low grade interstitial and endocardial inflammation along with CD68-positive macrophages and SARS-CoV-2 particles in the absence of myocyte necrosis. Ischemic and non-ischemic myocardial injury in COVID-19 was significantly associated with fatal outcomes especially in those with underlying cardiovascular disease associated with severe cardiac dysfunction and life-threatening arrhythmias and sudden death during the severe or the resolving phase of infection, in which persistent severe systemic inflammation has been proposed as the potential adverse mechanism[42].
Gastrointestinal symptoms are common in COVID-19 (ranging from 3% to 20%) and include loss of appetite, nausea, vomiting, abdominal pain, and diarrhoea (very rarely bloody stools reported in 4% of patients in the series by Wan et al[43]), along with respiratory symptoms. Nonetheless, a group of COVID-19 patients can present with isolated diarrhoea. The SARS-CoV-2 can be detected in the faeces in about 50% of infected subjects in the absence of a clear correlation between digestive symptoms and detection of the virus in stool. Han and colleagues described a unique sub-group of COVID-19 patients with mild or no respiratory disease and lower disease severity presenting with gastrointestinal symptoms. These patients had a higher likelihood of detection of viral RNA in their faeces with longer duration for viral clearance. The onset of respiratory symptoms and fever lagged behind digestive symptoms and, in some patients, never occurred[44]. Pan and colleagues showed that 18.6% of patients with COVID-19 presented with a gastrointestinal-specific symptom, including diarrhoea, vomiting, or abdominal pain, with a long time from onset to admission and diagnosis compared to those without digestive symptoms. With increasing disease severity, the gastrointestinal symptoms and signs worsened, and such patients had elevated liver enzymes, monocytopenia, and more coagulation abnormalities[45]. ACE-2 receptors were highly expressed in the proximal and distal enterocytes of the small intestine. Successful viral entry depends not only on the presence of this receptor but also on the transmembrane protease serine 2 (TMPRSS2) receptor, which is critical for the fusion of viral and cellular membranes. The ACE-2 and TMPRSS2 are co-expressed in the lung alveolar type 2 cells and upper esophageal epithelial lining as well as ileum and colon mucosa[46,47]. Pautrat et al[48] described a patient with SARS-CoV-2 infection, presenting as an appendicular syndrome (appendicitis on clinical examination without radiological evidence). However, even though the CT chest was suggestive of COVID-19, reports of RT-PCR confirmation was not reported by the authors. In patients with COVID-19, gastrointestinal symptoms are not uncommon, and CT imaging of the chest followed by RT-PCR confirmation from nasopharyngeal swabs is mandatory in the current pandemic setting.
The primary and direct involvement of the liver in COVID-19 has not yet been convincingly reported. The related signs of liver involvement mainly stem from secondary damage due to the disease or its treatment, and there is no clear proof that the virus causes hepatocyte damage[49]. A wide range of liver test abnormalities has been identified in patients with COVID-19 and most commonly involve a rise in alanine transaminase and aspartate transaminase. These liver test abnormalities have been identified in approximately 10% to 50% of patients, depending on the study series. Hyperbilirubinaemia and an increase in prothrombin time were notable in patients with severe disease and possibly represented advanced disease associated with multiple organ failure, sepsis, drug-induced liver injury due to experimental antivirals and antibiotics and severe refractory hypoxia. Acute liver failure has not been described in SARS-CoV-2 infection[50,51]. Liver histopathology of COVID-19, as described via post autopsy and minimally invasive post mortem biopsies, was characterized by microvesicular steatosis and non-specific lobular and portal inflammation, which could have been treatment-related, associated with the underlying comorbid condition or concomitant sepsis[52-54]. In a group of patients described from the Wuhan epicenter, one patient with severe COVID-19 was found to have severe hepatitis with a maximum elevation of serum alanine transaminase of 7590 U/L, which could have been secondary to hypoxic liver injury due to critical illness. Even though the virus entry ACE-2 receptors are highly upregulated in the liver, direct organ damage due to viral replication and tissue destruction has not been demonstrated. In this regard, the hepatic involvement of COVID-19 is probably secondary to multiple organ dysfunction associated with disease progression or treatments utilized, such as the use of lopinavir and ritonavir or high dose hydroxychloroquine, or the presence of underlying chronic liver disease. Authors have suggested that COVID-19-induced hepatic damage is only a “clinical distraction”, and focus needs to be maintained on managing the patient as a whole and not based on single organ systems[55-57]. Wander et al[58] described a 59-year-old COVID-19 patient with underlying human immunodeficiency virus infection, metabolic syndrome, and Grave’s disease, primarily presenting with acute non-icteric hepatitis. The patient recovered uneventfully in the hospital[58]. Huang et al[59] reported the fatal outcome of a liver transplant recipient after SARS-CoV-2 infection in whom multiple organ failure progressed rapidly. Nonetheless, primary liver involvement was not reported in this case. Clinicians caring for COVID-19 patients must evaluate liver tests at admission and closely monitor those with deteriorating liver functions, as the latter may be the harbinger of progressive respiratory disease, the beginning of multiple organ failure or an associated drug-induced liver injury.
Neurological involvement among patients with COVID-19 has been reported in multiple series worldwide. Almost all of the patients were critically ill, and the symptoms and signs included confusion, diffuse corticospinal tract involvement (exaggerated tendon reflexes, clonus, and extensor plantar responses), presence of dysexecutive syndrome with inattention, disorientation, and unorganized movements. Focal signs were notably absent in these patients, and MR imaging revealed enhancement of leptomeningeal spaces, bilateral frontotemporal hypoperfusion and acute ischemic stroke features with focal hyperintensity on diffusion-weighted images. Cerebrospinal fluid analysis was non-contributory and RT-PCR for viral RNA was negative in cerebrospinal fluid samples[60,61]. Neuropsychiatric manifestations evidenced by altered behaviour, dissociated and contextually flawed speech responses and psychosis have been reported in COVID-19 patients[62]. Other non-specific features include headache, altered level of consciousness and dizziness that are related to the severity of systemic illness, while specific features reported were loss of sense of smell or taste, myopathy, ataxia, stroke, and convulsions.
Furthermore, a plethora of severe neurological manifestations has been described with COVID-19 critical illness, most of which are partly related to the underlying chronic comorbid condition such as cerebrovascular events–ischemic or haemorrhagic stroke, hypoxic encephalopathy and drug-induced neurotoxicity[63-66]. Viral infiltration of the brain stem in patients with COVID-19 was demonstrated by Li et al[67]. The first case of SARS-CoV-2 infection and associated Guillan-Barre syndrome was reported by Zhao et al[68]. In this patient, the respiratory symptoms and signs along with fever occurred at a later stage during hospitalization. Contacts of the patient also tested positive for COVID-19. Recovery was complete with the use of supportive care and intravenous immunoglobulin. A similar case was reported from Iran in which a 65-year-old male presented with rapidly progressive acute bilateral symmetric quadriparesis. He tested positive for COVID-19, and further imaging revealed bilateral GGOs and consolidation. Immunoglobulin therapy improved the patient’s outcome[69]. A probable case of meningitis associated with the novel coronavirus infection was reported from Japan. The patient presented with convulsions followed by loss of consciousness. However, the authors were unable to confirm viral RNA detection on nasopharyngeal swab tests, and brain MR imaging revealed mesial temporal lobe and hippocampus hyperintensities. Serology for other viruses was non-contributory, and hence COVID-19 was made as a diagnosis of exclusion[70]. A similar case of intracerebral haemorrhage in a COVID-19 patient was also reported but could have been more of an association with critical illness rather than direct causation[71]. Other neurological presentations of COVID-19 reported include Miller-Fisher syndrome, polyneuritis cranialis, and acute onset encephalopathy[72,73]. In summary, COVID-19 may present with meningoencephalitis features, loss of smell and taste (described in the next section), and acute inflammatory demyelinating polyneuropathy is a subgroup of patients. Other manifestations are likely to be associated with severe disease and may not be due to direct viral effect.
The American Academy of Otolaryngology and the British Association of Otorhinolaryngology recommended that anosmia with or without dysgeusia be added to the list of primary screening symptoms for COVID-19 given the frequent association of these among patients reported from epicenters[74]. Eliezer et al[75] described a woman in her 40 s, with COVID-19, who presented with bilateral obstructive inflammation of olfactory clefts on MR imaging, which severely impaired olfactory function[75]. Olfactory dysfunction has been recognized in patients with severe disease and self-identified by patients after a prolonged latency period. Clinicians practicing in COVID-19 red zones must be aware that acute onset loss of smell and taste in the presence of a patent nasal airway should raise a high degree of suspicion for SARS-CoV-2 testing. Classic nasal cavity symptoms such as rhinorrhoea, itchy erythematous nasal cavity, and nasal congestion associated with common viral respiratory disease seldom occur in COVID-19. Hypogeusia and hyposmia in patients without prior ear, nose, or throat disorders are strong pointers toward the clinical diagnosis of COVID-19. The explanation for smell and taste dysfunction in COVID-19 is believed to be the direct virus damaging effects on gustatory receptors and olfactory mucosa. Olfactory and taste dysfunction can occur during the early or late course of the disease, and can also be the only presenting symptom that could portend severe disease in those with a prolonged disease course[76-79]. Acute otitis media with otalgia and tinnitus as the sole presentation of COVID-19 in a 35-year-old woman was reported from Turkey. Audiometry revealed conductive hearing loss with a type-b appearance on tympanometry and bilateral GGOs on chest imaging[80].
Cheema et al[81] reported a healthy young woman with COVID-19 presenting with right-sided keratoconjunctivitis and mild respiratory symptoms in the absence of fever. The conjunctival swab of the affected eye was positive for the SARS-CoV-2 virus[81]. An update issued by the American Academy of Ophthalmology discussed conjunctivitis as a presenting symptom of COVID-19 in affected patients, and large series on 1099 COVID-19 patients described conjunctival congestion as an associated symptom in 0.8%[82]. In the study by Wu et al[83], 31.6% of COVID-19 patients had ocular manifestations in the form of conjunctivitis with conjunctival hyperemia, chemosis, epiphora, or excessive secretions. Univariate analysis of patients with ocular manifestations demonstrated a higher likelihood of leucocytosis, raised procalcitonin, C-reactive protein level and lactate dehydrogenase compared to those without eye symptoms. Nonetheless, Seah et al[84] showed that the risk of SARS-CoV-2 transmission through tears was probably low. Guo et al[85] also described the reduced potential of SARS-CoV-2 transmission through conjunctival secretions as detectable viral load in the conjunctival sac of affected patients was very low in the absence of strong evidence for local site replication[85]. In COVID-19, the eye symptoms and signs are rare associations and even rarer index or presenting manifestations of the disease.
Mounting evidence from multiple countries suggests that SARS-CoV-2 infection has the potential to promote hypercoagulability at the local and systemic levels, even leading to instances of stroke in young persons. In severe progressive disease, this coagulation profile switches to one of disseminated intravascular coagulation, a harbinger of death. A middle-aged woman with COVID-19 who presented with severe pulmonary embolism with a clot in the patent foramen ovale was reported from France. Emergency embolectomy and extracorporeal life support failed to save the patient. Retrospective CT evaluation revealed multiple GGOs in both lungs[86]. In the absence of other classical respiratory or gastrointestinal symptoms, acute presentations linked to the thrombogenic state could be a presentation of COVID-19. An underlying thrombophilic state, with frequent pulmonary embolisms, was reported among SARS patients at necropsy[87]. Casey et al[88] described a 42-year-old man with COVID-19, who presented with acute shortness of breath, severe chest pain, and hemoptysis. CT angiography of the chest showed bilateral segmental pulmonary emboli with areas of consolidation of the right lower lobe. Anticoagulation and oxygen therapy improved symptoms, and patients were eventually discharged to home[88]. In severe COVID-19, it is presumed that local disseminated intravascular coagulation expressed in the lungs leads to activation of pulmonary thrombosis in the presence or absence of pneumonia followed by fibrinolysis activation and severe systemic inflammatory state[89]. Dolhnikoff et al[90] performed ultrasound-based minimally invasive autopsies on sample tissues from several organs in patients dying of COVID-19. They identified exudative and proliferative diffuse alveolar damage associated with epithelial viral cytopathic effects involving the alveoli and small airway epithelium along with lymphocytic infiltration. Multiple areas of fibrinous thrombi in small pulmonary arterioles in damaged and preserved lung areas were notable in 80% of patients. This was associated with endothelial tumefaction associated with the packing of pulmonary megakaryocytes within pulmonary capillaries[90]. Wang et al[91] found that patients at high risk of venous thromboembolism were older, required intensive unit admission, mechanical ventilation and had abnormal liver tests associated with higher levels of C-reactive protein. Zhang et al[92] reported three patients with COVID-19 in whom the disease course was complicated with coagulopathy and secondary antiphospholipid antibodies associated with a critical illness. In one patient, peripheral ischemia of upper limb digits and bilateral cerebral infarcts in multiple vascular territories evolved, complicating recovery. Similar to Ebola, cytomegalovirus, and the SARS virus, SARS-CoV-2 has the potential to induce severe thrombotic phenomena at the local sites and systemic circulation. Identifying COVID-19 patients at high risk of thromboembolism is important for the initiation of prophylactic support that could prevent sudden clinical deterioration[93,94].
In a cohort of COVID-19 patients in Lombardy, Italy, 20.4% were found to develop cutaneous manifestations not related to drug use or other treatment interventions. These included erythematous rash, widespread urticaria, and zoster-like vesicles. Trunk involvement was predominant, and itching seldom reported. The authors did not find any apparent correlation of dermatological manifestations with the disease severity[95]. In a report from Spain, a 32-year-old female with COVID-19 was found to develop an urticarial rash six days after the onset of respiratory symptoms. However, she had received hydroxychloroquine and azithromycin for four days before the onset of rash. The skin biopsy revealed a perivascular infiltrate of lymphocytes, some eosinophils, and upper dermal edema. The rash resolved in five days after antihistamine treatment[96]. In a large cohort of patients from China, only two out of 1099 were found to have skin lesions[82]. Similarly, another report from Spain described a young woman who presented at 14-d of COVID-19 diagnosis with pruritic, confluent erythematous-yellowish papules of both heels, which evolved into intense itchy erythematous plaques with thickening[97]. A report from France described pruritic disseminated erythematous plaques (urticarial) of the face with acral involvement preceding classic respiratory symptoms in a COVID-19 patient[98]. Bouaziz et al[99] described vascular skin lesions in COVID-19 patients. These included violaceous macules with “porcelain-like” appearance, livedo reticularis-like cutaneous eruption, non-necrotic and necrotic purpura and chilblain-like lesions with or without Raynaud’s phenomenon as well as eruptive cherry angioma[99]. Mahé et al[100] described an older woman who developed an erythematous rash of both antecubital fossae extending to the axillary folds and trunk after a period of loss of appetite and asthenia. Dermatology evaluation confirmed symmetrical drug-related intertriginous and flexural exanthema even though drug history was non-contributory. CT imaging of the chest showed bilateral GGOs, and RT-PCR for SARS-CoV-2 was positive. Complete resolution of symptoms occurred over three weeks[100]. In summary, even though rare, a small sub-group of COVID-19 patients can present with cutaneous manifestations akin to viral exanthem or urticaria. Nonetheless, cutaneous manifestations due to drug intake should be carefully excluded. The presence of dermatological manifestations in COVID-19 does not relate to disease severity or to clinical outcomes.
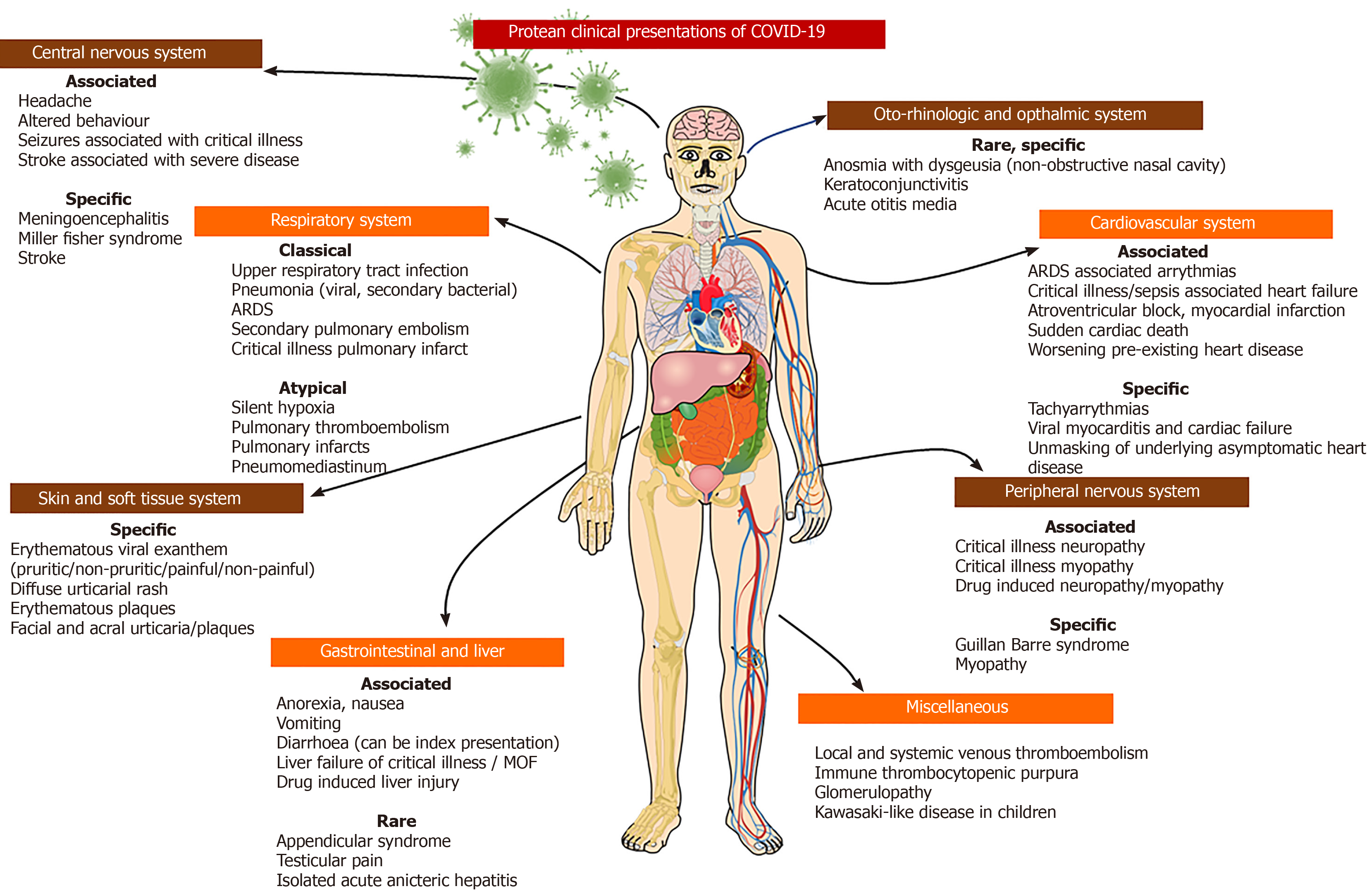
Direct kidney involvement is very rare, and any renal dysfunction associated with COVID-19 relates to the severity and onset of critical illness. Acute kidney injury can occur as part of drug therapy, severe systemic inflammation, multiple organ failure associated with severe ARDS, secondary bacterial sepsis and cardiogenic shock[101]. Larsen et al[102] described an African-American woman with stable chronic kidney disease who developed collapsing glomerulopathy after COVID-19. The patient presented with fever, cough, vomiting, and flank pain, which progressed to confusion along with advanced azotaemia and oliguria requiring mechanical ventilation and renal replacement therapy leading to complete recovery after a prolonged hospital stay. The SARS-CoV-2 nucleoprotein antigen was positive in a renal biopsy specimen, but in-situ hybridization analysis for the virus failed to show evidence of viral RNA in the kidney sample, suggesting the absence of direct renal involvement. In a previous series, post-mortem kidney biopsy in COVID-19 patients has typically demonstrated acute tubular injury without glomerulopathy[103]. Other anecdotal clinical presentations of COVID-19 reported in the literature include a form of abdominal pain syndrome with severe testicular pain, vaso-occlusive crisis with acute chest syndrome in sickle-cell disease, and possible immune thrombocytopenic purpura and leucoerythroblastic reactions[104-107]. A hyperinflammatory systemic disease akin to Kawasaki disease including shock syndrome and macrophage activation among children with COVID-19, has been reported from multiple centers[108,109]. A summary of the protean clinical manifestations of COVID-19 is shown in Figure 2.
The COVID-19 infection due to SARS-CoV-2 typically presents with acute onset respiratory symptoms with or without associated prodrome such as headache, lethargy, anorexia, diarrhoea, and arthralgia. Cardiovascular manifestations are usually related to underlying chronic comorbid conditions and the severity of infections. Nonetheless, rare presentations in the form of electrocardiographic abnormalities and myocarditis leading to cardiac failure require consideration. Gastrointestinal and hepatic manifestations are mostly therapy-related or associated with disease severity and critical illness. Anosmia and hypogeusia are typical and may occur without other classical features. Rare presentations include dermatological and thromboembolic phenomena as well as central and peripheral nervous system involvement, even though the latter is more common with multisystem involvement of COVID-19.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu Y S-Editor: Zhang L L-Editor: Webster JR P-Editor: Xing YX
| 1. | Singh A, Shaikh A, Singh R, Singh AK. COVID-19: From bench to bed side. Diabetes Metab Syndr. 2020;14:277-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | Wang H, Li X, Li T, Zhang S, Wang L, Wu X, Liu J. The genetic sequence, origin, and diagnosis of SARS-CoV-2. Eur J Clin Microbiol Infect Dis. 2020;39:1629-1635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 302] [Cited by in RCA: 285] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 3. | Nadeem MS, Zamzami MA, Choudhry H, Murtaza BN, Kazmi I, Ahmad H, Shakoori AR. Origin, Potential therapeutic targets and treatment for coronavirus disease (COVID-19). Pathogens. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 4. | Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 932] [Article Influence: 186.4] [Reference Citation Analysis (0)] |
| 5. | Yuen KS, Ye ZW, Fung SY, Chan CP, Jin DY. SARS-CoV-2 and COVID-19: The most important research questions. Cell Biosci. 2020;10:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 390] [Cited by in RCA: 353] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 6. | Zhang T, Wu Q, Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr Biol. 2020;30:1346-1351.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 870] [Cited by in RCA: 749] [Article Influence: 149.8] [Reference Citation Analysis (0)] |
| 7. | Lai CC, Liu YH, Wang CY, Wang YH, Hsueh SC, Yen MY, Ko WC, Hsueh PR. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Facts and myths. J Microbiol Immunol Infect. 2020;53:404-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 610] [Cited by in RCA: 554] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 8. | Ashour HM, Elkhatib WF, Rahman MM, Elshabrawy HA. Insights into the recent 2019 novel coronavirus (SARS-CoV-2) in light of past human coronavirus outbreaks. Pathogens. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 389] [Cited by in RCA: 356] [Article Influence: 71.2] [Reference Citation Analysis (1)] |
| 9. | Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1854] [Cited by in RCA: 2017] [Article Influence: 403.4] [Reference Citation Analysis (0)] |
| 10. | Khan S, Siddique R, Shereen MA, Ali A, Liu J, Bai Q, Bashir N, Xue M. Emergence of a novel coronavirus, severe acute respiratory syndrome coronavirus 2: Biology and therapeutic options. J Clin Microbiol. 2020;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 158] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 11. | Kannan S, Shaik Syed Ali P, Sheeza A, Hemalatha K. COVID-19 (Novel Coronavirus 2019) - recent trends. Eur Rev Med Pharmacol Sci. 2020;24:2006-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 136] [Reference Citation Analysis (0)] |
| 12. | Singhal T. A review of coronavirus disease-2019 (COVID-19). Indian J Pediatr. 2020;87:281-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2006] [Cited by in RCA: 1567] [Article Influence: 313.4] [Reference Citation Analysis (0)] |
| 13. | Seah I, Agrawal R. Can the coronavirus disease 2019 (covid-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocul Immunol Inflamm. 2020;28:391-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 369] [Cited by in RCA: 443] [Article Influence: 88.6] [Reference Citation Analysis (0)] |
| 14. | Li H, Liu SM, Yu XH, Tang SL, Tang CK. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int J Antimicrob Agents. 2020;55:105951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 692] [Cited by in RCA: 582] [Article Influence: 116.4] [Reference Citation Analysis (0)] |
| 15. | Giwa AL, Desai A, Duca A. Novel 2019 coronavirus SARS-CoV-2 (COVID-19): An updated overview for emergency clinicians. Emerg Med Pract. 2020;22:1-28. [PubMed] |
| 16. | Li H, Zhou Y, Zhang M, Wang H, Zhao Q, Liu J. Updated approaches against SARS-CoV-2. Antimicrob Agents Chemother. 2020;64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 158] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 17. | Ahn DG, Shin HJ, Kim MH, Lee S, Kim HS, Myoung J, Kim BT, Kim SJ. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19). J Microbiol Biotechnol. 2020;30:313-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 549] [Cited by in RCA: 563] [Article Influence: 112.6] [Reference Citation Analysis (0)] |
| 18. | Habibzadeh P, Stoneman EK. The novel coronavirus: A bird's eye view. Int J Occup Environ Med. 2020;11:65-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 173] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 19. | Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P, Pan P, Wang W, Hu D, Liu X, Zhang Q, Wu J. Coronavirus infections and immune responses. J Med Virol. 2020;92:424-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1056] [Cited by in RCA: 1167] [Article Influence: 233.4] [Reference Citation Analysis (0)] |
| 20. | Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529-539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1895] [Cited by in RCA: 1789] [Article Influence: 223.6] [Reference Citation Analysis (0)] |
| 21. | Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, Sun C, Sylvia S, Rozelle S, Raat H, Zhou H. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: A scoping review. Infect Dis Poverty. 2020;9:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1355] [Cited by in RCA: 1087] [Article Influence: 217.4] [Reference Citation Analysis (0)] |
| 22. | Henry BM. COVID-19, ECMO, and lymphopenia: A word of caution. Lancet Respir Med. 2020;8:e24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 181] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 23. | Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T, Brünink S, Schneider J, Schmidt ML, Mulders DG, Haagmans BL, van der Veer B, van den Brink S, Wijsman L, Goderski G, Romette JL, Ellis J, Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MP, Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5481] [Cited by in RCA: 4909] [Article Influence: 981.8] [Reference Citation Analysis (1)] |
| 24. | Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, Fang C, Huang D, Huang LQ, Huang Q, Han Y, Hu B, Hu F, Li BH, Li YR, Liang K, Lin LK, Luo LS, Ma J, Ma LL, Peng ZY, Pan YB, Pan ZY, Ren XQ, Sun HM, Wang Y, Wang YY, Weng H, Wei CJ, Wu DF, Xia J, Xiong Y, Xu HB, Yao XM, Yuan YF, Ye TS, Zhang XC, Zhang YW, Zhang YG, Zhang HM, Zhao Y, Zhao MJ, Zi H, Zeng XT, Wang YY, Wang XH; for the Zhongnan Hospital of Wuhan University Novel Coronavirus Management and Research Team, Evidence-Based Medicine Chapter of China International Exchange and Promotive Association for Medical and Health Care (CPAM). A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res. 2020;7:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 881] [Cited by in RCA: 1136] [Article Influence: 227.2] [Reference Citation Analysis (0)] |
| 25. | Lake MA. What we know so far: COVID-19 current clinical knowledge and research. Clin Med (Lond). 2020;20:124-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 442] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 26. | Cheng ZJ, Shan J. 2019 Novel coronavirus: Where we are and what we know. Infection. 2020;48:155-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 339] [Cited by in RCA: 311] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 27. | Sohrabi C, Alsafi Z, O'Neill N, Khan M, Kerwan A, Al-Jabir A, Iosifidis C, Agha R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int J Surg. 2020;76:71-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3213] [Cited by in RCA: 2651] [Article Influence: 530.2] [Reference Citation Analysis (0)] |
| 28. | Xie J, Tong Z, Guan X, Du B, Qiu H, Slutsky AS. Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive Care Med. 2020;46:837-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 367] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 29. | Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation, and Treatment of Coronavirus (COVID-19) In: StatPearls [Internet], Treasure Island (FL): StatPearls Publishing, 2020. [PubMed] |
| 30. | Yao W, Wang T, Jiang B, Gao F, Wang L, Zheng H, Xiao W, Yao S, Mei W, Chen X, Luo A, Sun L, Cook T, Behringer E, Huitink JM, Wong DT, Lane-Fall M, McNarry AF, McGuire B, Higgs A, Shah A, Patel A, Zuo M, Ma W, Xue Z, Zhang LM, Li W, Wang Y, Hagberg C, O'Sullivan EP, Fleisher LA, Wei H; collaborators. Emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China: lessons learnt and international expert recommendations. Br J Anaesth. 2020;125:e28-e37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 224] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 31. | Li Y, Xia L. Coronavirus Disease 2019 (COVID-19): Role of chest CT in diagnosis and management. AJR Am J Roentgenol. 2020;214:1280-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 652] [Article Influence: 130.4] [Reference Citation Analysis (0)] |
| 32. | Li M, Lei P, Zeng B, Li Z, Yu P, Fan B, Wang C, Li Z, Zhou J, Hu S, Liu H. Coronavirus Disease (COVID-19): Spectrum of CT findings and temporal progression of the disease. Acad Radiol. 2020;27:603-608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 33. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5776] [Article Influence: 1155.2] [Reference Citation Analysis (2)] |
| 34. | Wang W, Gao R, Zheng Y, Jiang L. COVID-19 with spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema. J Travel Med. 2020;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 35. | Atri D, Siddiqi HK, Lang J, Nauffal V, Morrow DA, Bohula EA. COVID-19 for the Cardiologist: A current review of the virology, clinical epidemiology, cardiac and other clinical manifestations and potential therapeutic strategies. JACC Basic Transl Sci. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 205] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 36. | Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, Metra M. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1111] [Cited by in RCA: 1269] [Article Influence: 253.8] [Reference Citation Analysis (0)] |
| 37. | Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 383] [Cited by in RCA: 469] [Article Influence: 117.3] [Reference Citation Analysis (0)] |
| 38. | He J, Wu B, Chen Y, Tang J, Liu Q, Zhou S, Chen C, Qin Q, Huang K, Lv J, Chen Y, Peng D. Characteristic electrocardiographic manifestations in patients with COVID-19. Can J Cardiol. 2020;36:966.e1-966.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 39. | Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2516] [Cited by in RCA: 2838] [Article Influence: 567.6] [Reference Citation Analysis (0)] |
| 40. | Chang D, Saleh M, Garcia-Bengo Y, Choi E, Epstein L, Willner J. COVID-19 infection unmasking Brugada syndrome. HeartRhythm Case Rep. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 41. | Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, Baldanti F, Paolucci S, Pelenghi S, Iotti GA, Mojoli F, Arbustini E. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911-915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 608] [Cited by in RCA: 745] [Article Influence: 149.0] [Reference Citation Analysis (0)] |
| 42. | Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: Acute and long-term implications. Eur Heart J. 2020;41:1798-1800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 464] [Cited by in RCA: 492] [Article Influence: 123.0] [Reference Citation Analysis (0)] |
| 43. | Wan Y, Li J, Shen L, Zou Y, Hou L, Zhu L, Faden HS, Tang Z, Shi M, Jiao N, Li Y, Cheng S, Huang Y, Wu D, Xu Z, Pan L, Zhu J, Yan G, Zhu R, Lan P. Enteric involvement in hospitalised patients with COVID-19 outside Wuhan. Lancet Gastroenterol Hepatol. 2020;5:534-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 44. | Han C, Duan C, Zhang S, Spiegel B, Shi H, Wang W, Zhang L, Lin R, Liu J, Ding Z, Hou X. Digestive symptoms in COVID-19 patients with mild disease severity: Clinical presentation, stool viral RNA testing, and outcomes. Am J Gastroenterol. 2020;115:916-923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 383] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 45. | Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical characteristics of covid-19 patients with digestive symptoms in Hubei, China: A descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115:766-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1160] [Cited by in RCA: 1203] [Article Influence: 240.6] [Reference Citation Analysis (0)] |
| 46. | D'Amico F, Baumgart DC, Danese S, Peyrin-Biroulet L. Diarrhea during COVID-19 infection: Pathogenesis, epidemiology, prevention, and management. Clin Gastroenterol Hepatol. 2020;18:1663-1672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 387] [Cited by in RCA: 378] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 47. | Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10:102-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1000] [Cited by in RCA: 935] [Article Influence: 187.0] [Reference Citation Analysis (0)] |
| 48. | Pautrat K, Chergui N. SARS-CoV-2 infection may result in appendicular syndrome: Chest CT scan before appendectomy. J Chir Visc. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Philips CA, Ahamed R, Augustine P. SARS-CoV-2 related liver impairment - perception may not be the reality. J Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 50. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1294] [Article Influence: 258.8] [Reference Citation Analysis (4)] |
| 51. | Feng G, Zheng KI, Yan QQ, Rios RS, Targher G, Byrne CD, Poucke SV, Liu WY, Zheng MH. COVID-19 and liver dysfunction: Current insights and emergent therapeutic strategies. J Clin Transl Hepatol. 2020;8:18-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 278] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 52. | Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver Int. 2020;40:1278-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 219] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 53. | Li J, Fan JG. Characteristics and mechanism of liver injury in 2019 coronavirus disease. J Clin Transl Hepatol. 2020;8:13-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 205] [Article Influence: 41.0] [Reference Citation Analysis (2)] |
| 54. | Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, Mou HM, Wang LH, Zhang HR, Fu WJ, Luo T, Liu F, Guo QN, Chen C, Xiao HL, Guo HT, Lin S, Xiang DF, Shi Y, Pan GQ, Li QR, Huang X, Cui Y, Liu XZ, Tang W, Pan PF, Huang XQ, Ding YQ, Bian XW. A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 461] [Reference Citation Analysis (0)] |
| 55. | Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 574] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 56. | Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 310] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 57. | Bangash MN, Patel J, Parekh D. COVID-19 and the liver: Little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 357] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 58. | Wander P, Epstein M, Bernstein D. COVID-19 presenting as acute hepatitis. Am J Gastroenterol. 2020;115:941-942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 59. | Huang JF, Zheng KI, George J, Gao HN, Wei RN, Yan HD, Zheng MH. Fatal outcome in a liver transplant recipient with COVID-19. Am J Transplant. 2020;20:1907-1910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 60. | Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: A systematic review. J Neurol Sci. 2020;413:116832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 520] [Cited by in RCA: 477] [Article Influence: 95.4] [Reference Citation Analysis (0)] |
| 61. | Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, Liu C, Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1219] [Cited by in RCA: 1252] [Article Influence: 250.4] [Reference Citation Analysis (0)] |
| 62. | Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar-Poli P, Zandi MS, Lewis G, David AS. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1673] [Cited by in RCA: 1518] [Article Influence: 303.6] [Reference Citation Analysis (0)] |
| 63. | Paybast S, Emami A, Koosha M, Baghalha F. Novel coronavirus disease (COVID-19) and central nervous system complications: What neurologist need to know. Acta Neurol Taiwan. 2020;29:24-31. [PubMed] |
| 64. | Baig AM. Neurological manifestations in COVID-19 caused by SARS-CoV-2. CNS Neurosci Ther. 2020;26:499-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 65. | Sharifi-Razavi A, Karimi N, Rouhani N. COVID-19 and intracerebral haemorrhage: Causative or coincidental? New Microbes New Infect. 2020;35:100669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 219] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 66. | Wang HY, Li XL, Yan ZR, Sun XP, Han J, Zhang BW. Potential neurological symptoms of COVID-19. Ther Adv Neurol Disord. 2020;13:1756286420917830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 67. | Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92:552-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1284] [Cited by in RCA: 1519] [Article Influence: 303.8] [Reference Citation Analysis (0)] |
| 68. | Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: Causality or coincidence? Lancet Neurol. 2020;19:383-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 665] [Cited by in RCA: 761] [Article Influence: 152.2] [Reference Citation Analysis (0)] |
| 69. | Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID-19 infection: A case report. J Clin Neurosci. 2020;76:233-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 371] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 70. | Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, Ueno M, Sakata H, Kondo K, Myose N, Nakao A, Takeda M, Haro H, Inoue O, Suzuki-Inoue K, Kubokawa K, Ogihara S, Sasaki T, Kinouchi H, Kojin H, Ito M, Onishi H, Shimizu T, Sasaki Y, Enomoto N, Ishihara H, Furuya S, Yamamoto T, Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1282] [Cited by in RCA: 1442] [Article Influence: 288.4] [Reference Citation Analysis (0)] |
| 71. | Li J, Long X, Zhu C, Hu S, Lin Z, Li J, Xiong N. A case of COVID-19 pneumonia with cerebral hemorrhage. Thromb Res. 2020;193:22-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 72. | Gutiérrez-Ortiz C, Méndez-Guerrero A, Rodrigo-Rey S, San Pedro-Murillo E, Bermejo-Guerrero L, Gordo-Mañas R, de Aragón-Gómez F, Benito-León J. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95:e601-e605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 526] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 73. | Filatov A, Sharma P, Hindi F, Espinosa PS. Neurological complications of coronavirus disease (COVID-19): Encephalopathy. Cureus. 2020;12:e7352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 327] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 74. | Xydakis MS, Dehgani-Mobaraki P, Holbrook EH, Geisthoff UW, Bauer C, Hautefort C, Herman P, Manley GT, Lyon DM, Hopkins C. Smell and taste dysfunction in patients with COVID-19. Lancet Infect Dis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 228] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 75. | Eliezer M, Hautefort C, Hamel AL, Verillaud B, Herman P, Houdart E, Eloit C. Sudden and complete olfactory loss function as a possible symptom of COVID-19. JAMA Otolaryngol Head Neck Surg. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 244] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 76. | Klopfenstein T, Kadiane-Oussou NJ, Toko L, Royer PY, Lepiller Q, Gendrin V, Zayet S. Features of anosmia in COVID-19. Med Mal Infect. 2020;50:436-439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 283] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 77. | Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: Common findings in COVID-19 patients. Laryngoscope. 2020;130:1787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 467] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 78. | Krajewska J, Krajewski W, Zub K, Zatoński T. COVID-19 in otolaryngologist practice: A review of current knowledge. Eur Arch Otorhinolaryngol. 2020;277:1885-1897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 79. | Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, Dequanter D, Blecic S, El Afia F, Distinguin L, Chekkoury-Idrissi Y, Hans S, Delgado IL, Calvo-Henriquez C, Lavigne P, Falanga C, Barillari MR, Cammaroto G, Khalife M, Leich P, Souchay C, Rossi C, Journe F, Hsieh J, Edjlali M, Carlier R, Ris L, Lovato A, De Filippis C, Coppee F, Fakhry N, Ayad T, Saussez S. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251-2261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1729] [Cited by in RCA: 1734] [Article Influence: 346.8] [Reference Citation Analysis (0)] |
| 80. | Fidan V. New type of corona virus induced acute otitis media in adult. Am J Otolaryngol. 2020;41:102487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 81. | Cheema M, Aghazadeh H, Nazarali S, Ting A, Hodges J, McFarlane A, Kanji JN, Zelyas N, Damji KF, Solarte C. Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID-19). Can J Ophthalmol. 2020;55:e125-e129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 256] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 82. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18848] [Article Influence: 3769.6] [Reference Citation Analysis (7)] |
| 83. | Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L, Wu K. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 726] [Cited by in RCA: 888] [Article Influence: 177.6] [Reference Citation Analysis (0)] |
| 84. | Seah IYJ, Anderson DE, Kang AEZ, Wang L, Rao P, Young BE, Lye DC, Agrawal R. Assessing viral shedding and infectivity of tears in coronavirus disease 2019 (COVID-19) patients. Ophthalmology. 2020;127:977-979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 299] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 85. | Guo D, Xia J, Shen Y, Tong J. SARS-CoV-2 may be related to conjunctivitis but not necessarily spread through the conjunctiva SARS-CoV-2 and conjunctiva. J Med Virol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 86. | Fabre O, Rebet O, Carjaliu I, Radutoiu M, Gautier L, Hysi I. Severe acute proximal pulmonary embolism and COVID-19: A word of caution. Ann Thorac Surg. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 87. | Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, Frank S, Turek D, Willi N, Pargger H, Bassetti S, Leuppi JD, Cathomas G, Tolnay M, Mertz KD, Tzankov A. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 892] [Cited by in RCA: 911] [Article Influence: 182.2] [Reference Citation Analysis (0)] |
| 88. | Casey K, Iteen A, Nicolini R, Auten J. COVID-19 pneumonia with hemoptysis: Acute segmental pulmonary emboli associated with novel coronavirus infection. Am J Emerg Med. 2020;38:1544.e1-1544.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 89. | Marongiu F, Grandone E, Barcellona D. Pulmonary thrombosis in 2019-nCoV pneumonia? J Thromb Haemost. 2020;18:1511-1513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 90. | Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, da Silva LFF, de Oliveira EP, Saldiva PHN, Mauad T, Negri EM. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost. 2020;18:1517-1519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 395] [Cited by in RCA: 436] [Article Influence: 87.2] [Reference Citation Analysis (0)] |
| 91. | Wang T, Chen R, Liu C, Liang W, Guan W, Tang R, Tang C, Zhang N, Zhong N, Li S. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7:e362-e363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 273] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 92. | Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H, Wang C, Zhao J, Sun X, Tian R, Wu W, Wu D, Ma J, Chen Y, Zhang D, Xie J, Yan X, Zhou X, Liu Z, Wang J, Du B, Qin Y, Gao P, Qin X, Xu Y, Zhang W, Li T, Zhang F, Zhao Y, Li Y, Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1532] [Cited by in RCA: 1606] [Article Influence: 321.2] [Reference Citation Analysis (0)] |
| 93. | Porfidia A, Pola R. Venous thromboembolism in COVID-19 patients. J Thromb Haemost. 2020;18:1516-1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 94. | Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3488] [Cited by in RCA: 3403] [Article Influence: 680.6] [Reference Citation Analysis (0)] |
| 95. | Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212-e213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 712] [Article Influence: 142.4] [Reference Citation Analysis (1)] |
| 96. | Fernandez-Nieto D, Ortega-Quijano D, Segurado-Miravalles G, Pindado-Ortega C, Prieto-Barrios M, Jimenez-Cauhe J. Comment on: Cutaneous manifestations in COVID-19: a first perspective. Safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol. 2020;34:e252-e254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 97. | Estébanez A, Pérez-Santiago L, Silva E, Guillen-Climent S, García-Vázquez A, Ramón MD. Cutaneous manifestations in COVID-19: a new contribution. J Eur Acad Dermatol Venereol. 2020;34:e250-e251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 98. | Henry D, Ackerman M, Sancelme E, Finon A, Esteve E. Urticarial eruption in COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34:e244-e245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 99. | Bouaziz JD, Duong T, Jachiet M, Velter C, Lestang P, Cassius C, Arsouze A, Domergue Than Trong E, Bagot M, Begon E, Sulimovic L, Rybojad M. Vascular skin symptoms in COVID-19: A French observational study. J Eur Acad Dermatol Venereol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 192] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 100. | Mahé A, Birckel E, Krieger S, Merklen C, Bottlaender L. A distinctive skin rash associated with coronavirus disease 2019? J Eur Acad Dermatol Venereol. 2020;34:e246-e247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 101. | Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol. 2020;16:308-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 336] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 102. | Larsen CP, Bourne TD, Wilson JD, Saqqa O, Sharshir MA. Collapsing glomerulopathy in a patient with coronavirus disease 2019 (COVID-19). Kidney Int Rep. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 261] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 103. | Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, Yi F, Yang HC, Fogo AB, Nie X, Zhang C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1054] [Cited by in RCA: 1267] [Article Influence: 253.4] [Reference Citation Analysis (0)] |
| 104. | Kim J, Thomsen T, Sell N, Goldsmith AJ. Abdominal and testicular pain: An atypical presentation of COVID-19. Am J Emerg Med. 2020;38:1542.e1-1542.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 105. | Nur E, Gaartman AE, van Tuijn CFJ, Tang MW, Biemond BJ. Vaso-occlusive crisis and acute chest syndrome in sickle cell disease due to 2019 novel coronavirus disease (COVID-19). Am J Hematol. 2020;95:725-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 106. | Mitra A, Dwyre DM, Schivo M, Thompson GR, Cohen SH, Ku N, Graff JP. Leukoerythroblastic reaction in a patient with COVID-19 infection. Am J Hematol. 2020;95:999-1000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 107. | Zulfiqar AA, Lorenzo-Villalba N, Hassler P, Andrès E. Immune Thrombocytopenic Purpura in a Patient with Covid-19. N Engl J Med. 2020;382:e43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 108. | Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, Bonanomi E, D'Antiga L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: An observational cohort study. Lancet. 2020;395:1771-1778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1578] [Cited by in RCA: 1665] [Article Influence: 333.0] [Reference Citation Analysis (0)] |
| 109. | Viner RM, Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;395:1741-1743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 335] [Article Influence: 67.0] [Reference Citation Analysis (0)] |