Published online Sep 6, 2020. doi: 10.12998/wjcc.v8.i17.3881
Peer-review started: March 25, 2020
First decision: June 8, 2020
Revised: June 21, 2020
Accepted: August 13, 2020
Article in press: August 13, 2020
Published online: September 6, 2020
Processing time: 162 Days and 17.7 Hours
Gallbladder sarcomatoid carcinoma is a rare and aggressive tumor, and little is known about its clinical behavior, prognosis, and optimal treatment.
From 1997 to 2017, we collected seven cases of gallbladder sarcomatoid carcinoma at our institution. The median patient age was 68.5 years. Six (85.7%) patients were female. Overall, 85.7% (6/7) of the tumors had a maximal diameter greater than 7 cm. Late TNM stage was associated with a significantly poor prognosis. All patients with advanced-stage (III/IV) disease died from metastases or disease progression shortly after surgery. One patient with stage IIIB disease who received adjuvant chemoradiotherapy (gemcitabine and capecitabine) achieved a progression-free survival (PFS) of 12 mo and overall survival of 15 mo, which might be the longest PFS reported among patients who ultimately experienced recurrence or metastasis.
Sarcomatoid carcinoma is a unique and aggressive gallbladder malignancy. Surgery is suggested as the first and only recognized treatment. There is a significant difference in prognosis between patients with early-stage and advanced-stage disease. Postoperative adjuvant therapy may bring survival benefits for locally advanced patients. Gemcitabine combined with fluorouracil and radiotherapy could be a potential strategy.
Core tip: From 1997 to 2017, we collected seven cases of gallbladder sarcomatoid carcinoma at our institution. By reporting these cases, this paper will enrich the clinical information of this rare tumor. TNM stage seemed to be a highly important prognostic indicator. There was a significant difference in prognosis between patients with early-stage and advanced-stage disease. For the first time, we report a patient treated with postoperative adjuvant therapy who achieved a progression-free survival for up to 12 mo. This approach may provide a potential strategy for gallbladder sarcomatoid carcinoma, which is a kind of rare and aggressive tumor with no standard treatment to date.
- Citation: Qin Q, Liu M, Wang X. Gallbladder sarcomatoid carcinoma: Seven case reports. World J Clin Cases 2020; 8(17): 3881-3889
- URL: https://www.wjgnet.com/2307-8960/full/v8/i17/3881.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i17.3881
Gallbladder cancer is the fifth most common malignancy of the gastrointestinal tract[1]. It is also the most aggressive cancer of the biliary tract with the shortest median survival time[2]. Adenocarcinoma is the most common type, while sarcomatoid carcinoma is rare and even more aggressive than other pathological types. Gallbladder sarcomatoid carcinoma accounts for only 4.3% of gallbladder cancers[3] and has often been confused with carcinosarcoma in previous reports. If we exclude the reports that may have confused sarcomatoid carcinoma and carcinosarcoma, there are approximately 20 cases of sarcomatoid carcinoma of the gallbladder described in the English-language literature worldwide[3-8]. Because of the rarity of this tumor, little is known about its clinical behavior, prognosis, and optimal treatment.
Despite rapid advances in surgery, chemotherapy, radiotherapy, and biomedicine in the past several decades, gallbladder cancer remains an invasive cancer with a discouraging prognosis, and there is no standard treatment for gallbladder sarcomatoid carcinoma[9]. Surgery is suggested as the main and only recognized treatment for sarcomatoid carcinoma of the gallbladder[6]. However, even with radical excision, patients still develop recurrence quickly, and postoperative adjuvant treatment might be necessary[6]. However, there is no standard adjuvant treatment, and only a few patients have received adjuvant chemotherapy in previous reports with unfavorable results[3].
To enrich this information, we retrospectively reviewed the data of patients with gallbladder sarcomatoid carcinoma at our institution over the past 20 years. We found seven patients, among whom one treated with chemoradiotherapy after surgery achieved a progression-free survival (PFS) of 12 mo and overall survival (OS) of 15 mo, which might be the longest PFS reported among patients who ultimately experienced recurrence or metastasis.
A total of seven patients were diagnosed with gallbladder sarcomatoid carcinoma and treated at our institution from 1997 to 2017. The median age was 68.5 years (ranging from 50 to 83 years). Six patients were female, and only one was male. All seven patients presented to hospital complaining of abdominal pain.
Although the duration of abdominal pain prior to admission ranged between 20 d and 4 years, the majority (71.4%) experienced pain for less than 2 mo (Table 1).
| Characteristic | No recurrence | Recurrence or metastasis | |||||
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | |
| Age | 83 | 79 | 50 | 51 | 76 | 65 | 76 |
| Sex (F/M) | M | F | F | F | F | F | F |
| Duration of pain | 1 mo | 6 mo | 4 yr | 1 mo | 1 mo | 20 d | 2 mo |
Case 1 and case 6 had undergone inguinal herniorrhaphy and appendectomy respectively. Case 2 had a history of hypertension.
Both case 6 and case 7 had mild tenderness in the right upper abdomen and no obvious rebound pain. No obvious abnormality was found in the physical examination of the other patients.
The laboratory examinations revealed that only case 2 and case 4 had liver dysfunction (Table 2) .The preoperative serum levels of several tumor markers [carbohydrate antigen (CA) 19-9, CA-125, carcinoembryonic antigen (CEA), and alpha-fetoprotein (AFP)] are shown in Table 2. The CEA and CA19-9 levels for both patients without recurrence were normal, and the CA-125 level was slightly elevated in one of these patients. Two (40%) patients with recurrence or metastasis had elevated serum CEA levels. Three (60%) patients with recurrence or metastasis had significantly high CA19-9 levels, with the highest one exceeding 1000 U/mL. Three (60%) patients with recurrence or metastasis had notably elevated CA-125, two of whom had high levels of both CA19-9 and CA-125. All the seven patients, with or without recurrence, showed normal AFP levels.
| No recurrence | Recurrence or metastasis | ||||||
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | |
| DBIL | 12.2↑ | 4.5 | 3.8 | 7.2 | 3.3 | 5.6 | 3.8 |
| ALT | 11 | 10 | 10 | 75↑ | 18 | 8 | 38 |
| AST | 21 | 15 | 21 | 62 | 22 | 14 | 25 |
| CEA | 1.05 | 1.37 | N/A | 2.54 | 47.4↑ | 0.29 | 5.74↑ |
| CA19-9 | 22.7 | 19.46 | 125↑ | > 1000↑ | 446.8↑ | 7.8 | 24.42 |
| CA-125 | 40.12 | 7.19 | 102.1↑ | 115.8↑ | N/A | 43.77 | 272.3↑ |
| AFP | 4.15 | 1.94 | 0.69 | 8.77 | 3.22 | 0.79 | N/A |
Case 1 and case 5 underwent contrast-enhanced abdominal MRI, and the rest of the patients underwent contrast-enhanced abdominal computed tomography (CT). Gallbladder mass was found in all patients. Granulomatous hyperplasia was considered in case 1 and malignant tumor was considered in other patients.
The seven patients were eventually diagnosed with gallbladder sarcomatoid carcinoma. A total of 57.1% (4/7) of the tumors were located in the gallbladder body, one located in the body and choledochus, one in the fundus and neck, and one in the fundus. A total of 85.7% (6/7) of the tumors had a maximal diameter greater than 7 cm. Two patients without recurrence had early-stage disease; one had stage I disease with a tumor restricted to the lamina propria, and the other had stage II disease with a tumor restricted to the connective tissue without extension beyond the serosa. The other five patients had advanced-stage disease, including one with stage IIIB, three with stage IVB, and one with stage III/IV with unknown lymph node status (Table 3).
| Characteristic | No recurrence | Recurrence or metastasis | |||||
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | |
| Position | Body and choledochus | Body | Body | Fundus and neck | Body | Body | Fundus |
| Diameter | 3 cm | 7.2 cm | 7 cm | 7 cm | 9 cm | 10 cm | 7 cm |
| Operation | P | R | R | R | R | R (colon) | P |
| Depth | T1 | T2 | T2 | T3 | T3 | T3 | T3 |
| Lymph node | N01 | N0 | N1 | N2 | Nx | N2 | Nx |
| Metastasis | M0 | M0 | M0 | M0 | M0 | M0 | M1 |
| Stage | I | II | IIIB | IVB | III/IV | IVB | IVB |
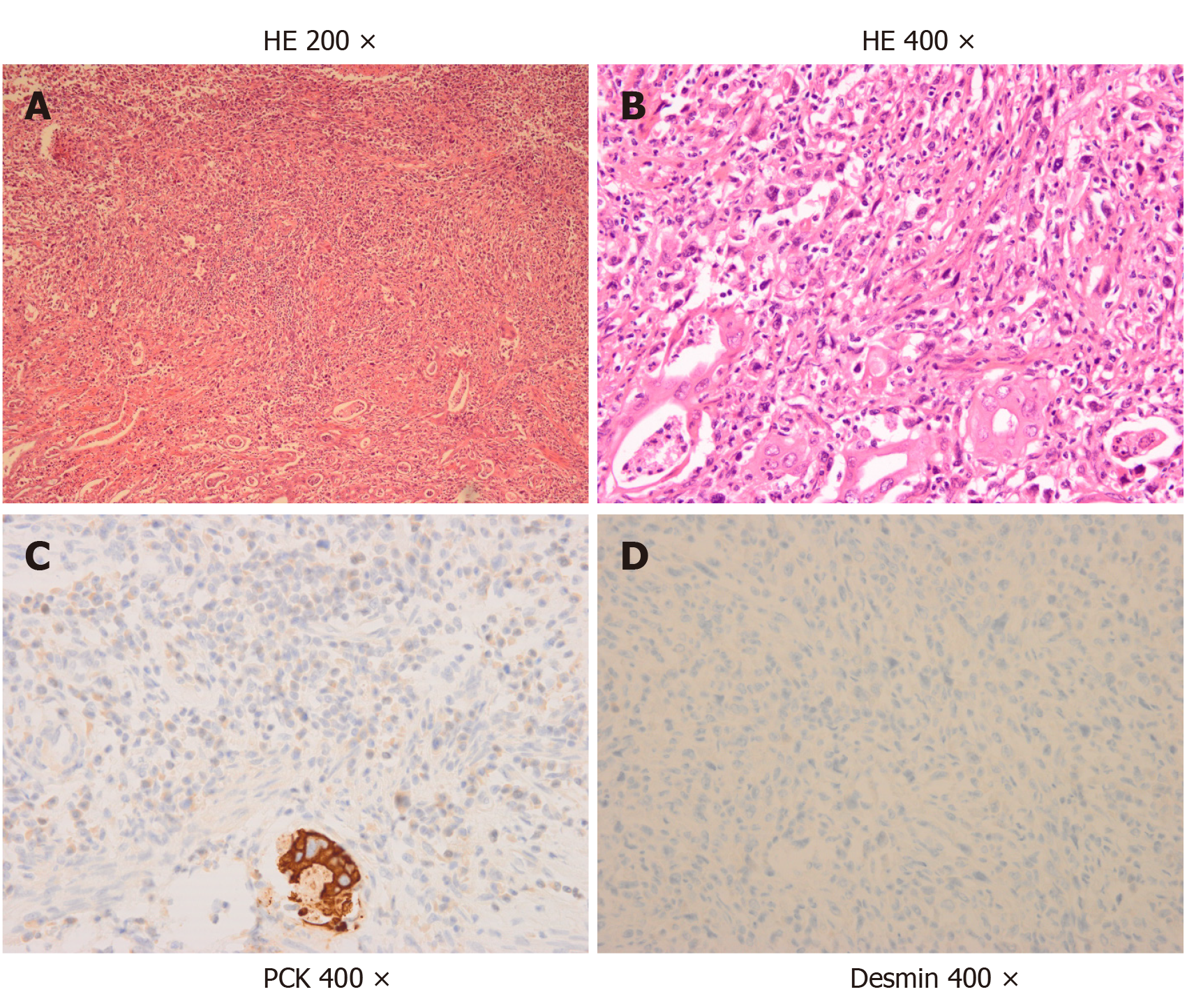
The pathological appearance of the sarcomatoid carcinoma samples is shown in Figure 1 and Table 4. Hematoxylin and eosin staining showed poorly differentiated carcinoma with spindle-shaped cells and a commonly pleomorphic morphology, which were typical figures of sarcomatoid carcinoma (Figure 1A and B). According to immunohistochemical staining, all seven patients strongly and diffusely expressed pan-cytokeratin (Figure 1C). Other epithelioid markers, such as CK-7 and CK-19, were also detected in some patients. Sarcoma-like spindle cells were negative for desmin in all seven patients (Figure 1D, Table 4) and negative for a-SMA in six patients (Table 4). All of these immunohistochemical results suggested that the sarcoma-like component was essentially carcinoma. Sarcomatoid carcinoma was usually pleomorphic. Adenocarcinoma and adenosquamous carcinoma can be found after extensive sampling. In the cases we collected, we also found some mixed components of carcinoma. Adenocarcinoma was common (3/7), with adenosquamous sometimes occurring concurrently (2/7).
| No recurrence | Recurrence or metastasis | ||||||
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | |
| Carcinoma | Adenocarcinoma | NM | NM | Adenosquamous | Adenosquamous | Adenocarcinoma | Adenocarcinoma |
| PCK | (+) | (+) | (+) | (+) | (+) | (+) | (+) |
| Cytokeratin | CK19+ | CK7 (+), CK20 (-) | CK19 (focal+), CK7 (-) | CK7 (+), CK19 (+) | NT | CK7 (focal+), CK20 (focal+) | NT |
| EMA | NT | (+) | (focal +) | NT | NT | + | NT |
| Vimentin | NT | NT | NT | NT | NT | (focal +) | NT |
| Desmin | (-) | (-) | (-) | (-) | (-) | (-) | (-) |
| a-SMA | (-) | (-) | NT | (-) | (-) | (-) | (-) |
| Other positive markers | S-100 (±) | P63 (focal +) | CAM5.2 (focal +) | ||||
| Other negative markers | Syn (-), CgA (-) | HCC (-), CD34 (-) | DOG1 (-), CD117 (-), CD34 (-) | ||||
All patients underwent surgical procedures. Case 1 underwent cholecystectomies without lymphadenectomy because intraoperative frozen section analysis resulted in a diagnosis of “adenoma”, which was finally proven to be sarcomatoid carcinoma by paraffin section analysis. Case 7, who had stage IV disease with peritoneal metastasis and intraperitoneal hematoma, underwent palliative cholecystectomy. The other five patients underwent radical surgery, which included resection of the primary gallbladder tumor, involved extrahepatic biliary tract, and portions of liver tissue (cases 2/3/4/5), lymphadenectomy, and right hemicolectomy (case 6). No serious surgical complications or death occurred.
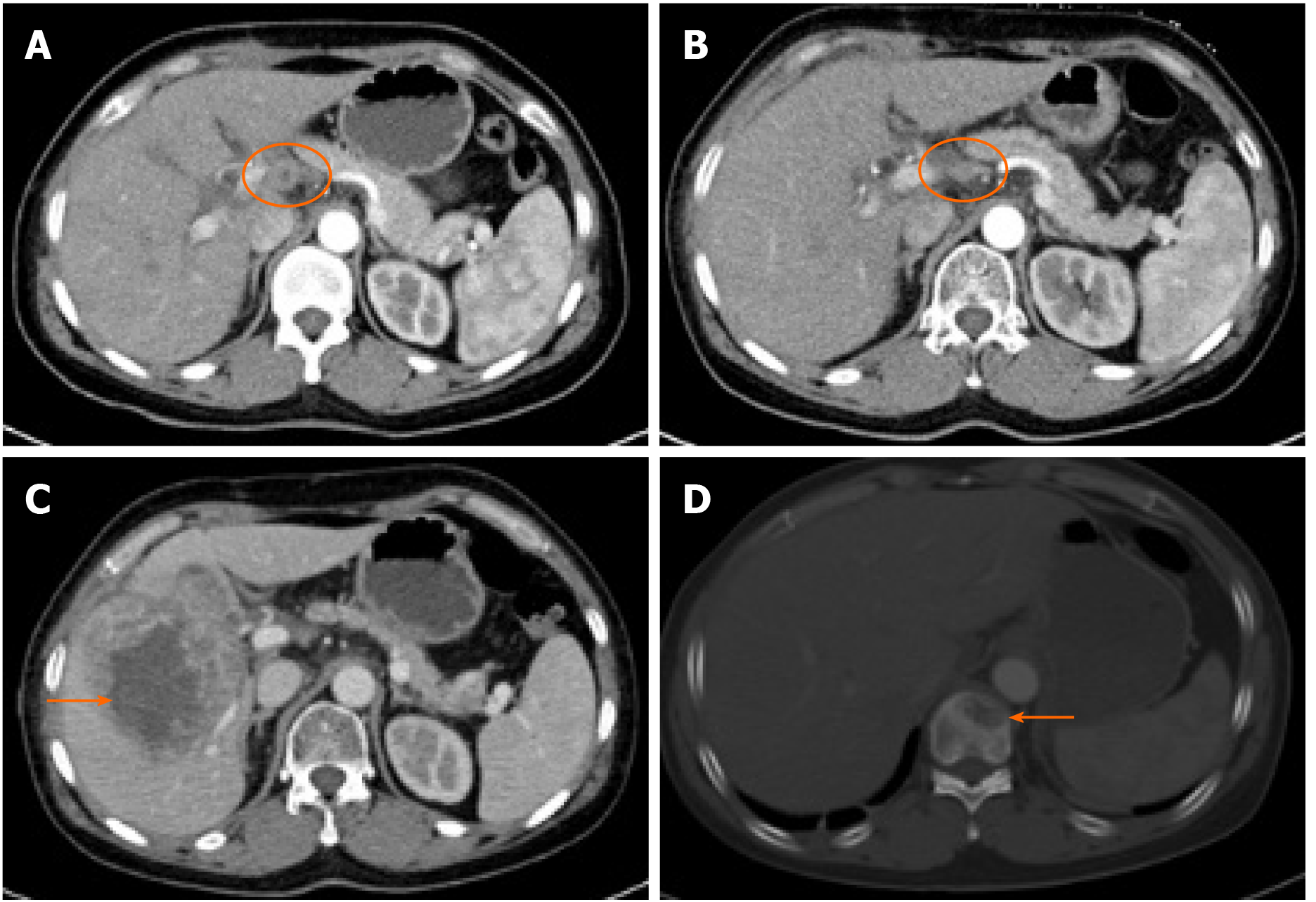
Case 3 was a 50-year-old woman who underwent cholecystectomy, lymphade-nectomy, and partial resection of the liver. She received adjuvant chemotherapy and radiotherapy 1 mo after surgery for stage T2N1M0 (IIIB) disease. She was treated with gemcitabine and capecitabine in 21-d cycles. After two cycles of chemotherapy, she received radiotherapy (5040 cGy to the tumor bed and regional lymph nodes). Two other cycles of chemotherapy were administered after radiotherapy. One month after the completion of all the adjuvant therapy, a CT scan was carried out to reassess the enlarged lymph node that was found before adjuvant therapy (Figure 2A), which had already shrunk without recurrence or metastases (Figure 2B). Three months after the completion of adjuvant therapy, the patient came to the hospital with abdominal distention, and the CT scan found metastases in the liver (Figure 2C) and thoracic vertebra (Figure 2D). She was treated with FOLFOXIRI as second-line chemotherapy for two cycles. She did not visit our hospital for antitumor therapy again because of her gradually declining general medical condition. She died 2 mo after second-line chemotherapy. With serial adjuvant therapies, she achieved a PFS of 12 mo and OS of 15 mo, which are the longest reported periods to date in such patients.
The follow-up time ranged from 1.5 years to 5 years. Tumor node metastasis (TNM) stage seemed to be a very important prognostic indicator. There was a significant difference in prognosis between patients with early-stage and advanced-stage disease. Two patients with early-stage (I/II) disease were still alive without recurrence for 5 years and 3 years. Neither patient received adjuvant therapy after the operation. All five patients with advanced-stage (III/IV) disease died from metastases or disease progression shortly after surgery. Four of five patients without adjuvant therapy developed metastases or progressed within 2 mo and died within 3 mo, while only one patient with stage IIIB disease who received adjuvant chemotherapy and radiotherapy achieved a PFS of 12 mo and OS of 15 mo (Table 5). Liver metastases were most common (4/5), followed by bone and peritoneal metastases.
| No recurrence | Recurrence or metastasis | ||||||
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | |
| Stage | I | II | IIIB | IVB | III/IV | IIIB | IVB |
| Adjuvant therapy | No | No | Yes | No | No | No | No |
| Recurrence or metastasis | No | No | Liver and bone | Liver | Liver | Liver | Peritoneum |
| PFS | > 5 yr | > 3 yr | 12 mo | 2 mo | 2 mo | 1 mo | - |
| OS | > 5 yr | > 3 yr | 15 mo | 2.6 mo | 2.5 mo | 2 mo | 1.8 mo |
Gallbladder sarcomatoid carcinoma is an extremely rare malignancy with aggressive behavior, representing only 4.3% of all gallbladder cancers, and has often been confused with carcinosarcoma in previous reports, as long as they have both epithelioid and sarcomatoid components[10]. In our opinion, there are two different kinds of malignant neoplasms. Kim et al[4] found that in sarcomatoid carcinoma, the tumor cells of the sarcomatous area coexpress CK and vimentin immuno-histochemically and ultrastructurally showed desmosome-like junctions and aggregates of cytoplasmic intermediate filaments. These findings suggested that the sarcomatoid components in sarcomatoid carcinoma were essentially carcinoma. According to the World Health Organization classification of digestive system tumors[11], carcinosarcoma is composed of carcinoma and sarcoma components. The sarcoma components can be chondrosarcoma, fibrosarcoma, leiomyosarcoma and so on. In this situation, immunohistochemistry shows that sarcomatoid and epithelioid components express interstitial and epithelioid markers, respectively[12]. Although sarcoma-like spindle cells were mixed with carcinoma tissue in our report, the cells actually expressed epithelioid markers and not sarcomatoid markers on immunohistochemistry. These results indicated that the sarcoma-like component was essentially carcinoma, and our pathologists defined those as sarcomatoid carcinoma rather than carcinosarcoma.
Females seem to be more likely to develop gallbladder tumors than males. Among all cases of gallbladder cancer, female patients are three times more common than male patients, and in sarcomatoid carcinoma, the trend is much more pronounced, as female patients are five to six times more common[2]. Advanced age is considered to be a high-risk factor for gallbladder cancer[2]. The median age was 68.5 years, and approximately 71.4% (5/7) of the patients in our center were over 65 years old, which is higher than the 40% in previous reports[3-8]. Nevertheless, interestingly, we found that two patients who have not yet developed recurrence were as old as 80, which may suggest that advanced age is a predictor of a better prognosis in gallbladder sarcomatoid carcinoma. Almost every patient with gallbladder sarcomatoid carcinoma shows symptoms of abdominal pain. However, most of the patients in our report had advanced-stage disease, and the tumor was larger than 7 cm. Visiting the hospital for an abdominal CT or ultrasound examination after the onset of abdominal symptoms is too late. For older patients, it may be more possible and economical to detect gallbladder lesions at an earlier stage by routine abdominal sonography, especially high-resolution[13] or contrast-enhanced[14] sonography, before the onset of symptoms.
The elevations in CEA, CA19-9, and CA-125 appear to be indicators of a poor prognosis in patients with gallbladder sarcomatoid carcinoma. CEA and CA19-9 are recommended tumor markers associated with gallbladder cancer in the National Comprehensive Cancer Network (NCCN) guidelines. The elevations in CEA and CA19-9 in gallbladder sarcomatoid carcinoma reported in the previous literature were not obvious[4,5,7,8]. In our report, three (60%) patients with recurrence or metastasis had significantly high CA19-9 levels, with the highest level exceeding 1000 U/mL. CA-125, initially thought to be a specific biomarker for ovarian cancer, is considered a potential biomarker of digestive tract neoplasms and is associated with a poor survival[15,16]. There were three (60%) patients with recurrence or metastasis who had significantly high CA-125 levels, and the number of patients with stage IV disease was approximately seven times higher than normal. Furthermore, the two patients without recurrence with stage I or stage II disease showed normal CEA, CA19-9, and CA-125 levels. Unfortunately, we did not continue to monitor the changes in the above tumor markers after progression and cannot further explain their correlations with the disease.
TNM stage seems to be an important indicator for the prognosis of gallbladder neoplasm[17]. In our report, the prognosis of patients with early-stage disease (stage I and stage II) was much better than that of patients with advanced-stage disease. The patients with stage I and stage II disease did not have recurrence for more than 5 years and 3 years, respectively. However, most of the patients who had advanced-stage disease were found to have recurred at approximately 2 mo and died quickly thereafter. These data are similar to those reported in the previous literature. The majority of patients with advanced stages disease recurred between 2 and 3 mo, and the survival time was between 5 and 12 mo in previous reports[3-8]. The data of patients with early-stage disease were not clear because there were no previous reports. However, no mention of long-term survival in patients with advanced-stage disease has been found until now.
Surgery is suggested as the first and only recognized treatment for sarcomatoid carcinoma of the gallbladder. Radical cholecystectomy refers to extensive resection of the gallbladder bed with a rim of liver tissues and peripheral metastatic lymph nodes or tissues. However, even with such a wide excision range, patients with late stage disease still recurred quickly after surgery, and the liver was the most common site, which may be because the serosa is lacking on the side that the gallbladder embeds in the liver. In total, two patients in our report and six in the past literature recurred only 2 mo after the operation[3-5,8]. These frustrating results suggest that surgical treatment is not enough for patients with locally advanced disease and that postoperative adjuvant treatment may be appropriate. Because of the rarity of gallbladder sarcomatoid carcinoma, there was no previously recommended adjuvant treatment. We considered that sarcomatoid carcinoma of the gallbladder was a kind of carcinoma, not sarcoma, so we referred to the adjuvant treatment regimen for gallbladder cancer. A nomogram suggested that certain subsets of patients with at least stage T2 or N1 disease will gain survival benefits from adjuvant chemoradiotherapy[18]. Wang et al[19] suggested that adjuvant radiotherapy provides a survival benefit in node-positive or ≥ T2 gallbladder cancer. The combination of gemcitabine and capecitabine followed by concurrent capecitabine and radiotherapy (45 Gy to regional lymph nodes; 54 to 59.4 Gy to the tumor bed) was proven to be well tolerated and promising by Ben-Josef et al[20]. Gemcitabine and cisplatin were used in a randomized, multinational phase III trial and were thought to increase the 24-mo postoperative disease-free survival (DFS) rate from 35% to 55% in gallbladder cancer[21]. Moreover, adjuvant chemotherapy with gemcitabine and adjuvant radiotherapy are still recommended by the NCCN clinical practice guidelines. Therefore, we treated the patient with a stage T2N1M0 tumor with gemcitabine and capecitabine for two cycles followed by radiotherapy (5040 cGy to the tumor bed and regional lymph nodes). Two additional cycles of the same chemotherapy as before were administered after the radiotherapy. With the combination of adjuvant chemotherapy and radiotherapy, the PFS of this patient was 12 mo, which is much longer than that of patients with advanced-stage gallbladder sarcomatoid carcinoma reported in the literature. To the best of our knowledge, this is the first detailed report of postoperative adjuvant treatment for gallbladder sarcomatoid carcinoma, and the patient got a PFS of 12 mo, which is the longest reported period to date. Gemcitabine combined with fluorouracil and radiotherapy could be considered as an adjuvant treatment for patients with locally advanced gallbladder sarcomatoid carcinoma. Unfortunately, liver and bone metastases occurred 3 mo in the patient after the completion of adjuvant therapy, suggesting that our adjuvant regimen could be further optimized. These optimizations may include an increase in the dose of radiotherapy at the liver site, as well as appropriate maintenance chemotherapy.
Gallbladder sarcomatoid carcinoma is a unique gallbladder malignancy with a poor prognosis. Surgery is suggested as the first and only recognized treatment. There is a significant difference in prognosis between patients with early-stage and advanced-stage disease. Postoperative adjuvant therapy may bring survival benefits for locally advanced patients. Gemcitabine combined with fluorouracil and radiotherapy could be a choice that should be further tested and optimized.
This study was generously supported by the Department of Pathology, West China Hospital of Sichuan University.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Filippou D, Kai K S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Li JH
| 1. | Bartlett DL. Gallbladder cancer. Semin Surg Oncol. 2000;19:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Kanthan R, Senger JL, Ahmed S, Kanthan SC. Gallbladder Cancer in the 21st Century. J Oncol. 2015;2015:967472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 3. | Liu KH, Yeh TS, Hwang TL, Jan YY, Chen MF. Surgical management of gallbladder sarcomatoid carcinoma. World J Gastroenterol. 2009;15:1876-1879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Kim MJ, Yu E, Ro JY. Sarcomatoid carcinoma of the gallbladder with a rhabdoid tumor component. Arch Pathol Lab Med. 2003;127:e406-e408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Takahashi Y, Fukushima J, Fukusato T, Shiga J. Sarcomatoid carcinoma with components of small cell carcinoma and undifferentiated carcinoma of the gallbladder. Pathol Int. 2004;54:866-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Hu ZH, Li ZW, Shen L, Zhang M, Zheng SS. Surgical therapy and prognosis of sarcomatoid carcinoma of the gallbladder. Hepatobiliary Pancreat Dis Int. 2010;9:175-179. [PubMed] |
| 7. | Kataria K, Yadav R, Seenu V. Sarcomatoid carcinoma of the gall bladder. J Surg Case Rep. 2012;2012:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Doval DC, Azam S, Mehta A, Pruthi A, Batra U, Choudhury KD, Kumar K. A report of sarcomatoid carcinoma of the gallbladder treated with palliative deocetaxel and gemcitabine chemotherapy. J Gastrointest Cancer. 2014;45 Suppl 1:270-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Rahman R, Simoes EJ, Schmaltz C, Jackson CS, Ibdah JA. Trend analysis and survival of primary gallbladder cancer in the United States: a 1973-2009 population-based study. Cancer Med. 2017;6:874-880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Lopez-Beltran A, Pacelli A, Rothenberg HJ, Wollan PC, Zincke H, Blute ML, Bostwick DG. Carcinosarcoma and sarcomatoid carcinoma of the bladder: clinicopathological study of 41 cases. J Urol. 1998;159:1497-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 163] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Hamilton S.R. ALA. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. IARC Press. 2000;203-217. |
| 12. | Bloxham CA, Bennett MK, Robinson MC. Bladder carcinosarcomas: three cases with diverse histogenesis. Histopathology. 1990;16:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Kim JH, Lee JY, Baek JH, Eun HW, Kim YJ, Han JK, Choi BI. High-resolution sonography for distinguishing neoplastic gallbladder polyps and staging gallbladder cancer. AJR Am J Roentgenol. 2015;204:W150-W159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Wang H, Ling W, Luo Y. Contrast-enhanced ultrasound findings of gallbladder adenocarcinoma with sarcomatoid carcinoma accompanied by intrahepatic metastasis: A case report and literature review. Medicine (Baltimore). 2018;97:e10773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Streppel MM, Vincent A, Mukherjee R, Campbell NR, Chen SH, Konstantopoulos K, Goggins MG, Van Seuningen I, Maitra A, Montgomery EA. Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum Pathol. 2012;43:1755-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Luo T, Chen W, Wang L, Zhao H. CA-125 is a potential biomarker to predict surgically incurable gastric and cardia cancer: A retrospective study. Medicine (Baltimore). 2016;95:e5297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg. 2000;232:557-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 311] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 18. | Wang SJ, Lemieux A, Kalpathy-Cramer J, Ord CB, Walker GV, Fuller CD, Kim JS, Thomas CR. Nomogram for predicting the benefit of adjuvant chemoradiotherapy for resected gallbladder cancer. J Clin Oncol. 2011;29:4627-4632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 19. | Wang SJ, Fuller CD, Kim JS, Sittig DF, Thomas CR, Ravdin PM. Prediction model for estimating the survival benefit of adjuvant radiotherapy for gallbladder cancer. J Clin Oncol. 2008;26:2112-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Ben-Josef E, Guthrie KA, El-Khoueiry AB, Corless CL, Zalupski MM, Lowy AM, Thomas CR, Alberts SR, Dawson LA, Micetich KC, Thomas MB, Siegel AB, Blanke CD. SWOG S0809: A Phase II Intergroup Trial of Adjuvant Capecitabine and Gemcitabine Followed by Radiotherapy and Concurrent Capecitabine in Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma. J Clin Oncol. 2015;33:2617-2622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 274] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 21. | Stein A, Arnold D, Bridgewater J, Goldstein D, Jensen LH, Klümpen HJ, Lohse AW, Nashan B, Primrose J, Schrum S, Shannon J, Vettorazzi E, Wege H. Adjuvant chemotherapy with gemcitabine and cisplatin compared to observation after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA-1 trial) - a randomized, multidisciplinary, multinational phase III trial. BMC Cancer. 2015;15:564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 163] [Article Influence: 16.3] [Reference Citation Analysis (0)] |