Published online Sep 6, 2020. doi: 10.12998/wjcc.v8.i17.3853
Peer-review started: June 6, 2020
First decision: June 19, 2020
Revised: July 2, 2020
Accepted: July 30, 2020
Article in press: July 30, 2020
Published online: September 6, 2020
Processing time: 89 Days and 20.8 Hours
Facial cosmetic procedures become popular for people with a desire to have a younger appearance, and cosmetic technology has developed rapidly over the past several decades. However, increasing complications related to cosmetic injections have been reported, and infection is one of the most serious problems and can cause anxiety and facial injury. We here report a case of Majocchi's granuloma (MG) caused by Trichophyton rubrum after facial injection of hyaluronic acid.
A 37-year-old woman presented to our hospital with a history of red papules, nodules, and abscesses on her left zygomatic arch for 2 mo. She had received a cosmetic injection of hyaluronic acid on the left side of her face prior to the appearance of the lesions. MG caused by Trichophyton rubrum after facial injection of hyaluronic acid was diagnosed based on morphology and molecular biological identification. In vitro antifungal susceptibility testing was conducted according to the Clinical and Laboratory Standards Institute M38-A2 method. Minimal inhibitory concentrations were used to evaluate the antifungal susceptibility. The antifungal agents and their minimal inhibitory concentrations for the strain were terbinafine (< 0.5 μg/mL), itraconazole (0.06 μg/mL), amphotericin B (0.25 μg/mL), fluconazole (32 μg/mL), voriconazole (0.125 μg/mL), posaconazole (0.125 μg/mL), and isavuconazole (0.06 μg/mL). We initially administered 250 mg/d oral terbinafine for 2 mo, but the patient still had painful papules, nodules and abscesses on her face. Then, we adjusted the treatment to itraconazole 400 mg/d for 8 wk based on the in vitro antifungal susceptibility testing results. The skin lesions improved significantly, and there was no recurrence during follow-up.
This case revealed that facial injection of hyaluronic acid may cause serious MG. Antifungal susceptibility testing should be considered in the treatment of MG caused by Trichophyton rubrum.
Core tip: Facial cosmetic procedures become popular due to the demand to attain a younger appearance and the development of cosmetic technology over the past decades. The risk of infection is increasing because of improper disinfection of the patient’s skin, incorrect injection technique, decreased general immunity, and the presence of pathogens. Here, we describe a case of Majocchi’s granuloma caused by Trichophyton rubrum, and the patient was successfully treated with itraconazole.
- Citation: Liu J, Xin WQ, Liu LT, Chen CF, Wu L, Hu XP. Majocchi's granuloma caused by Trichophyton rubrum after facial injection with hyaluronic acid: A case report. World J Clin Cases 2020; 8(17): 3853-3858
- URL: https://www.wjgnet.com/2307-8960/full/v8/i17/3853.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i17.3853
With the increasing popularity of cosmetic injections, such as hyaluronic acid and botulinum injection, an increasing number of related complications after cosmetic injections have been reported recently[1,2]. Hyaluronic acid fillers may cause early and immediate adverse reactions, such as needle marks or pinpoint bleeding, erythema or redness, swelling and edema, pain, tenderness, and ecchymosis, in 0.05% to 2% of cases[3]. Delayed adverse reactions, including hypersensitivity and granulomatous reaction, are reported to occur in up to 0.6% of cases[4].
Among the complications, the risk of infection increased by improper disinfection of the patient’s skin, incorrect injection techniques, decreased general immunity, and the presence of pathogens[5]. To date, the difficulty in the study of Majocchi's granuloma (MG) from an infection lies in the accurate diagnosis of the organism and the identification of susceptibility to antifungal testing in vitro, which are important to guide the treatment of MG caused by pathogens. We report a case of MG caused by Trichophyton rubrum infection after facial injection of hyaluronic acid.
A 37-year-old woman presented with red papules, nodules, and abscesses on her left zygomatic arch for 2 mo.
The patient’s symptoms started 2 mo prior to evaluation. The scale and size of the skin lesions on her face gradually increased, and some of the nodules and plaques ruptured and caused pain.
The patient received cosmetic injection of hyaluronic acid on the left side of her face in a small clinic prior to the appearance of lesions. The patient was diagnosed with a skin infection at the local hospital, but the lesions did not improve after antibiotic ointment treatment. She had no history of fungal infections or other systemic diseases, no history of risk from her residence, no contact with pets, and no history of tuberculosis.
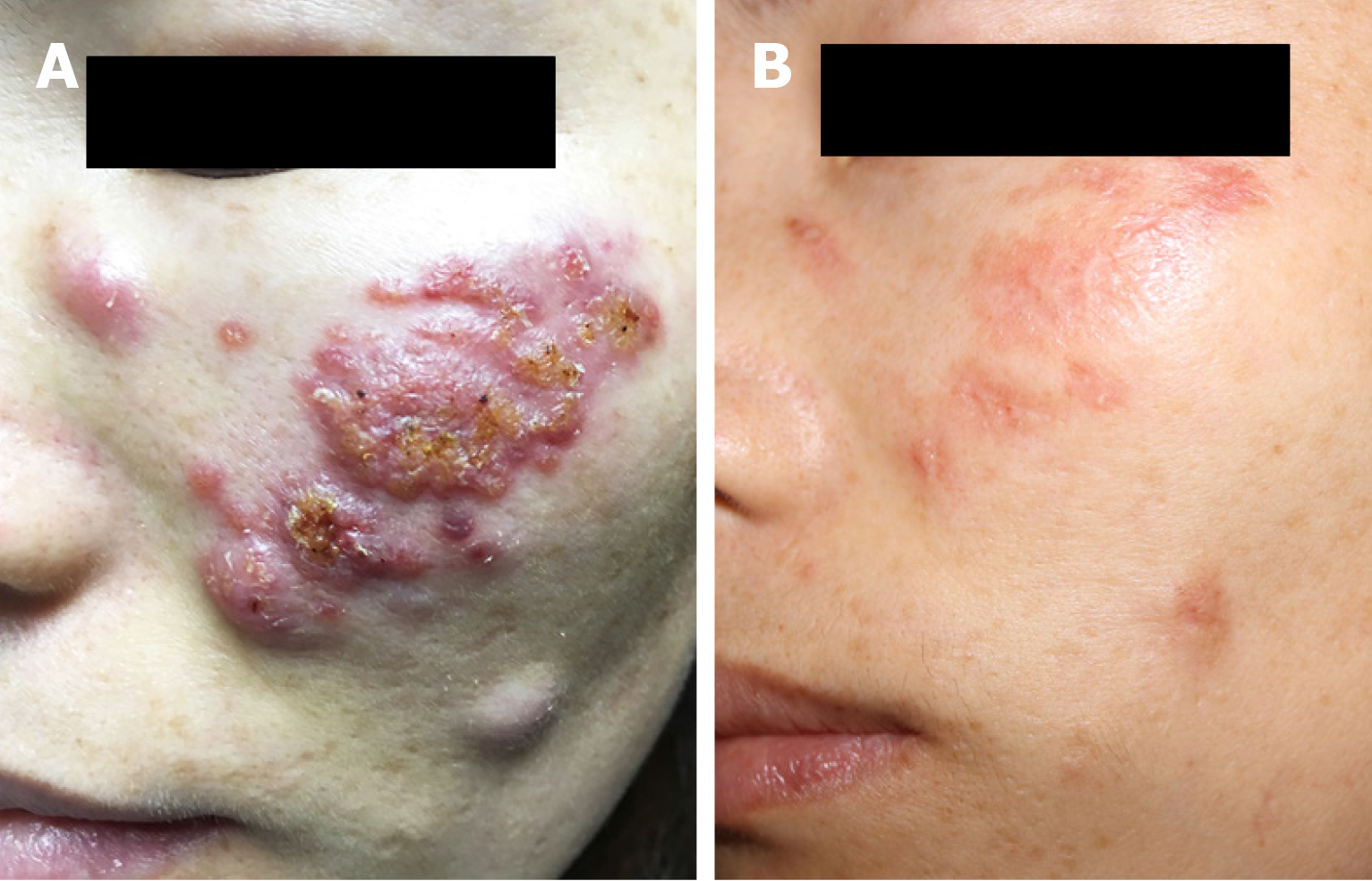
Dermatological examination revealed red papules, nodules, and abscesses on the left side of her face. The size of the nodules varied, the largest nodule was approximately 0.8 cm × 0.6 cm and the smallest nodule was approximately 0.3 cm × 0.2 cm. Some nodules and abscesses produced purulent secretions. Necrotic tissue was attached to the wound, causing burning, pinching pain in the patient (Figure 1A).
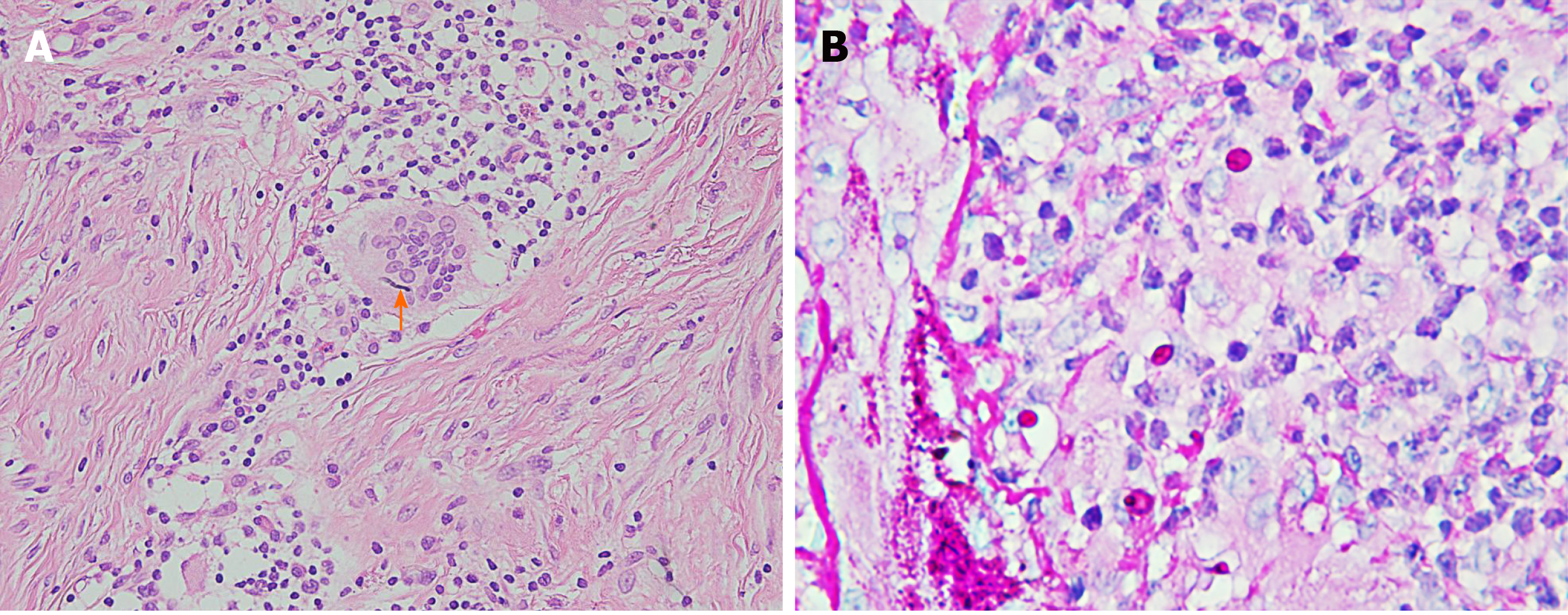
Laboratory examinations revealed a leukocyte count of 4 × 109/L, hemoglobin level of 110 g/L, and platelet count of 170 × 109/L. There were no abnormal results in the renal and hepatic function tests. Tests for hepatitis B, hepatitis C, human immunodeficiency virus, cytomegalovirus and Epstein-Barr virus were negative. Histological examination revealed diffuse multinucleated giant cells as well as infiltration of lymphocytes and neutrophils into the dermis. The pathological findings of the skin biopsy included acanthosis and papilloma by hematoxylin-eosin staining (Figure 2A) and dermal granuloma and cytoplasmic branched septate hyphae by periodic acid-Schiff staining (Figure 2B). The results of the T-SPOT-TB test, acid-fast stain, human immunodeficiency virus test, and rapid plasma reagin test were also negative.
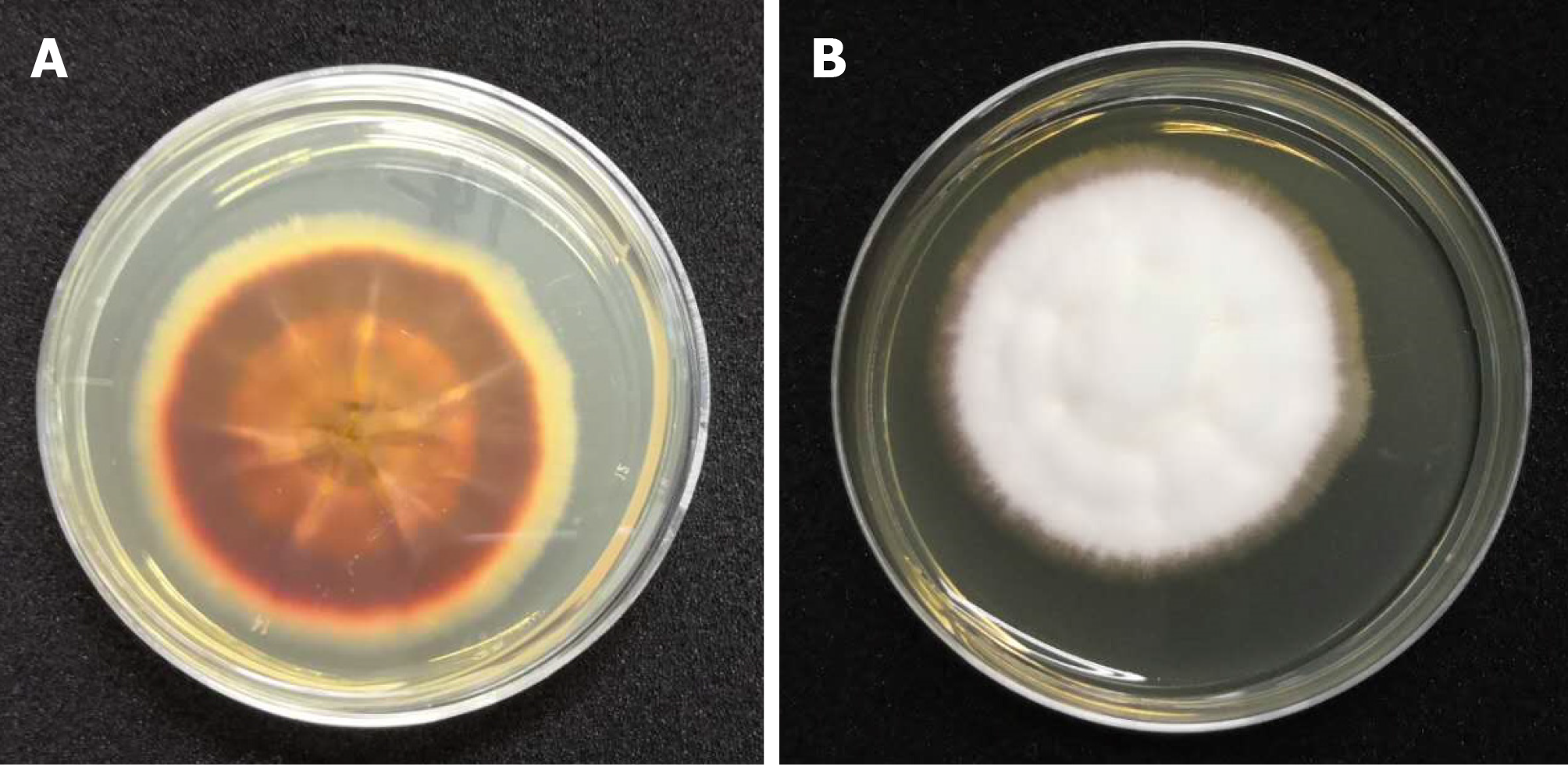
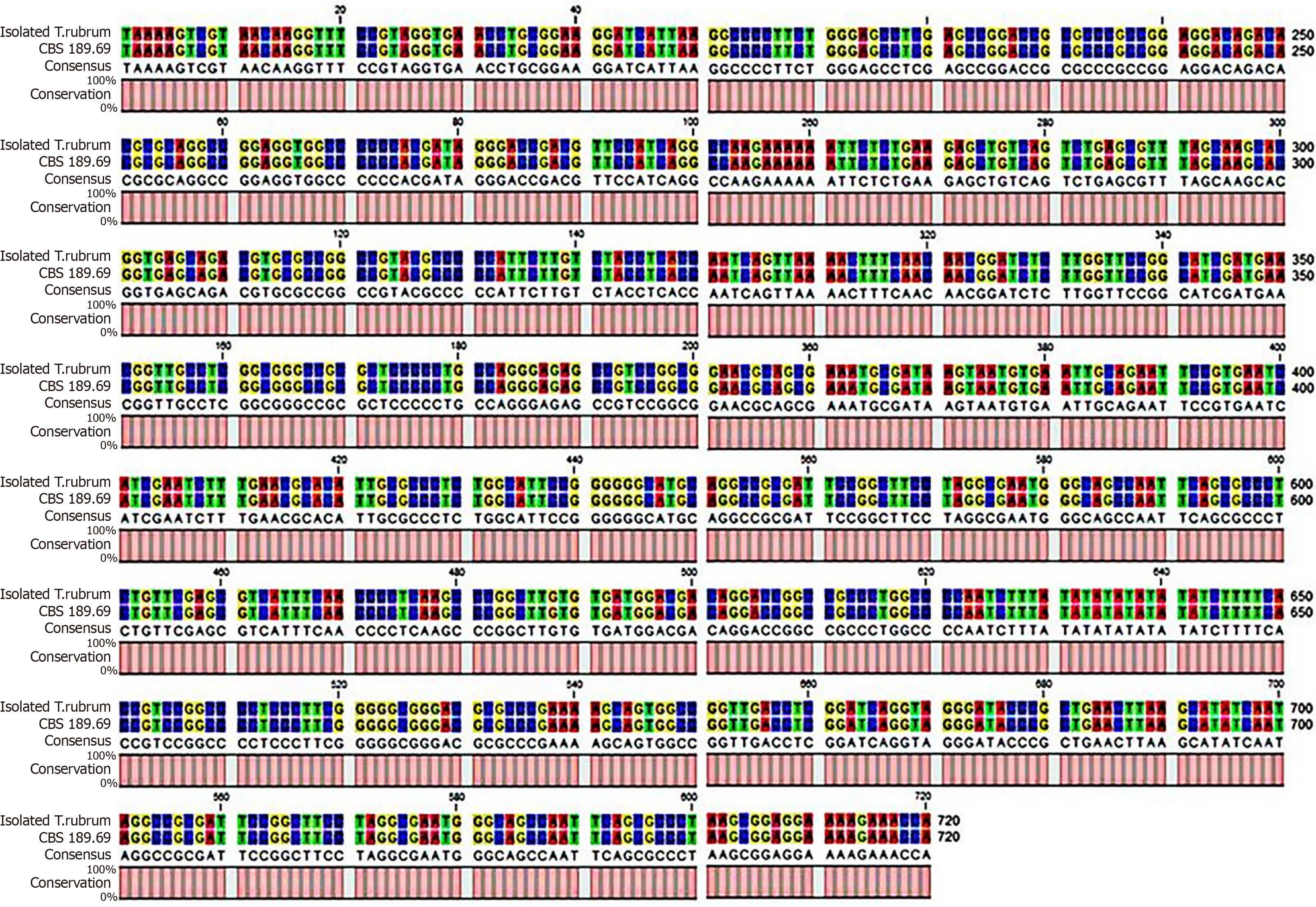
Skin biopsy samples taken from the active borders were cultured in Sabouraud dextrose agar and incubated at 28 °C for 2 wk. Septated hyaline hyphae and chains of arthrospores were visualized on 10% KOH wet mount preparations. The organism grew radially and produced reddish-brown and yellow pigments at the same time (Figure 3). We further extracted the fungal deoxyribonucleic acid and employed primers ITS-1 (5’-TCCGTAGGTGAACCTGCGG-3’) and ITS-4 (5’-TCCTCCGCTTATTGATATGC-3’) to perform polymerase chain reaction amplification. The amplification product was sequenced and then analyzed with BLAST in GenBank, and the genetic sequence was more than 99% similar to the standard strain of Trichophyton rubrum CBS 189.69 (Figure 4). Therefore, the fungus was finally identified as Trichophyton rubrum. We performed in vitro antifungal susceptibility testing according to the Clinical and Laboratory Standards Institute M38-A2 method[6] and found that the isolated strain of Trichophyton rubrum was sensitive to most antifungal agents, including terbinafine (< 0.5 μg/mL), itraconazole (0.06 μg/mL), amphotericin B (0.25 μg/mL), fluconazole (32 μg/mL), voriconazole (0.125 μg/mL), posaconazole (0.125 μg/mL), and isavuconazole (0.06 μg/mL).
A diagnosis of MG caused by Trichophyton rubrum after facial injection of hyaluronic acid was made based on the cosmetic injection history, the presence of skin lesions, morphological and molecular biological results and histological examination.
The patient was initially started on terbinafine 250 mg/d for 2 mo, but the painful papules, nodules and abscesses on her face did not improve. Then, we adjusted the treatment to itraconazole 400 mg/d for 8 wk based on the in vitro antifungal susceptibility testing results.
The skin lesions improved significantly after treatment with itraconazole 400 mg/d for 8 wk, and there was no recurrence during the follow-up (Figure 1B).
Facial cosmetic procedures become popular because of people’s desire for a younger appearance and the development of cosmetic technology over the past several decades[7]. To date, hyaluronic acid is reported to be safe and well tolerated and has become one of the most important fillers for cosmetic rejuvenescence. However, complications, such as infection, anaphylaxis and embolism after facial injection, are more common as more cosmetic procedures are performed, especially cosmetic injections administered in small unlicensed clinics.
Dermatophytes are widespread and are among the most common cause of superficial fungal infections worldwide[8]. Trichophyton rubrum is currently recognized as the most common fungal pathogen among dermatophyte infections and is often intractable. MG is an inflammatory and granulomatous dermatophyte infection, and the main pathogen that causes MG is Trichophyton rubrum. It has been reported that the number of cases of MG has been rising over the years[9]. In the present case, the patient and her family members had no history of dermatophytosis. She had no contact with others except her family members after undergoing cosmetic injection of hyaluronic acid. She presented with multiple painful abscesses on the left side of her face, and the lesion was located at the site of cosmetic injection. A contaminant from the operating room of the unqualified clinic where the injection procedure was performed likely caused the Trichophyton rubrum infection.
Systemic treatment of Trichophyton rubrum infection mainly includes allylamines, azoles, polyenes and hydroxypyridones. In general, terbinafine (250 mg/d), as a representative allylamine antifungal agent, is the preferred systemic antifungal drug for the treatment of MG and is used in 40.8% of cases. When the infectious agent cannot be isolated, terbinafine should be the first-choice treatment because organisms resistant to itraconazole or griseofulvin have been reported to be responsive to terbinafine. Wang et al[10] presented two cases of MG with different clinical features. Both patients had satisfactory relief after treatment with terbinafine. Su et al[11] also successfully treated a case of Trichophyton rubrum infection characterized by MG and deeper dermatophytosis with oral terbinafine 250 mg/d, and the patient's skin lesion disappeared. However, terbinafine-resistant strains have been found in cases of drug-resistant dermatophyte infection, including Trichophyton rubrum infection, in recent years[12-14]. Other systemic antifungal drugs to treat dermatophyte infections include itraconazole, griseofulvin, ketoconazole, voriconazole, and posaconazole[15-19]. Gupta et al[20] found that two pulses of oral itraconazole therapy appeared to be effective in the treatment of MG. In the case we reported here, the patient was unresponsive to terbinafine and was successfully treated with itraconazole, which should indicate the need for doctors to select treatment agents by performing in vitro antifungal susceptibility testing.
Mycology examinations are important to identify the causative agent, especially the type of invasive dermatophytosis, and can determine the treatment. In the case of MG and other invasive dermatophyte infections that are difficult to treat, such as mycetoma and pseudomycetoma, terbinafine is the preferred choice for treatment. Therefore, it is of key importance to accurately diagnose the disease-causing agents, especially in immunocompromised patients. In addition, cosmetic injections should be given in a sterile environment, and people should be aware of the risk of fungal infection in the pursuit of beauty.
This case revealed that facial injection of hyaluronic acid may cause serious MG. Antifungal susceptibility testing should be conducted to help select proper agents during clinical treatment.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Chinese Journal of Dermatology and Venereology, Editorial board member; Fungi Group of Guangdong Dermatology and Venereal Diseases Society, Member.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mastoraki A S-Editor: Zhang L L-Editor: MedE-Ma JY P-Editor: Xing YX
| 1. | Ozturk CN, Li Y, Tung R, Parker L, Piliang MP, Zins JE. Complications following injection of soft-tissue fillers. Aesthet Surg J. 2013;33:862-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (1)] |
| 2. | Shin JY, An MY, Roh SG, Chang SC, Lee NH. Unusual Aspergillus Infection after Dermal Filler Injection. J Craniofac Surg. 2017;28:2066-2067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Weinberg MJ, Solish N. Complications of hyaluronic acid fillers. Facial Plast Surg. 2009;25:324-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Lowe NJ, Maxwell CA, Lowe P, Duick MG, Shah K. Hyaluronic acid skin fillers: adverse reactions and skin testing. J Am Acad Dermatol. 2001;45:930-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 174] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Jahn K, Homey B, Gerber PA. [Management of complications after aesthetic hyaluronic acid injections]. Hautarzt. 2014;65:851-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Wayne P. Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. CLSI Document M38-A2. Clinical and Laboratory Standards Institute, 2008. Available from: https://infostore.saiglobal.com/en-us/Standards/CLSI-M38-A2-2ED-2008-357324_SAIG_CLSI_CLSI_813977/. |
| 7. | Fedok FG. Advances in minimally invasive facial rejuvenation. Curr Opin Otolaryngol Head Neck Surg. 2008;16:359-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Weitzman I, Summerbell RC. The dermatophytes. Clin Microbiol Rev. 1995;8:240-259. [PubMed] |
| 9. | Boral H, Durdu M, Ilkit M. Majocchi's granuloma: current perspectives. Infect Drug Resist. 2018;11:751-760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Wang X, Yang Y, Li R, Yu J. Two Cases of Dermatophytic Granuloma Successfully Treated with Terbinafine. Mycopathologia. 2018;183:611-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Su H, Li L, Cheng B, Zhu J, Zhang Q, Xu J, Zhu M. Trichophyton rubrum Infection Characterized by Majocchi's Granuloma and Deeper Dermatophytosis: Case Report and Review of Published Literature. Mycopathologia. 2017;182:549-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Mukherjee PK, Leidich SD, Isham N, Leitner I, Ryder NS, Ghannoum MA. Clinical Trichophyton rubrum strain exhibiting primary resistance to terbinafine. Antimicrob Agents Chemother. 2003;47:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 214] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 13. | Favre B, Ghannoum MA, Ryder NS. Biochemical characterization of terbinafine-resistant Trichophyton rubrum isolates. Med Mycol. 2004;42:525-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Osborne CS, Leitner I, Favre B, Ryder NS. Amino acid substitution in Trichophyton rubrum squalene epoxidase associated with resistance to terbinafine. Antimicrob Agents Chemother. 2005;49:2840-2844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Ilkit M, Durdu M, Karakaş M. Majocchi's granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Lyra MR, Muniz Álvarez B, Lanziano AL, Imbeth MAA, Sá AM, Cuzzi T, Oliveira JC, Schubach AO. Exfoliative erythroderma and palmoplantar hyperkeratosis associated with Majocchi's granuloma by Trichophyton tonsurans in a patient with AIDS. Rev Iberoam Micol. 2017;34:185-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Swali R, Ramos-Rojas E, Tyring S. Majocchi granuloma presenting as a verrucous nodule of the lip. Proc (Bayl Univ Med Cent). 2018;31:115-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Mazur M, Lodyga M, Łańczak A, Adamski Z. Majocchi's granuloma (granuloma trichophyticum) in a guinea pig owner: A case report and literature review. J Mycol Med. 2018;28:523-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Rallis E, Katoulis A, Rigopoulos D. Pubic Majocchi's Granuloma Unresponsive to Itraconazole Successfully Treated with Oral Terbinafine. Skin Appendage Disord. 2016;1:111-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Gupta AK, Groen K, Woestenborghs R, De Doncker P. Itraconazole pulse therapy is effective in the treatment of Majocchi's granuloma: a clinical and pharmacokinetic evaluation and implications for possible effectiveness in tinea capitis. Clin Exp Dermatol. 1998;23:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |