Published online Sep 6, 2020. doi: 10.12998/wjcc.v8.i17.3847
Peer-review started: April 23, 2020
First decision: June 13, 2020
Revised: June 23, 2020
Accepted: July 30, 2020
Article in press: July 30, 2020
Published online: September 6, 2020
Processing time: 133 Days and 21.8 Hours
Ulcerative colitis (UC) is defined as a chronic inflammatory bowel disease that can occur in any part of the large bowel. In addition, UC affects only the large bowel except for backwash ileitis and pouchitis, whereas Crohn's disease (CD) affects the entire digestive tract. Inflammatory bowel disease (IBD) patients tend to be diagnosed with CD or indeterminate colitis when combined with gastric lesion. However, in recent years, some UC patients are reported to have various degrees of lesions in gastroduodenum. Here, we report a case of gastroduodenitis associated with UC (GDUC).
A 25-year-old man with a history of Klippel-Trenaunay syndrome presented to the hospital with mucopurulent bloody stool and epigastric persistent colic pain for 2 wk. Continuous superficial ulcers and spontaneous bleeding were observed under colonoscopy. Subsequent gastroscopy revealed mucosa with diffuse edema, ulcers, errhysis, and granular and friable changes in the stomach and duodenal bulb, which were similar to the appearance of the rectum. After ruling out other possibilities according to a series of examinations, a diagnosis of GDUC was considered. The patient hesitated about intravenous corticosteroids, so he received a standardized treatment with pentasa of 3.2 g/d. After 0.5 mo of treatment, the patient’s symptoms achieved complete remission. Follow-up endoscopy and imaging findings showed no evidence of recurrence for 26 mo.
The occurrence of gastrointestinal involvement in UC is rare, which may open a new window for studying the etiology and pathogenesis of UC. Physicians should consider broad differential diagnosis by endoscopic biopsy and laboratory examinations.
Core tip: Gastroduodenitis associated with ulcerative colitis (GDUC) is rare. We here report a case of GDUC. The Diagnosis and treatment process was described in detail, which may open a new window for studying the etiology and pathogenesis of UC. We also discussed the relationship between UC and gastric lesions. Physicians should consider broader differential diagnosis by endoscopic biopsy and laboratory examinations.
- Citation: Yang Y, Li CQ, Chen WJ, Ma ZH, Liu G. Gastroduodenitis associated with ulcerative colitis: A case report. World J Clin Cases 2020; 8(17): 3847-3852
- URL: https://www.wjgnet.com/2307-8960/full/v8/i17/3847.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i17.3847
Ulcerative colitis (UC) is a chronic nonspecific inflammatory disease of the colon and rectum. Its etiology is unclear. Some studies have confirmed that the disease is related to genetic, infectious and autoimmune factors[1]. Bloody diarrhea is the most common early symptom. Other symptoms include abdominal pain, hematochezia, weight loss, vomiting and so on. UC generally affects rectum and colon. The upper digestive tract is not generally considered to be the target organ in UC. In recent years, it has been reported that gastric and duodenal mucosal lesions may also occur in patients with UC[2-5]. We describe a rare case of UC involving gastroduodenum, and demonstrates that the manifestations of the gastroduodenum under endoscopy, histopathology and radiography are similar to the lesions of colorectum.
A 25-year-old man presented to the hospital with mucopurulent bloody stool and epigastric persistent colic pain.
The patient’s symptoms started 2 wk ago. He was treated with a 2-wk course of standard anti-acid treatment as well as symptomatic therapies, such as spasmolysis and antibiotics, which were ineffective in alleviating his symptoms.
The patient was previously diagnosed with Klippel-Trenaunay syndrome.
The patient's vital signs were stable. Physical examination showed epigastric tenderness.
Laboratory results showed a significant elevation of white-cell count and C-reactive protein value, which were respectively 18.8 × 109/L and 59 mg/L. Tests for perinuclear antineutrophil cytoplasmic antibodies and anti-Saccharomyces cerevisiae antibodies were negative. Neither Helicobacter Pylori nor Epstein-Barr virus infection was detected.
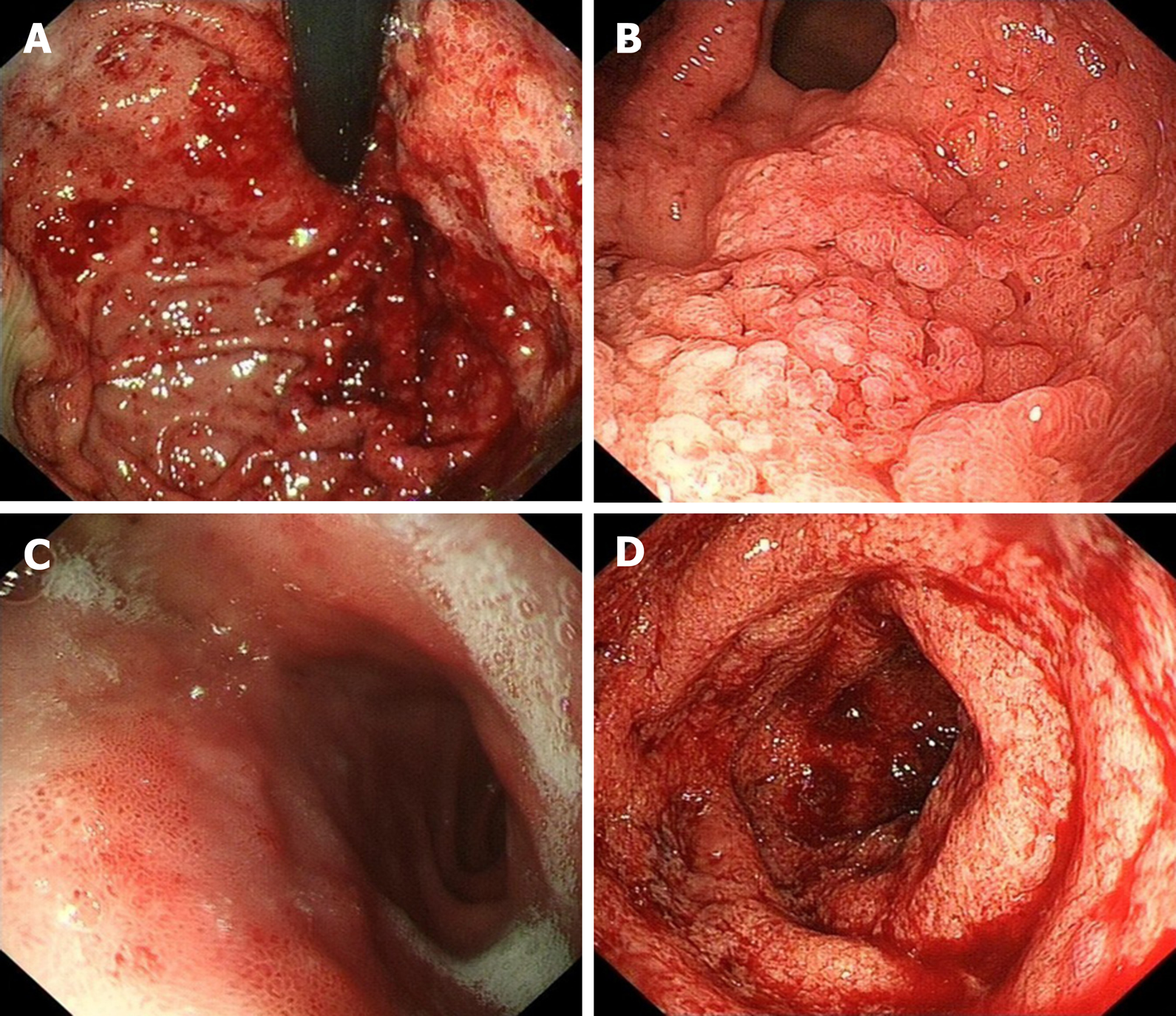
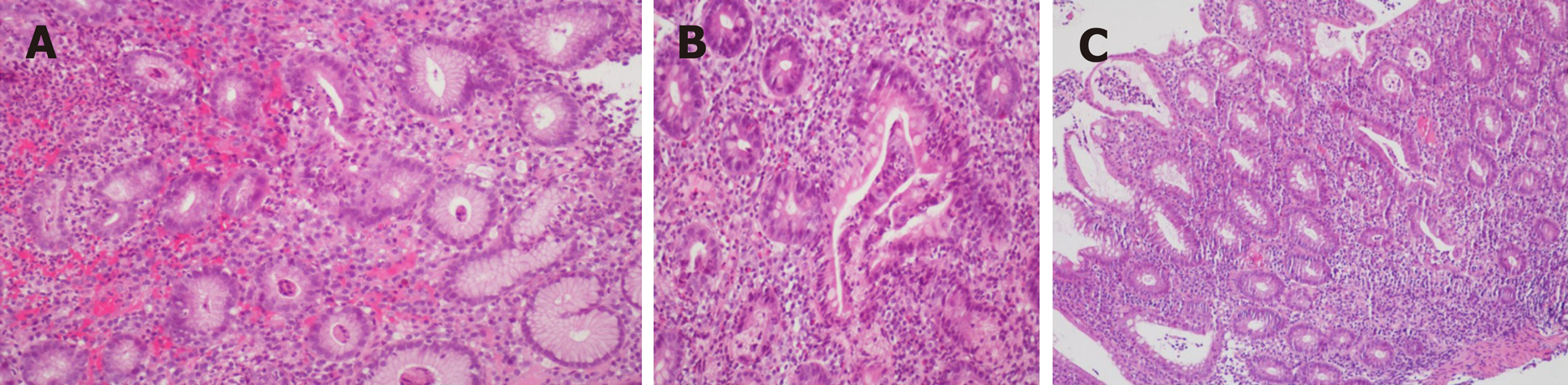
Endoscopy revealed mucosa with diffuse edema, ulcers, errhysis, and granular and friable changes in the stomach (Figure 1A and B) and duodenal bulb (Figure 1C), which were similar to the appearance of the rectum (Figure 1D). Biopsy specimens from the gastroduodenum (Figure 2A and B) and colorectum (Figure 2C) showed diffuse inflammation, acute cryptitis, and abscesses. Disease activity and extent were determined, and the activity of the mucosal inflammation was scored using the Mayo endoscopic subscore (Table 1).
| Locations | MES | |
| Before treatment | After treatment | |
| Gastroduodenum | 3 | 0 |
| Rectum | 3 | 0 |
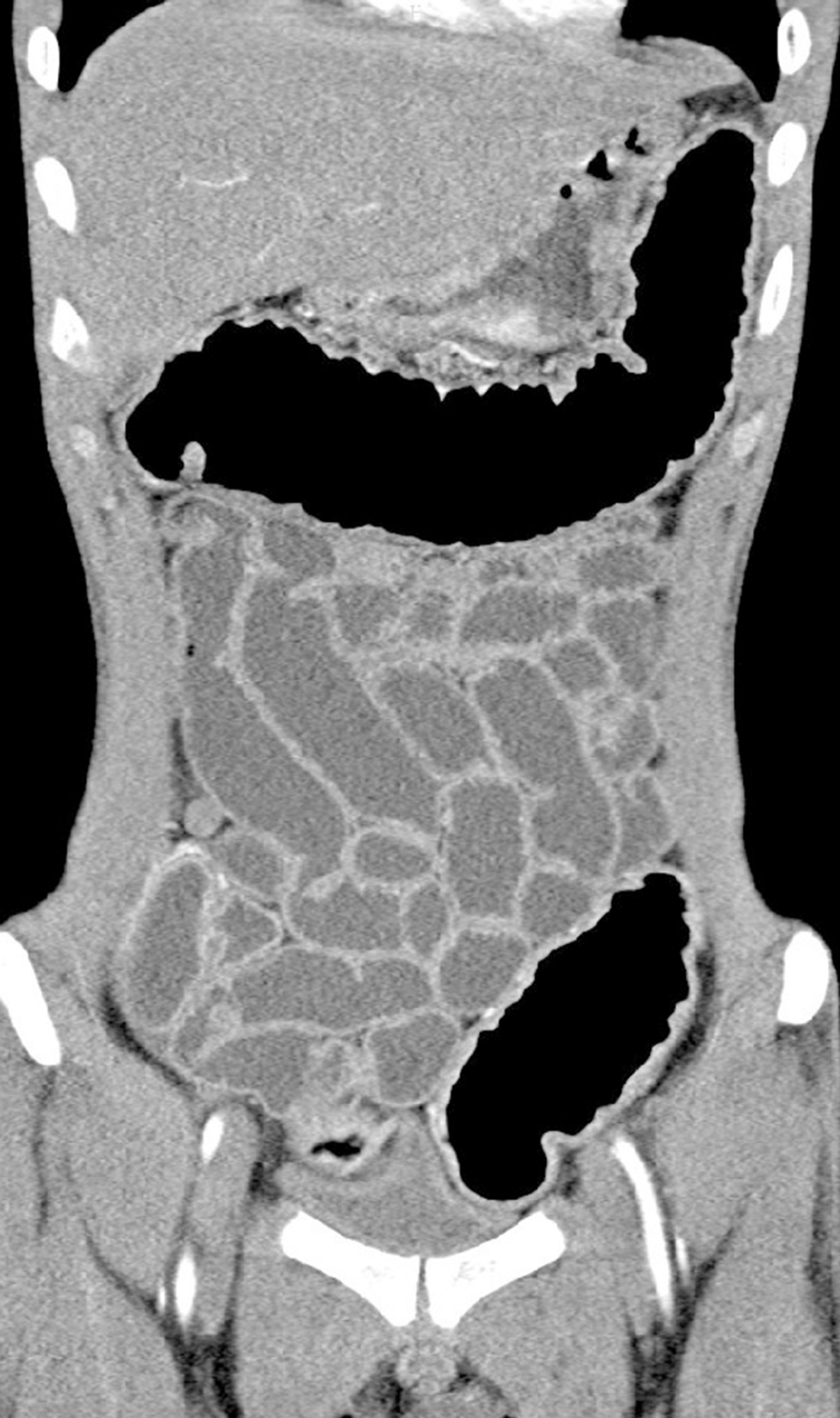
In order to determine whether other parts of the digestive tract were also involved, enteroscopy was suggested, but the patient refused. We identified the intactness of the small intestine through looking into the terminal ileum by colonoscopy and computed tomography (CT). Diffuse thickening of the stomach and colorectal wall was seen on CT, while the small intestine was not involved (Figure 3).
Gastroduodenitis associated with ulcerative colitis (GDUC).
The patient received a standardized treatment with pentasa of 3.2 g/d.
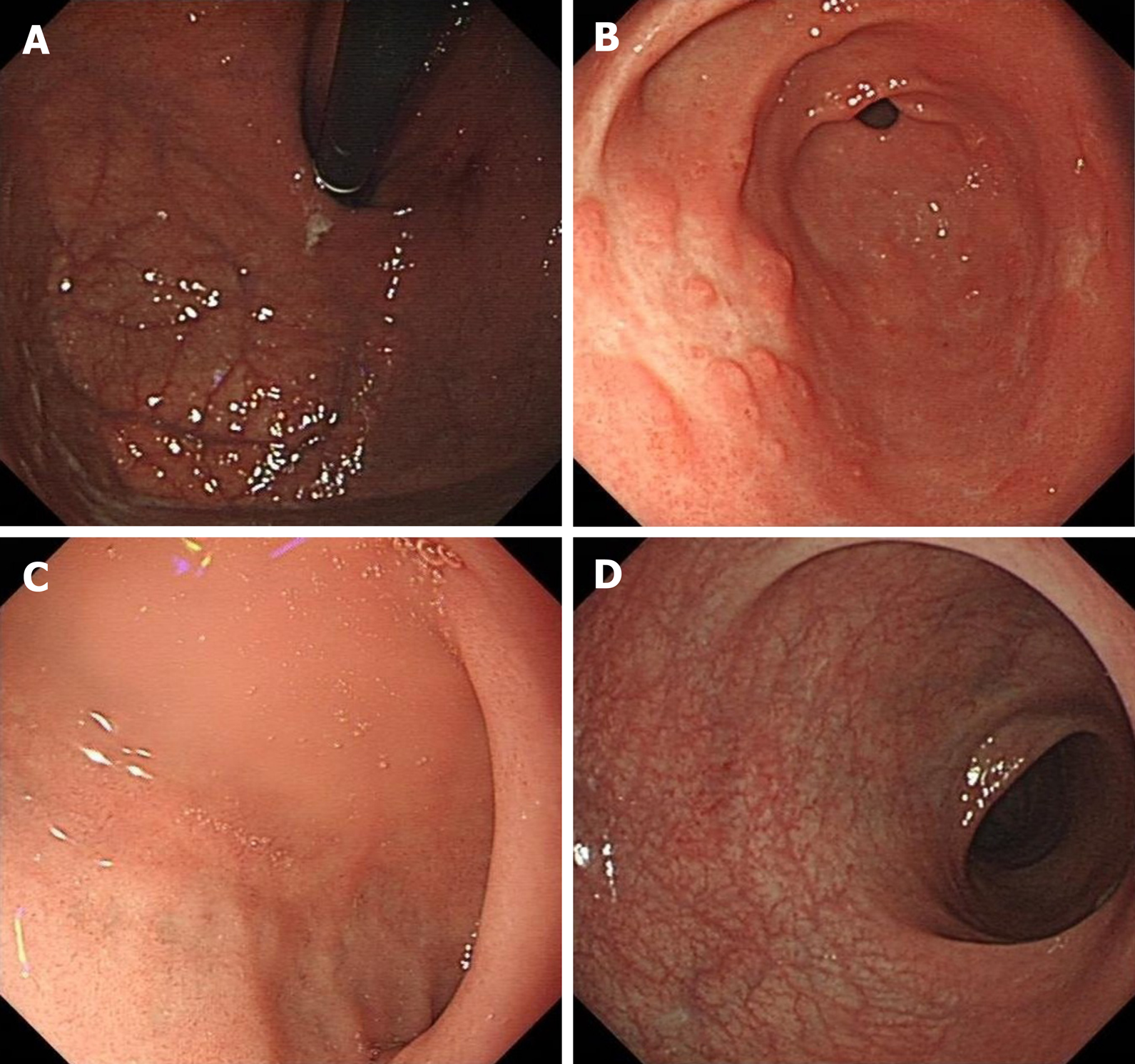
After 0.5 mo of treatment, the patient’s symptoms achieved complete remission. The follow-up endoscopy showed no significant abnormalities in the fundus of the stomach, duodenum, or rectum. Polypoid hyperplasia was observed in the gastric antrum (Figure 4). The Mayo endoscopic subscore suggested that the mucosal inflammation was “normal or inactive disease” (Table 1). The follow-up endoscopy and imaging examination showed no evidence of recurrence for 26 mo.
In the past years, there has been a consensus that UC is limited to colon and rectum, and total colorectal resection is thus regarded as radical treatment. Little is known about UC involving gastroduodenum. In recent years, there have been some reports describing the involvement of the upper digestive tract in UC[6-9]. The concept of GDUC was first proposed by Hori et al[10], but the diagnosis standard is not rigorous enough due to the scanty reports. More extensive colitis and a lower dose of prednisolone administration might be the main risk factors for GDUC[9,11].
Although the etiology of GDUC is unclear, several studies have revealed that it may be associated with the imbalance of immune response of genetically susceptible hosts to bacterial antigens, resulting in an excessive autoimmune response to the gastroduodenal epithelium[3,10]. Remarkably, inflammatory cells of the gastrointestinal tract in patients with GDUC were recruited from primary colorectal lesions via memory T cells[3,10].
Upper gastrointestinal lesions of GDUC are usually recognized by endoscopy, and these lesions are defined as friable mucosa, granular mucosa, or, conditionally, multiple aphthae[10]. Pathologic examination of UC reveals that the lesions are limited to the mucosal layer with Paneth cell metaplasia, mucin depletion, distortion of crypt architecture, crypt abscesses, and infiltrates of the mucosa with inflammatory cells. It is noticeable that the gastroduodenal lesions in the present case possessed similar pathological features with the colorectal lesion, which is consistent with the previous reports[3,9-11]. Furthermore, Hisabe et al[3] proposed diagnostic criteria for GDUC: (1) Improvement of the lesions with treatment of UC; and/or (2) Resemblance of the lesions to UC in pathological findings. In our case, diagnosis was established based on the initial treatment failure, exclusion of autoimmune, nonautoimmune conditions and infection, the high degree of similarity between the gastroduodenal and colorectal manifestations, and the good response to mesalamine. Therefore, more studies are needed to explore the pathogenesis of UC in the future.
Our report describes a case of GDUC. The occurrence of gastrointestinal involvement in UC is rare. This report may open a new window for studying the etiology and pathogenesis of UC. We also discussed the relationship between UC and gastric lesions. Physicians should consider broader differential diagnosis by endoscopic biopsy and laboratory examinations.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rosen SA S-Editor: Wang DM L-Editor: MedE-Ma JY P-Editor: Zhang YL
| 1. | Gould NJ, Davidson KL, Nwokolo CU, Arasaradnam RP. A systematic review of the role of DNA methylation on inflammatory genes in ulcerative colitis. Epigenomics. 2016;8:667-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Kato R, Iwamuro M, Hiraoka S, Takashima S, Inokuchi T, Takahara M, Kondo Y, Tanaka T, Okada H. Evaluation of the Upper Gastrointestinal Tract in Ulcerative Colitis Patients. Acta Med Okayama. 2018;72:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Hisabe T, Matsui T, Miyaoka M, Ninomiya K, Ishihara H, Nagahama T, Takaki Y, Hirai F, Ikeda K, Iwashita A, Higashi D, Futami K. Diagnosis and clinical course of ulcerative gastroduodenal lesion associated with ulcerative colitis: possible relationship with pouchitis. Dig Endosc. 2010;22:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Li M, Liu Y, Cui J, Qin H, Shi Y, Zhang S, Zhao Y. Ulcerative colitis with mucosal lesions in duodenum: Two case reports. Medicine (Baltimore). 2019;98:e15035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Rubenstein J, Sherif A, Appelman H, Chey WD. Ulcerative colitis associated enteritis: is ulcerative colitis always confined to the colon? J Clin Gastroenterol. 2004;38:46-51. [PubMed] [DOI] [Full Text] |
| 6. | Valdez R, Appelman HD, Bronner MP, Greenson JK. Diffuse duodenitis associated with ulcerative colitis. Am J Surg Pathol. 2000;24:1407-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Ikeuchi H, Hori K, Nishigami T, Nakano H, Uchino M, Nakamura M, Kaibe N, Noda M, Yanagi H, Yamamura T. Diffuse gastroduodenitis and pouchitis associated with ulcerative colitis. World J Gastroenterol. 2006;12:5913-5915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Terashima S, Hoshino Y, Kanzaki N, Kogure M, Gotoh M. Ulcerative duodenitis accompanying ulcerative colitis. J Clin Gastroenterol. 2001;32:172-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Sasaki M, Okada K, Koyama S, Yoshioka U, Inoue H, Fujiyama Y, Bamba T. Ulcerative colitis complicated by gastroduodenal lesions. J Gastroenterol. 1996;31:585-589. [PubMed] [DOI] [Full Text] |
| 10. | Hori K, Ikeuchi H, Nakano H, Uchino M, Tomita T, Ohda Y, Hida N, Matsumoto T, Fukuda Y, Miwa H. Gastroduodenitis associated with ulcerative colitis. J Gastroenterol. 2008;43:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | García Gavilán MDC, López Vega MC, Sánchez IM. Ulcerative colitis with gastric and duodenal involvement. Rev Esp Enferm Dig. 2017;109:535-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |