Published online Sep 6, 2020. doi: 10.12998/wjcc.v8.i17.3797
Peer-review started: February 26, 2020
First decision: April 21, 2020
Revised: May 10, 2020
Accepted: August 14, 2020
Article in press: August 14, 2020
Published online: September 6, 2020
Processing time: 190 Days and 16.6 Hours
Data regarding the neuroradiology features of the West Nile virus neuroinvasive disease (WNV NID) is rather scarce. To contribute to the knowledge of the WNV NID, we present a patient with a combination of encephalitis and acute flaccid paresis, with cauda equina arachnoiditis as the main magnetic resonance (MR) finding.
A 72-year-old female patient was admitted due to fever, headache and gait instability. During the first several days she developed somnolence, aphasia, urinary incontinence, constipation, and asymmetric lower extremities weakness. Cerebrospinal fluid analysis indicated encephalitis. Native brain computed tomography and MR were unremarkable, while spinal MR demonstrated cauda equina enhancement without cord lesions. Virology testing revealed WNV IgM and IgG antibodies in serum and cerebrospinal fluid, which confirmed acute WNV NID. The treatment was supportive. After two months only a slight improvement was noticed but cognitive impairment, loss of sphincter control and asymmetric inferior extremities weakness remained. The patient died after a month on chronic rehabilitation.
Cauda equina arachnoiditis is a rare, but possible neuroradiological feature in acute flaccid paresis form of WNV NID.
Core tip: Data regarding neuroradiology features of the West Nile virus neuroinvasive disease is scarce and cauda equina arachnoiditis is rare. We discuss a case of West Nile virus encephalitis and acute flaccid paresis of the lower extremities, with cauda equina arachnoiditis with focus on diagnostic management.
- Citation: Santini M, Zupetic I, Viskovic K, Krznaric J, Kutlesa M, Krajinovic V, Polak VL, Savic V, Tabain I, Barbic L, Bogdanic M, Stevanovic V, Mrzljak A, Vilibic-Cavlek T. Cauda equina arachnoiditis – a rare manifestation of West Nile virus neuroinvasive disease: A case report. World J Clin Cases 2020; 8(17): 3797-3803
- URL: https://www.wjgnet.com/2307-8960/full/v8/i17/3797.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i17.3797
West Nile virus (WNV) infection is an emerging zoonosis spreading across Europe over the last two decades[1-4]. The most severe clinical presentation is WNV neuroinvasive disease (WNV NID), affecting less than 1% of infected persons. WNV NID usually presents as meningitis, encephalitis, acute flaccid paralysis, or as a combination of the conditions above[5]. Although rare, WNV NID can be life-threatening, may require long-term hospitalization and rehabilitation, and leave long-lasting neurological sequelae[6]. The epidemiology, clinical presentation, and virology diagnostic methods of WNV infection are well established[7]. However, data on the WNV NID imaging is rather scarce, based on small case series and individual case reports. In WNV NID, brain magnetic resonance (MR) images may be normal or may indicate nonspecific findings in the basal ganglia, thalami, mesial temporal areas, brainstem, and cerebellum. Spinal MR images may show abnormalities affecting various levels of the spinal or, in rare cases, cauda equina enhancement[8]. To contribute to the knowledge of the WNV NID, we present a patient with a combination of encephalitis and α-fetoprotein, with cauda equina arachnoiditis as the main MR finding.
A 72-year-old patient sought medical attention for high fever, headache, and gait instability.
In July 2018, a 72-year-old female, was admitted to a local hospital due to a three-day fever, progressive frontal and occipital headache, and gait instability.
At admission, the patient had a fever of 39.0 °C, while other vital signs were normal. She appeared weary, slow in verbal and motoric responses, but completely oriented. Meningeal signs were negative. Neurological examination revealed symmetric fine intention hand tremor and instability while sitting and walking. Physical examination was otherwise unremarkable, besides irregular pulse due to atrial fibrillation. The patient's laboratory data at admission are presented in Table 1.
| Parameter | Day tested 41 | Day tested 10 | Reference range |
| Cerebrospinal fluid examination | |||
| Cell count/mm3 | 99 | 90 | 0-5 |
| Mononuclear cells (%) | 80 | 96 | Mononuclear 100% |
| Proteins (g/L) | 1.143 | 1.0 | 0.17–0.37 |
| Glucose (% of blood glucose) | 32% | 52% | 60%-70% |
| Blood examination | |||
| C-reactive protein (mg/L) | 0.2 | 7.3 | < 5.0 |
| White blood cells (× 109/L) | 9.1 | 9.7 | 4.0–10.0 |
| Platelets (× 109/L) | 156 | 150 | 100-400 |
| Red blood cells (× 1012/L) | 4.73 | 5.59 | 4.4-5.8 |
| Hemoglobin (g/L) | 128 | 149 | 120–180 |
| Bilirubin (μmol/L) | - | 20 | 3.0–20.0 |
| Aspartate-aminotransferase (U/L) | 37 | 32 | 11–38 |
| Alanine-aminotransferase (U/L) | 27 | 31 | 12–48 |
| Gamma-glutamyltransferase (U/L) | - | 77 | 11–55 |
| Lactate dehydrogenase (U/L) | 206 | 234 | < 241 |
Her non-contrast brain multi-slice computed tomography was normal. The spinal tap was postponed for 48 h due to anticoagulation therapy (dabigatran). Empirical therapy with acyclovir 1 g q8 h intravenously and doxycycline 100 mg q12 h orally were administered.
On the 6th day, the patient developed somnolence, aphasia, disorientation, urinary retention with asymmetric lower extremity weakness (weaker right leg). Repeated brain multi-slice computed tomography was unremarkable. Spinal tap results are presented in Table 1. The diagnosis of encephalitis was established. Due to neurologic deterioration, the patient was transferred to the Department for Intensive Care Medicine and Neuroinfectology in a tertiary institution.
Past medical history disclosed arterial hypertension, type 2 diabetes, hyperlipidemia, and permanent atrial fibrillation. The patient was taking amlodipine, bisoprolol, metformin, simvastatin and dabigatran.
The patient was transferred to a tertiary institution in severely impaired general condition. Her vital signs were as follows: Temperature 36 °C, heart rate 160 beats per minute, respiratory rate 16 breaths per minute, blood pressure 140/85 mmHg and oxygen saturation in room air 95%. She was unable to walk or sit, she was somnolent, opening eyes on demand and demonstrating signs of Wernicke’s aphasia (impaired comprehension with meaningless speech), without obvious signs of cranial nerves’ dysfunction. Muscle tone, strength and deep tendon reflexes were normal on upper extremities, while low on inferior extremities, more on the right side. Plantar response was “silent” on both sides. Abdominal cutaneous reflexes were normal. The patient had urinary catheter placed due to urinary retention. Rectal tone was decreased, the patient was incontinent for feces, and anal wink test was absent. No tremor was noticed. Sensory exam could not be performed due to the patient’s limited communication abilities. The remaining physical examination was unremarkable, besides absolute arrhythmia due to atrial fibrillation.
The patient's laboratory results at admission to the tertiary institution are listed in Table 1. The cerebrospinal fluid (CSF) Gram-stain was unremarkable, the 16S rDNA test of CSF was negative, and the culture did not yield any pathogen. The CSF was further analyzed by polymerase chain reaction and proven negative for herpes simplex virus 1/2, varicella-zoster virus, enteroviruses and Listeria monocytogenes. Anti-HIV, HIV antigen and Treponema pallidum hemagglutination assay were negative. Serology for Borrelia burgdorferi in serum and CSF was negative.
CSF, serum and urine samples were tested for the presence of neuroinvasive arboviruses: Tick-borne encephalitis (TBEV), WNV, Usutu (USUV), Toscana (TOSV), Tahyna ˙(TAHV) and Bhanja virus (BHAV). CSF, urine and serum samples were tested using a reverse-transcriptase polymerase chain reaction (RT-PCR): TBEV (Schwaiger et al[9]), WNV (Tang et al[10]), USUV (Nikolay et al[11]), TOSV (Weidmann et al[12], 2008), TAHV (Li et al[13], 2015) and BHAV (Matsuno et al[14], 2013). In addition, CSF and serum samples were tested for the presence of TBEV, WNV and USUV IgM and IgG antibodies using commercial enzyme-linked immunosorbent assays (TBEV, WNV, USUV - Euroimmun, Lübeck, Germany) and indirect immunofluorescence assay (TOSV - Euroimmun, Lübeck, Germany). According to the European Union case definition[7], WNV infection was confirmed by detection of IgM and IgG antibodies in both serum and CSF samples. Detection of low IgG avidity index as well as dynamics of IgG antibodies in consecutive serum samples (days 8, 15 and 22) further supported acute WNV infection (Table 2). WNV RNA was not detected in CSF, urine nor serum samples.
| Virus | Day tested | Serology | RT-PCR | |||||
| Serum ELISA | CSF ELISA | Serum IFA IgM/IgG | IgG avidityc | CSF | Urine | Serum | ||
| TBEV | 8 | Neg (0.32)/Neg (4.61) | Neg (0.24)/Neg (< 2) | NT | NT | Neg | Neg | Neg |
| WNV | 8 | Pos (4.40)/Pos (29.31) | Pos (4.61)/Pos (44.67) | NT | Low (3%) | Neg | Neg | Neg |
| 15 | Pos (4.18)/Pos (115.23) | |||||||
| 22 | Pos (4.47)/Pos (143.64) | |||||||
| USUV | 8 | NT/Neg (10.95) | NT/Neg (12.04) | NT | NT | Neg | Neg | Neg |
| TOSV | 8 | NT | NT | Neg/Neg | NT | Neg | Neg | Neg |
| TAHV | 8 | NT | NT | NT | NT | Neg | Neg | Neg |
| BHAV | 8 | NT | NT | NT | NT | Neg | Neg | Neg |
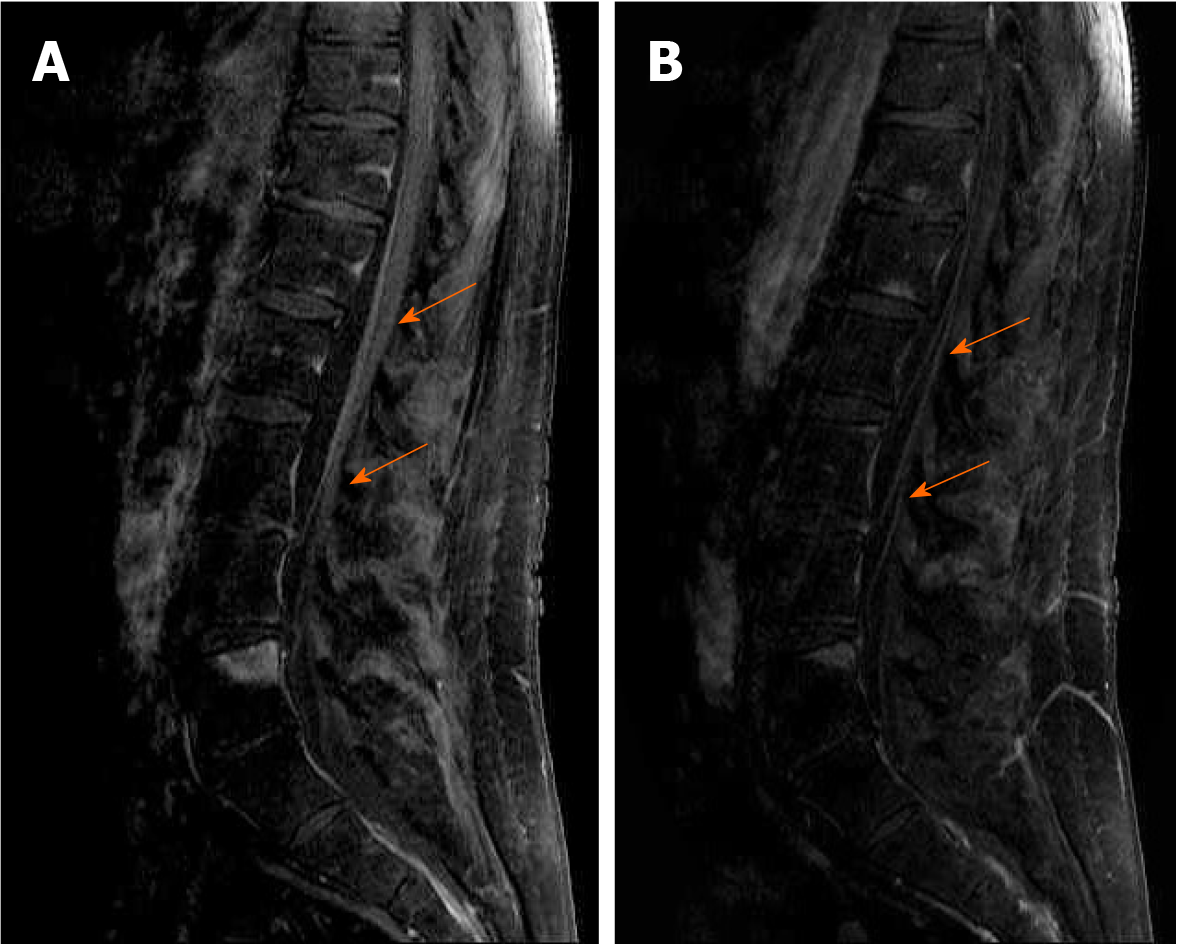
Magnetic resonance (MR) imaging of the brain and lumbosacral spine was performed using a 1.5T MR scanner (Symphony; Siemens Medical Solutions, Erlangen, Germany). The brain MR protocol consisted of sagittal T1-weighted spin-echo, axial T2-weighted fast-spin echo, axial T2-weighted fast-spin echo fat saturated, axial fluid-attenuated inversion recovery, pre- and post-contrast axial T1-weighted spin-echo fat saturated. Brain MR demonstrated nonspecific microvascular lesions in the periventricular regions and deep white matter. The lumbosacral MR protocol consisted of sagittal and axial T1 and T2 weighted image; postcontrast sagittal fat saturated T1 weighted image. It demonstrated intensive cauda equina enhancement as shown in Figure 1 (A and B).
The electroencephalogram was diffusely irregular and diffusely slow. The electromyoneurography was not performed due to technical issues at the time.
The patient was retired, living alone and taking care of herself. Exposure history revealed that the patient lived in a rural suburb in the Karlovac County, about 200 m from the Kupa River. She denied contact with animals. A few weeks before the illness, she noticed frequent mosquito bites and no tick bites. She denied traveling during the past months, as well as vaccination.
The final diagnosis of WNV encephalitis with acute flaccid paresis of the lower extremities due to cauda equina arachnoiditis was established.
The presented case was identified as an index case of WNV infection in Croatia in 2018, diagnosed in Karlovac County, where no autochthonous WNV infections were previously reported[15].
The patient was initially empirically treated with acyclovir 1 g q8 h intravenously and doxycycline 100 mg q12 h orally, until the diagnosis of WNV neuroinvasive disease was confirmed. Afterwards, supportive therapy and physical rehabilitation were applied.
The patient was hospitalized for eight weeks with a limited recovery. She recovered from aphasia but remained with a severe cognitive dysfunction. She was able to sit unassisted, but unable to walk. Lower extremity weakness slightly improved, but the patient remained incontinent for urine and feces. The hospitalization was complicated with healthcare-associated urinary tract infection and pneumonia. After two months the patient was discharged to a chronic rehabilitation facility where she succumbed to another pneumonia episode after a one-month period.
The presented WNV NID case raises several discussion points. The present knowledge about WNV NID neuroradiological findings is based on a rather small number of images. PubMed database search, using combination of terms “West Nile virus”, “WNV”, “encephalitis”, “myelitis”, “cauda equina”, and “magnetic resonance“ (MR), has identified seven publications in total (five case series and two individual cases). Four case series originated from the United States (three in 2002, one comprising patients from 1996 to 2016), and one from Serbia (in 2012)[6,8,16-18]. The total number of patients included was 147, the total number of patients with brain MR performed was 75, while the total number of patients with spinal MR was 33. Individual case reports present patients from 2012, one from the United States and one from Korea[19,20].
Most of the analyzed studies date back to the early 2000s[5,8,16,17]. Therefore, it would probably be useful to examine future WNV NID patients with new MR technologies to better characterize this clinical entity.
When analyzing only patients with abnormal spinal MR from the publications previously mentioned, the isolated cauda equina lesions were observed in seven, while combined spinal cord and cauda equina lesions were observed in three patients. Therefore, taking into consideration a rather small number of published cases, the question arises whether WNV has a predilection for the cauda equina. To provide an answer to this question, detailed neuroradiological examinations of the WNV NID patients are required. Clinicians as well as radiologists must be aware of this possibility, even though it is rare, and include a postcontrast scan of the spinal cord, cauda equina and nerve roots in the scanning protocol.
Only one study so far deals with the correlation of WNV NID neuroradiological findings and short-term disease outcomes[8]. It demonstrates that patients with fluid-attenuated inversion recovery and T2 weighted image brain and brain-stem changes have the worst outcomes, while the patients with spinal cord and nerve roots involvement have moderate-to-severe residual deficits. Indeed, new research demonstrating the correlation between clinical presentation, neuroradiological findings, and short and long-term outcomes is warranted.
Finally, the clinicians should have in mind that cauda equina enhancement is also described in the subarachnoid spread of neoplasms, central nervous system sarcoidosis, Guillain-Barré syndrome, and other viral central nervous system infections[21-23].
Our case has one limitation. The electromyoneurography was not performed due to technical issues at the time. This diagnostic procedure is highly recommended to elucidate the complex neurophysiology of the weakness in WNV NID[24].
Nevertheless, our case is a rare and detailed description of WNV NID, presenting as encephalitis with cauda equina arachnoiditis. We believe it contributes to the knowledge of MR appearances in WNV NID.
Besides, this is the westernmost case since the first appearance of WNV NID in Croatia in 2012[25,26], occurring in Karlovac County, bordering the Republic of Slovenia.
Cauda equina arachnoiditis is occasionally described on spinal magnetic resonance in patients with WNV NID. Therefore, there is a possibility that WNV has a predilection for the cauda equina area. We propose that magnetic resonance of the entire neuroaxis should be carried out in WNV NID patients to better characterize this relevant emerging clinical entity and its outcomes.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Baymakova M S-Editor: Zhang L L-Editor: A P-Editor: Li JH
| 1. | Holt E. West Nile virus spreads in Europe. Lancet Infect Dis. 2018;18:1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | European Centre for Disease Prevention and Control (ECDC). West Nile virus infection. Available from: https://www.ecdc.europa.eu/en/west-nile-virus-infection Cited 25 February 2020. |
| 3. | Baymakova M, Trifonova I, Panayotova E, Dakova S, Pacenti M, Barzon L, Lavezzo E, Hristov Y, Ramshev K, Plochev K, Palu G, Christova I. Fatal Case of West Nile Neuroinvasive Disease in Bulgaria. Emerg Infect Dis. 2016;22:2203-2204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Baymakova M, Christova I, Panayotova E, Trifonova I, Chobanov A, Daskalov I, Popov GT, Plochev K. West Nile virus infection with neurological disorders: a case report and a brief review of the situation in Bulgaria. Acta Clin Croat. 2019;58:546-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Davis LE, DeBiasi R, Goade DE, Haaland KY, Harrington JA, Harnar JB, Pergam SA, King MK, DeMasters BK, Tyler KL. West Nile virus neuroinvasive disease. Ann Neurol. 2006;60:286-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 234] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 6. | Hawkes MA, Carabenciov ID, Wijdicks EFM, Rabinstein AA. Critical West Nile Neuroinvasive Disease. Neurocrit Care. 2018;29:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | European Centre for Disease Prevention and Control (ECDC). EU case definitions. Available from: https://www.ecdc.europa.eu/en/surveillance-and-disease-data/eu-case-definitions Cited 25 February 2020. |
| 8. | Ali M, Safriel Y, Sohi J, Llave A, Weathers S. West Nile virus infection: MR imaging findings in the nervous system. AJNR Am J Neuroradiol. 2005;26:289-297. [PubMed] |
| 9. | Schwaiger M, Cassinotti P. Development of a quantitative real-time RT-PCR assay with internal control for the laboratory detection of tick borne encephalitis virus (TBEV) RNA. J Clin Virol. 2003;27:136-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 259] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 10. | Tang Y, Anne Hapip C, Liu B, Fang CT. Highly sensitive TaqMan RT-PCR assay for detection and quantification of both lineages of West Nile virus RNA. J Clin Virol. 2006;36:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Nikolay B, Weidmann M, Dupressoir A, Faye O, Boye CS, Diallo M, Sall AA. Development of a Usutu virus specific real-time reverse transcription PCR assay based on sequenced strains from Africa and Europe. J Virol Methods. 2014;197:51-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Weidmann M, Sanchez-Seco MP, Sall AA, Ly PO, Thiongane Y, Lô MM, Schley H, Hufert FT. Rapid detection of important human pathogenic Phleboviruses. J Clin Virol. 2008;41:138-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Li H, Cao YX, He XX, Fu SH, Lyu Z, He Y, Gao XY, Liang GD, Wang HY. Real-time RT-PCR Assay for the detection of Tahyna Virus. Biomed Environ Sci. 2015;28:374-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 14. | Matsuno K, Weisend C, Travassos da Rosa AP, Anzick SL, Dahlstrom E, Porcella SF, Dorward DW, Yu XJ, Tesh RB, Ebihara H. Characterization of the Bhanja serogroup viruses (Bunyaviridae): a novel species of the genus Phlebovirus and its relationship with other emerging tick-borne phleboviruses. J Virol. 2013;87:3719-3728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Vilibic-Cavlek T, Savic V, Sabadi D, Peric L, Barbic L, Klobucar A, Miklausic B, Tabain I, Santini M, Vucelja M, Dvorski E, Butigan T, Kolaric-Sviben G, Potocnik-Hunjadi T, Balenovic M, Bogdanic M, Andric Z, Stevanovic V, Capak K, Balicevic M, Listes E, Savini G. Prevalence and molecular epidemiology of West Nile and Usutu virus infections in Croatia in the 'One health' context, 2018. Transbound Emerg Dis. 2019;66:1946-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 16. | Jeha LE, Sila CA, Lederman RJ, Prayson RA, Isada CM, Gordon SM. West Nile virus infection: a new acute paralytic illness. Neurology. 2003;61:55-59. [PubMed] |
| 17. | Petropoulou KA, Gordon SM, Prayson RA, Ruggierri PM. West Nile virus meningoencephalitis: MR imaging findings. AJNR Am J Neuroradiol. 2005;26:1986-1995. [PubMed] |
| 18. | Popovic N, Milosevic B, Urosevic A, Poluga J, Popovic N, Stevanovic G, Milosevic I, Korac M, Mitrovic N, Lavadinovic L, Nikolic J, Dulovic O. Clinical characteristics and functional outcome of patients with West Nile neuroinvasive disease in Serbia. J Neurol. 2014;261:1104-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | DeQuesada I 2nd, Saindane A. West Nile virus presenting as flaccid paralysis: Case report and literature review. Radiol Case Rep. 2012;7:705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Hwang J, Ryu HS, Kim H, Lee SA. The first reported case of West Nile encephalitis in Korea. J Korean Med Sci. 2015;30:343-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Marjelund S, Jaaskelainen A, Tikkakoski T, Tuisku S, Vapalahti O. Gadolinium enhancement of cauda equina: a new MR imaging finding in the radiculitic form of tick-borne encephalitis. AJNR Am J Neuroradiol. 2006;27:995-997. [PubMed] |
| 22. | Yousem DM, Zimmerman R, Grossman RI. Neuroradiology: The Requisites. Philadelphia, PA: Mosby/Elsevier, 2010: 192-227. |
| 23. | Coskun A, Kumandas S, Paç A, Karahan OI, Guleç M, Baykara M. Childhood Guillain-Barré syndrome. MR imaging in diagnosis and follow-up. Acta Radiol. 2003;44:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Leis AA, Stokic DS. Neuromuscular manifestations of west nile virus infection. Front Neurol. 2012;3:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 25. | Pem-Novosel I, Vilibic-Cavlek T, Gjenero-Margan I, Pandak N, Peric L, Barbic L, Listes E, Cvitkovic A, Stevanovic V, Savini G. First outbreak of West Nile virus neuroinvasive disease in humans, Croatia, 2012. Vector Borne Zoonotic Dis. 2014;14:82-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Vilibic-Cavlek T, Kaic B, Barbic L, Pem-Novosel I, Slavic-Vrzic V, Lesnikar V, Kurecic-Filipovic S, Babic-Erceg A, Listes E, Stevanovic V, Gjenero-Margan I, Savini G. First evidence of simultaneous occurrence of West Nile virus and Usutu virus neuroinvasive disease in humans in Croatia during the 2013 outbreak. Infection. 2014;42:689-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |