Published online Sep 6, 2020. doi: 10.12998/wjcc.v8.i17.3763
Peer-review started: May 8, 2020
First decision: June 7, 2020
Revised: June 8, 2020
Accepted: August 6, 2020
Article in press: August 6, 2020
Published online: September 6, 2020
Processing time: 118 Days and 23.5 Hours
The main pathophysiological basis of coronavirus disease 2019 (COVID-19) causing respiratory failure is a cytokine storm and interleukin-6 (IL-6) is an important component of the COVID-19 cytokine storm. As a specific antagonist of IL-6, tocilizumab may block the cytokine storm of COVID-19. The Diagnosis and Treatment Guidelines of New Coronavirus Pneumonia (7th Edition) includes tocilizumab as a recommended drug for immunotherapy in severe and critical COVID-19 patients. However, the specific clinical efficacy of tocilizumab in the treatment of COVID-19 patients is worth studying.
To determine the clinical efficacy of tocilizumab in inhibiting the cytokine storm in COVID-19.
In total, 19 severe and critical COVID-19 patients were enrolled in this study, and were treated with tocilizumab in Optical Valley Campus of Hubei Maternal and Child Health Care Hospital from February 20 to March 31, 2020. The imaging manifestations and clinical data before and after treatment were analyzed retrospectively, including routine peripheral venous blood tests, routine blood biochemical tests, coagulation test, C-reactive protein (CRP), IL-6, and arterial blood gas analysis.
Of the 19 patients in this group, 13 (68.4%) had significantly improved symptoms of COVID-19 (5 patients were discharged directly and 8 patients were transferred after improvement) following treatment. One case was invalid, 1 case was exacerbated, and 4 deaths (21.1%) were observed (all critical cases). The lymphocyte count, CRP, lactic acid, oxygenation index, fibrinogen (FIB) and IL-6 levels were significantly different in the improved group.
Tocilizumab treatment is effective against IL-6 in COVID-19 patients, but it does not completely inhibit the inflammation and cytokine storm in all patients with COVID-19.In the clinical treatment of COVID-19 patients, attention should be paid to the timing of drug administration and other adjuvant treatments.
Core tip: Tocilizumab treatment is effective against IL-6 in Coronavirus Disease 2019 (COVID-19) patients, but it does not completely inhibit the inflammation and cytokine storm in all patients with COVID-19. In the clinical treatment of COVID-19 patients, attention should be paid to the timing of drug administration and other adjuvant treatments.
- Citation: Zeng J, Xie MH, Yang J, Chao SW, Xu EL. Clinical efficacy of tocilizumab treatment in severe and critical COVID-19 patients. World J Clin Cases 2020; 8(17): 3763-3773
- URL: https://www.wjgnet.com/2307-8960/full/v8/i17/3763.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i17.3763
The global pandemic of Coronavirus Disease 2019 (COVID-19) is a serious threat to people’s lives[1,2]. Some studies[3-5] have found that the main pathophysiological basis of COVID-19 causing respiratory failure and even hypoxic death, is immune cells released by mononuclear/macrophage cells after infection with the new coronavirus (SARS-CoV-2) in the blood which further activate the body's immune cells (such as monocytes, macrophages, T cells, B cells, etc.) or non-immune cells (such as endothelial cells), causing these cells to release a large number of cytokines (cytokine storm), thereby seriously destroying the ventilation function of lungs. Interleukin-6 (IL-6) is an important component of the COVID-19 cytokine storm. As a specific antagonist of IL-6, tocilizumab may block the cytokine storm in COVID-19 and prevent the severe to critical transition in patients. The Diagnosis and Treatment Guidelines of New Coronavirus Pneumonia (7th Edition) includes tocilizumab as a recommended drug for immunotherapy in patients with severe COVID-19[6,7]. We analyzed the clinical data of 19 severe and critical COVID-19 patients who were treated with tocilizumab in Optical Valley Campus of Hubei Maternal and Child Health Care Hospital, in order to analyze the effect of tocilizumab on inhibition of the COVID-19 cytokine storm and provide a theoretical reference for the clinical treatment of patients with severe COVID-19.
Inclusion criteria: The Guidelines of the 7th Edition were used for the diagnosis and clinical classification of COVID-19[8]. Patients were eligible if they met the following criteria: Age > 18 years, positive nucleic acid test, fever or respiratory symptoms, imaging findings of viral pneumonia, oxygen saturation (SaO2) at rest less than 93% on admission, and IL-6 in peripheral blood > 30 pg/mL (upper limit of normal value: 10 pg/mL).
Exclusion criteria were as follows: (1) Previous medical history combined with organ transplantation and immune system diseases; (2) Pregnant or lactating women; (3) Suffering from mental illness; and (4) Disagree with treatment of tocilizumab.
In total, 19 severe and critical patients (12 males and 7 females, aged 55-94 years, median age: 76.00 ± 10.76 years, clinical classification at admission: 11 severe cases and 8 critical cases) with COVID-19 who met the above criteria were included in the study. This study conforms to the Declaration of Helsinki (2000) of the World Medical Association. All patients signed consent forms or their family members agreed by phone, and this was recorded. The clinical data were analyzed retrospectively.
On the basis of routine treatment, all patients stopped immunoglobulin treatment for more than 3 d. The recommended dose of 8 mg/kg of tocilizumab (Roche Pharma Ltd., Switzerland) was diluted with 0.9% sodium chloride solution to 100 mL, protected from light, and intravenously infused for more than 1 h. Observation indices were as follows: (1) Chest computed tomography or chest radiography before and after tocilizumab treatment; and (2) Routine peripheral venous blood tests, routine blood biochemical tests, coagulation test, C-reactive protein (CRP), IL-6 and arterial blood gas analysis were carried out before and after tocilizumab treatment at 1 d, 3 d, 5 d, and 10 d, respectively.
According to the treatment process and final outcome of the included patients, patients were divided into the "effective group" and "ineffective group". The above observation indices in the two groups were then determined and a retrospective controlled study was carried out to analyze the specific therapeutic effect and the reasons for the differences in the therapeutic effect of tocilizumab in the treatment of COVID-19 patients. In addition, rational clinical application, timing of drug administration, observation items and auxiliary treatment were also investigated.
Statistical analysis of the data was performed using the SPSS 24.0 software package. Counted data were expressed as a percentage (%), and compared using the χ2 test. Normally distributed continuous variables are presented as the mean ± SD, and skewed continuous variables are presented as the median (interquartile range). t-test was used to compare the mean values of two groups of measurement data with normal distribution and equal variances assumed, t’-test was used for equal variances not assumed; One-Way ANOVA was used to compare the mean values of the same item at different times in the same group. Comparisons of skewed distribution measurement data was performed by the Mann-Whitney U test or Kruskal-Wallis H test. In the correlation analysis of measurement data in accordance with skewed distribution, Spearman rank correlation was adopted. P < 0.05 represents a significant difference.
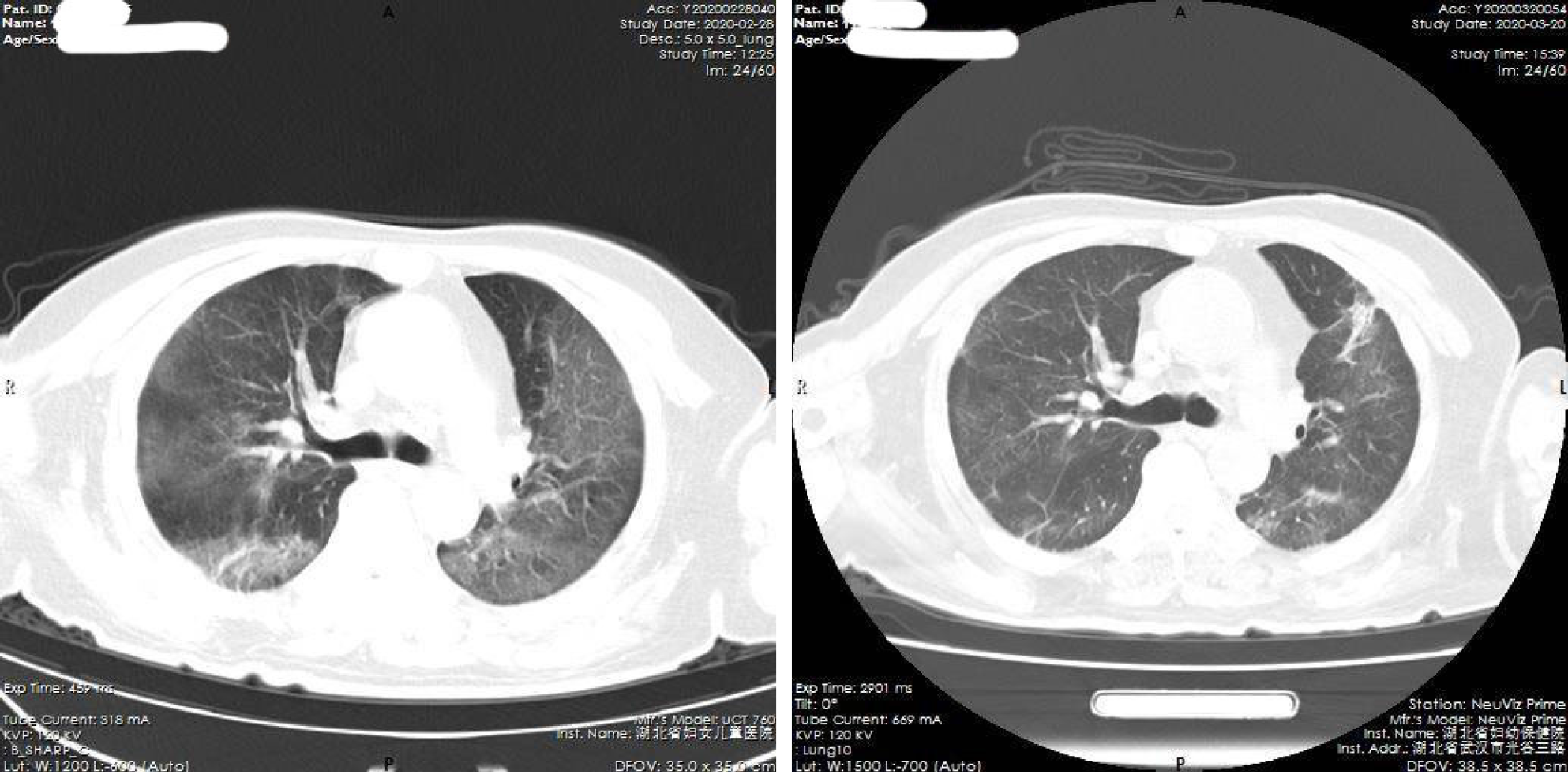
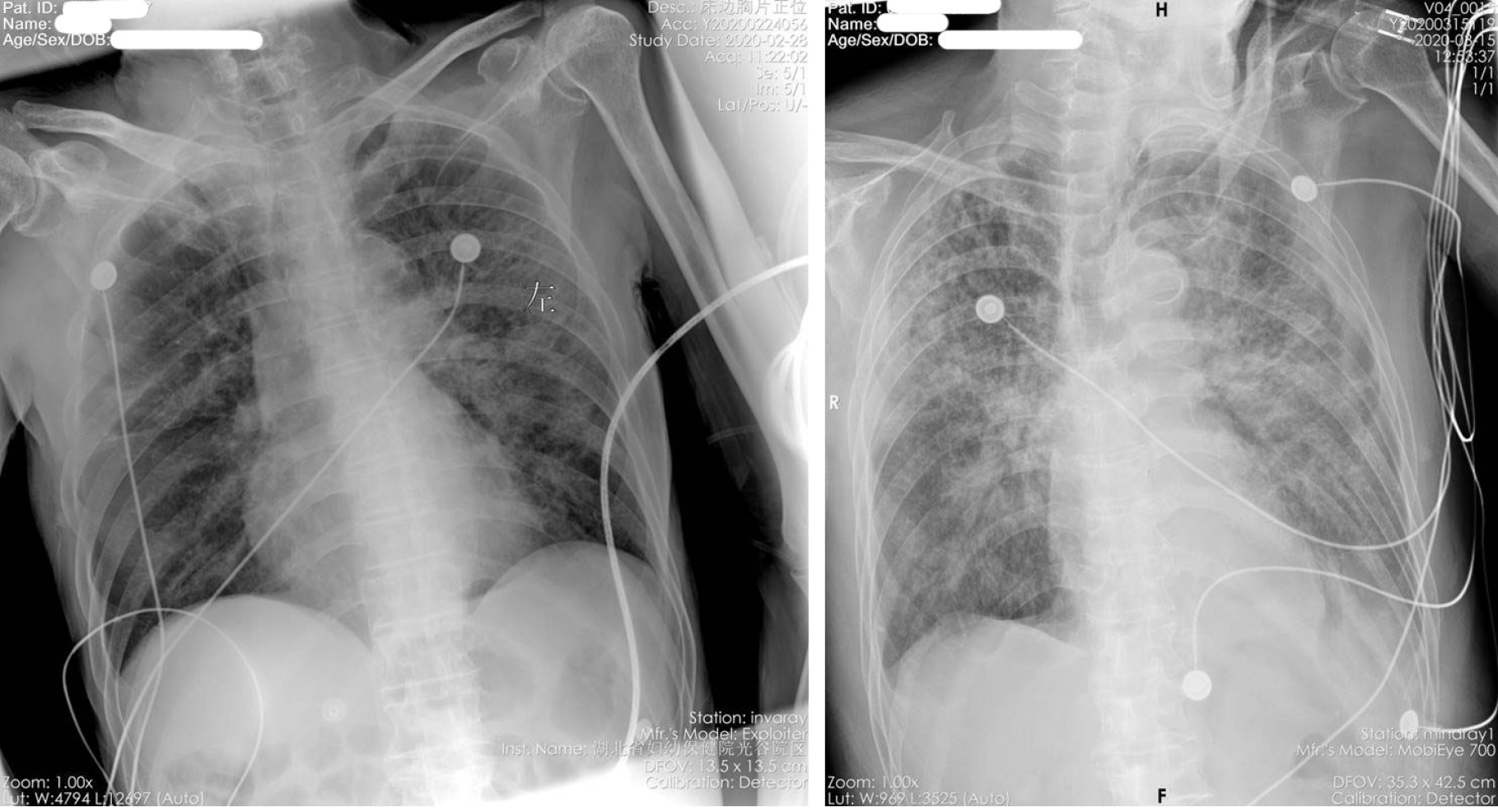
All 19 severe and critical COVID-19 patients underwent tocilizumab therapy. In 13 patients (68.4%), the symptoms were significantly improved, and 5 patients were discharged from hospital, 8 were transferred after improvement, 1 was transferred after exacerbation, treatment was ineffective in 1, and 4 patients died (21.1%). The patients who died all had critical COVID-19, including: 1 case of lung tumor with bone metastasis combined with pathological fracture which was suspected before admission, and the patient died of respiratory failure 2 d after tocilizumab treatment. One patient had COVID-19 combined with atrial fibrillation and pleural effusion, and died of respiratory failure 2 d after tocilizumab treatment. Two patients died of gastrointestinal bleeding on the 7th and 12th days after tocilizumab treatment. Thirteen cases with obvious improvement of symptoms were included in the "effective group". Six cases of invalid or worsening disease were included in the "ineffective group". Imaging examination showed that after treatment with tocilizumab, pulmonary viral pneumonia improved significantly in the effective group, and inflammation was absorbed (Figure 1 and Figure 2). However, the ineffective group showed that viral inflammation of the lung was obviously aggravated and exudation was increased.
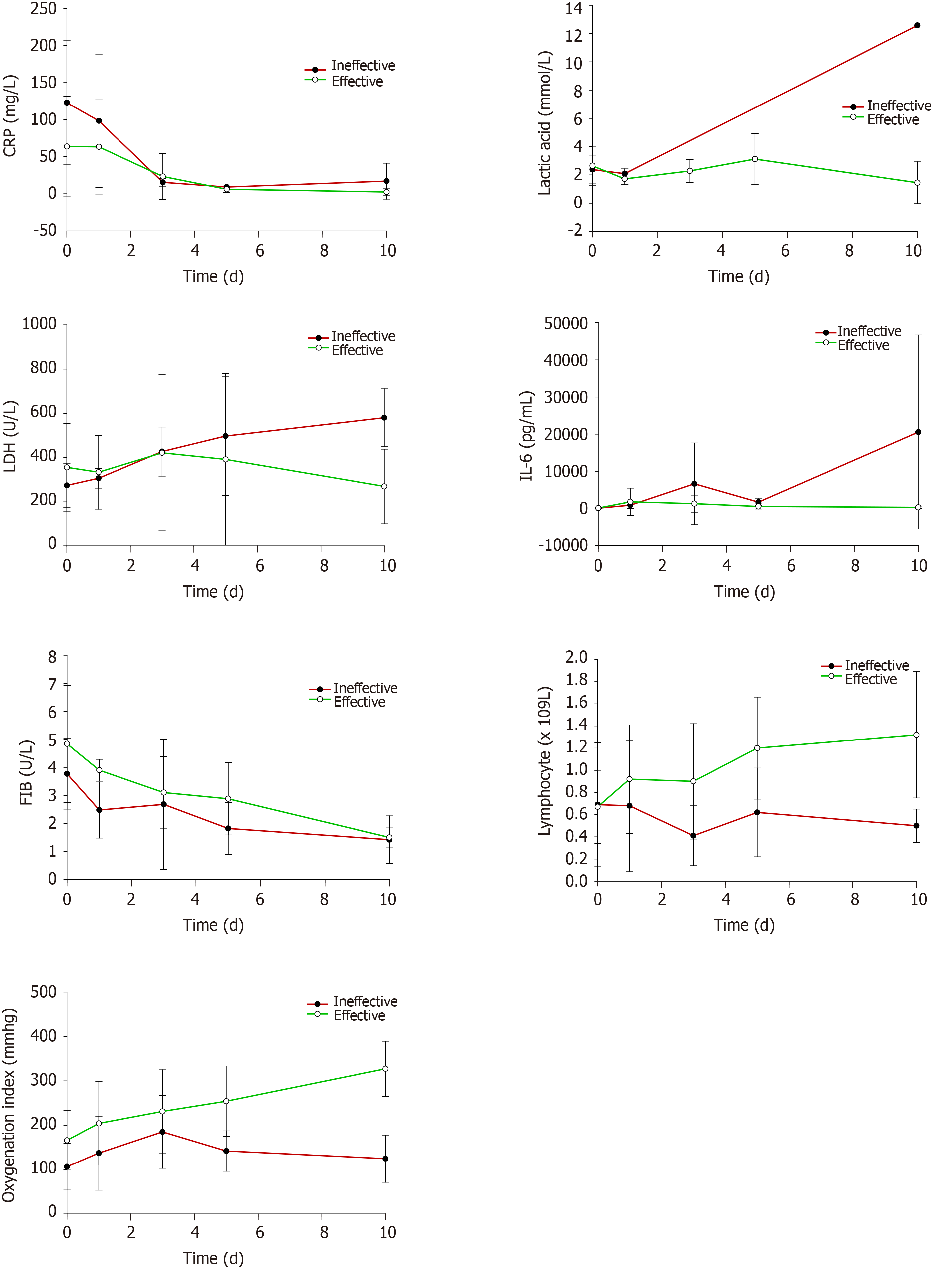
Routine peripheral venous blood tests, routine blood biochemical tests, coagulation test, CRP, IL-6 and arterial blood gas were analyzed before and after treatment in the two groups (Table 1). The results showed that after tocilizumab treatment, the lymphocyte count and oxygenation index of the improved patients increased significantly, while CRP and FIB showed an overall downward trend. Although the levels of lactic acid and IL-6 temporarily increased, there was an overall decline. These findings showed that the cytokine storm and inflammation in COVID-19 patients can be controlled by tocilizumab treatment. However, the ineffective group showed that CRP was different to lactic acid, lactate dehydrogenase (LDH) and IL-6. CRP showed a general downward trend, while lactic acid, LDH and IL-6 showed an overall upward trend, which suggested that tocilizumab did not inhibit the cytokine storm in these patients.
| Group | Age (yr) | M:F | Critical: severe | Laboratory test items | Before treatment | 1st day after treatment | 3rd day after treatment | 5th day after treatment | 10th day after treatment | Statistical value | P value | |
| Effective | 55-94 (74 ± 10.66) | 9:4 | 3:10 | SaO2 (%) | 80-93 (86.38 ± 5.21) | |||||||
| Lymphocyte (× 109/L) | 0.30-1.56 (0.67 ± 0.33) | 0.51-2.07 (0.92 ± 0.49) | 0.29-1.75 (0.90 ± 0.52) | 0.59-1.91 (1.20 ± 0.46) | 0.58-2.38 (1.32 ± 0.57) | 3.76 | 0.0085 | |||||
| WBC (× 109/L) | 3.5-15.1 (9.47 ± 4.11) | 5.1-13.7 (8.8 ± 3.25) | 3.4-19.7 (9.08 ± 4.72) | 3.3-21.6 (9.13 ± 5.40) | 3.7-17.7 (9.6 ± 5.35) | 0.06 | 0.9927 | |||||
| PLT (× 109/L) | 80-456 (220.92 ± 110.19) | 70-396 (226.55 ± 115.44) | 54-370 (192.4 ± 96.51) | 53-384 (226.25 ± 112.38) | 46-324 (208.1 ± 77.42) | 0.26 | 0.9026 | |||||
| CRP (mg/L) | 10.85-235.29 (63.76 ± 67.83) | 4.43-231.35 (63.31 ± 64.77) | 3.83-99.75 (23.36 ± 30.93) | 1.03-15.33 (6.11 ± 4.44) | 0.14-14.61 (2.42 ± 4.33) | 5.91 | 0.0004 | |||||
| Lactic acid (mmol/L) | 1.10-6.20 (2.65 ± 1.38) | 1.10-2.10 (1.72 ± 0.41) | 1.40-3.20 (2.28 ± 0.82) | 1.40-5.80 (3.12 ± 1.81) | 0.40-2.50 (1.45 ± 1.48) | 3.64 | 0.0102 | |||||
| LDH (U/L) | 187-633 (356 ± 198.2) | 206-609 (333.8 ± 166.38) | 207-949 (421.5 ± 353.52) | 185-1085 (391.8 ± 388.05) | 192-520 (269.75 ± 168.68) | 0.59 | 0.6704 | |||||
| Oxygenation index (mmHg) | 67-300 (166 ± 67.13) | 98-385 (204.08 ± 94.22) | 98-395 (231.17 ± 93.95) | 122-380 (254.25 ± 79.4) | 216-397 (327.42 ± 62.02) | 7.35 | 0.0001 | |||||
| FIB (g/L) | 2.05-9.46 (4.84 ± 2.09) | 3.55-4.57 (3.9 ± 0.39) | 1.16-4.66 (3.1 ± 1.29) | 1.41-5.64 (2.88 ± 1.29) | 0.97-2.13 (1.5 ± 0.37) | 12.56 | 0.0000 | |||||
| IL-6 (pg/mL) | 30.37-609.36 (107.17 ± 154.91) | 36.59-12600.94 (1793.42 ± 3675.7) | 73.80-7295.1 (1286.84 ± 2291.07) | 24.05-2502.67 (541.42 ± 687.93) | 14-1109.47 (286.33 ± 342.37) | 11.71 | 0.0016 | |||||
| D-dimer (mg/L) | 0.98-12.21 (3.5 ± 3.71) | 0.66-26.11 (5.62 ± 10.09) | 0.78-20.26 (5.75 ± 6.73) | 0.73-29.48 (6.4 ± 9.53) | 1.28-4.2 (2.2 ± 0.89) | 0.81 | 0.5260 | |||||
| Ineffective | 57-88 (83 ± 11.93)1 | 4:2 | 5:11 | SaO2 (%) | 70-88 (77.67 ± 8.89)1 | |||||||
| Lymphocyte (× 109/L) | 0.15-1.74 (0.69 ± 0.56) | 0.08-1.59 (0.68 ± 0.59) | 0.36-0.17 (0.41 ± 0.27)1 | 0.18-0.98 (0.62 ± 0.4)1 | 0.29-0.66 (0.5 ± 0.15)1 | 0.29 | 0.8816 | |||||
| WBC (× 109/L) | 3.7-26.0 (10.68 ± 8.02) | 2.9-22.1 (9.92 ± 8.72) | 4-11.8 (8.33 ± 3.71) | 7.5-10.3 (9.03 ± 1.49) | 4.7-14.1 (8 ± 4.22) | 0.21 | 0.9290 | |||||
| PLT (× 109/L) | 41-338 (160.67 ± 102.66) | 11-313 (153.8 ± 110.39) | 113-301 (171.5 ± 84.47) | 76-287 (137.65 ± 100.42) | 40-276 (147.75 ± 98.65) | 0.10 | 0.9817 | |||||
| CRP (mg/L) | 39.78-241.53 (122.8 ± 83.7) | 34.65-254.52 (98.38 ± 90.03) | 9.51-20.89 (15.44 ± 5.26) | 7.45-11.86 (9.08 ± 1.52) | 3.2-53.33 (17.05 ± 24.22)1 | 5.51 | 0.0025 | |||||
| Lactic acid (mmol/L) | 1.1-3.4 (2.38 ± 0.96) | 1.7-2.36 (2.09 ± 0.34) | Untested | Untested | 12.58 (12.58)1 | 194.84 | 0.0000 | |||||
| LDH (U/L) | 203-345 (274 ± 100.41) | 275-338 (306.5 ± 44.55) | 320-542 (427.33 ± 111.18) | 308-687 (497.5 ± 267.99) | 488-673 (580.5 ± 130.82)1 | 4.37 | 0.0082 | |||||
| Oxygenation index (mmHg) | 51-180 (106.34 ± 52.53) | 59.1-266 (137.02 ± 83.46) | 82-283 (185 ± 82.13) | 94-200 (141.75 ± 45.49)1 | 84-200 (124.5 ± 53.12)1 | 1.20 | 0.3374 | |||||
| FIB (g/L) | 1.59-4.87 (3.77 ± 1.26) | 1.34-3.21 (2.48 ± 1) | 0.85-5.29 (2.68 ± 2.32) | 1.09-3.1 (1.82 ± 0.93) | 0.88-2.4 (1.42 ± 0.85) | 1.77 | 0.1817 | |||||
| IL-6 (pg/mL) | 30.63-157.08 (78.27 ± 43.81) | 167.08-2124.42 (878.54 ± 911.52)1 | 559.96-23138.48 (6644.37 ± 11006.23)1 | 990.81-2725.70 (1703.35 ± 907.99)1 | 44.95-50000 (20562.42 ± 26144.85)1 | 3.01 | 0.0478 | |||||
| D-Dimer (mg/L) | 0.40-53.81 (11.63 ± 20.8)1 | 1.29-69.56 (29.7 ± 35.55)1 | 3.16-24.6 (10.91 ± 11.89) | 5.44-15.63 (9.81 ± 6.74) | 1.75-6.88 (4.06 ± 2.6) | 0.83 | 0.5223 | |||||
The same factors were statistically analyzed between the two groups (Table 1). The results showed that the ineffective cases were significantly older than the effective cases, and most were critical, with more bacterial infections and hypercoagulable states. The patients who improved were admitted with less serious illness than those who failed. After tocilizumab treatment, the lymphocytes and oxygenation index in the ineffective group not only did not increase significantly, but showed a downward trend. However, CRP, LDH and IL-6 levels in these patients were statistically significantly higher than in those who improved.
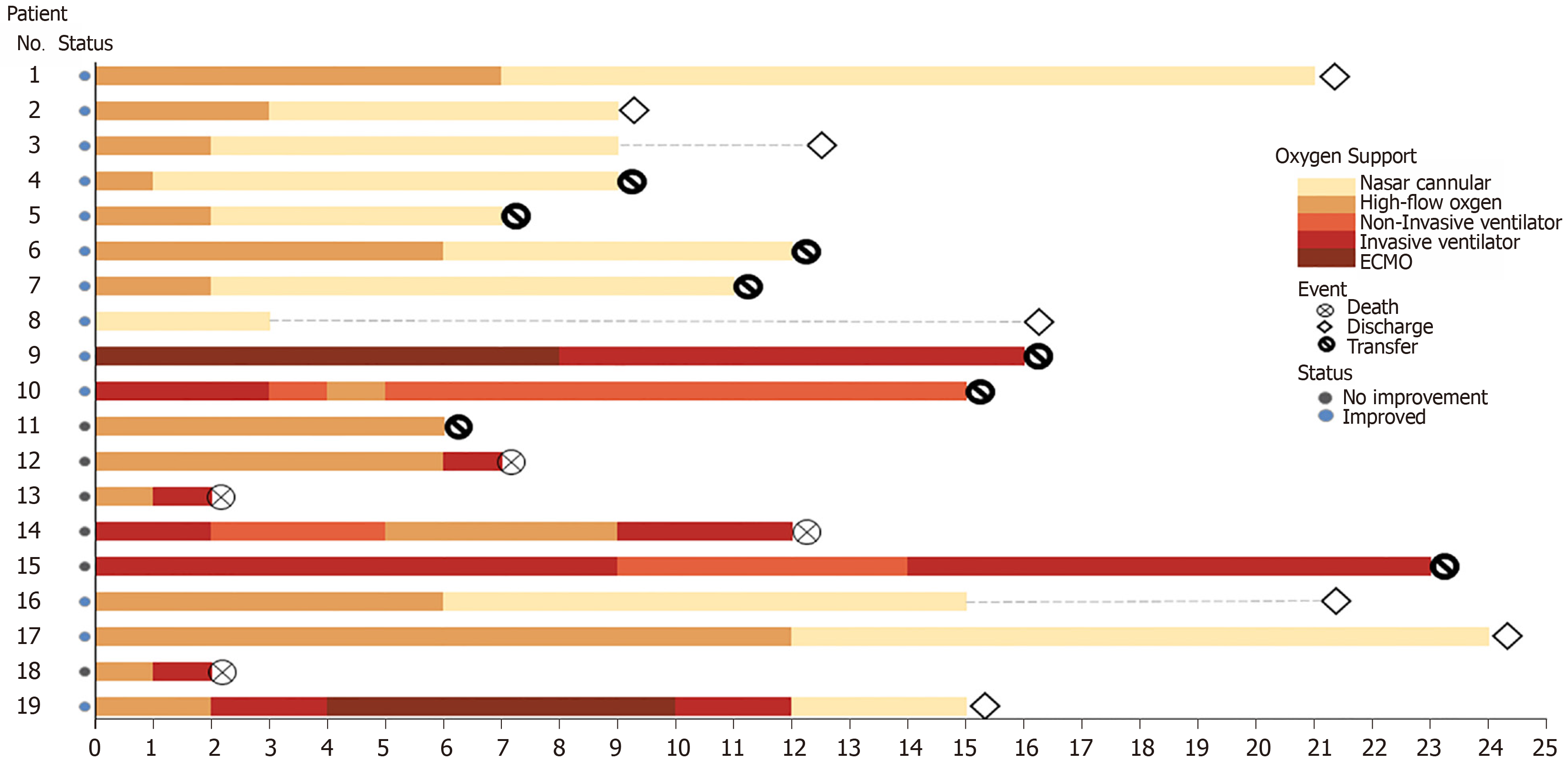
Oxygen therapy in this group of patients consisted of invasive ventilator therapy during treatment in 8 patients (42.1%), high-flow oxygen therapy in 16 (84.2%), and noninvasive ventilator therapy in 3 (15.7%). Following tocilizumab treatment, the changes in patients' oxygen therapy are shown in Figure 3. Among the 14 patients who underwent high-flow oxygen therapy before tocilizumab treatment, 10 patients (71.4%) improved. The improvement rate of invasive ventilator therapy before and after tocilizumab treatment was only 33.3%. During the course of treatment, of the 8 patients who underwent invasive ventilator therapy, 2 cases were treated with extracorporeal membrane oxygenation and were successfully weaned from the machine to the invasive ventilator, 3 cases improved and changed to a non-invasive ventilator, and 2 patients with infection resumed invasive ventilator therapy (Table 2). These findings suggest that the improvement rate after oxygen therapy was closely related to the patient's basic condition.
| The number of patients before treatment with tocilizumab | |||||
| ECMO (n = 1) | Invasive ventilator (n = 3) | High flow oxygen (n = 14) | Nasal catheter oxygen (n = 1) | ||
| Number of patients after treatment with tocilizumab | Death | 0 | 1 | 3 | 0 |
| ECMO | 0 | 0 | 0 | 0 | |
| Invasive ventilator | 1 | 1 | 0 | 0 | |
| Noninvasive ventilator | 0 | 1 | 0 | 0 | |
| High flow oxygen | 0 | 0 | 1 | 0 | |
| Nasal catheter oxygen | 0 | 0 | 6 | 0 | |
| Non oxygen inhalation | 0 | 0 | 4 | 1 | |
| Improved | 1 (100%) | 1 (33.3%) | 10 (71.4%) | 1 (100%) | |
It has been reported that SARS-CoV-2 enters lung cells through endocytosis mediated by the ACE2 receptor, proliferates in large quantities, and releases more virus by budding or inducing programmed cell death[9,10]. After being recognized by the pattern recognition receptor on the body’s immune cells, a large number of cytokines are released through signal transduction to activate more immune cells to participate in virus clearance, thus forming a cytokine storm. The over-activated immune system will certainly kill a large number of normal lung cells, seriously destroy the ventilation function of the lung, leading to respiratory failure, and death due to hypoxia. The cytokine storm caused by COVID-19 is mainly related to IL-1B, IL-6, IL-12, interferon γ, interferon-gamma-inducible protein-10 and monocyte chemotactic protein-1. The expression of IL-6 is greater than that of tumor necrosis factor-α and IL-1. A high concentration of IL-6 can induce various pathological functions related to thrombosis, vascular leakage and myocardial dysfunction, resulting in tissue hypoxia, hypotension, multiple organ dysfunction and disseminated intravascular coagulation. Therefore, IL-6 is generally used as a biomarker of disease severity and prognosis in the COVID-19 cytokine storm. In this study, the lymphocyte count, white blood cell count, platelets, lactic acid, LDH, FIB, IL-6 and D-dimer were selected as the observation indices.
In the present study, we found that most of the patients with COVID-19 had a reduced IL-6 level and inflammation indices after treatment with tocilizumab, and the results of pulmonary imaging showed that inflammation had improved. However, 8 patients showed an abnormal elevation of IL-6 (> 1000) 3 d after tocilizumab administration, and all showed disease aggravation, especially an increase in lung inflammation and exudation. Four of these patients died. This suggests that the source of inflammation in patients with COVID-19 is not only due to the virus infection, but also due to secondary infection and other factors. Inhibition of IL-6 by tocilizumab alone does not completely inhibit all inflammation and the cytokine storm in patients with COVID-19. Targeted treatment and other adjuvant therapy measures should also be introduced to inhibit the inflammatory response. Therefore, we administered methylprednisolone 40-80 mg/d on the basis of tocilizumab inhibition of IL-6 treatment, by intravenous drip for 3-5 d, supplemented with DFPP plasma exchange to clear inflammatory factors, or infusion of plasma from patients who had recovered from COVID-19, or intravenous infusion of ulinastatin and other measures, which ultimately significantly improved the condition of these patients. Therefore, in the clinical treatment of COVID-19 patients, the changes in relevant inflammatory indices should be closely monitored. If the cytokine storm cannot be inhibited, we should timely identify other causes of the inflammatory cascade for targeted treatment, with additional treatment measures in order to avoid missing the opportunity of treatment. In this group, we found that the therapeutic effect of tocilizumab was not so good in elderly critical patients. Therefore, tocilizumab should be administered as much as possible before the patient develops serious secondary complications. Otherwise, if the best opportunity of treatment is missed, the clinical effect will be limited.
Cytokine storm is an excessive immune phenomenon produced by the body due to external stimuli. Unrestricted mass release of cytokines leads to systemic inflammation. Infections can trigger cytokine storms, but many diseases can also induce cytokine storms. It has been reported that the level of IL-6 in patients with COVID-19 does not decrease but increases after tocilizumab treatment[11-13]. The higher the IL-6 level, the worse the prognosis. We also found that the level of IL-6 in all cases increased significantly on the 1st day and the 3rd day after tocilizumab treatment, while the level of IL-6 in improved patients decreased significantly, but still increased significantly in the ineffective group. This is consistent with the above report. These findings suggest that the source of IL-6 may not only be due to the virus infection, but also due to other complications such as secondary bacterial infection. The side effects of tocilizumab include coagulation dysfunction and severe hypoxemia, which may endanger the life of patients. The 4 patients who died all had significantly increased IL-6 after tocilizumab treatment and 2 of these patients died of respiratory failure, and the other 2 died of gastrointestinal bleeding. Is it not known whether gastrointestinal bleeding is a side effect of tocilizumab and is worthy of vigilance and further investigation (Figure 4).
The main pathophysiological basis of Coronavirus disease 2019 (COVID-19) causing respiratory failure, is the cytokine storm, and interleukin-6 (IL-6) is an important component of the COVID-19 cytokine storm. As a specific antagonist of IL-6, tocilizumab may block the cytokine storm in COVID-19.
The Diagnosis and Treatment Guidelines of New Coronavirus Pneumonia (7th Edition) includes tocilizumab as a recommended drug for immunotherapy in severe COVID-19 patients. However, the specific clinical efficacy of tocilizumab in the treatment of COVID-19 is worth studying.
This study aimed to determine the clinical efficacy of tocilizumab in inhibiting the cytokine storm in COVID-19.
In total, 19 severe and critical COVID-19 patients who were treated with tocilizumab were included in this study. The imaging manifestations and the clinical data before and after treatment were analyzed retrospectively, including routine peripheral venous blood tests, routine blood biochemical tests, coagulation test, C-reactive protein (CRP), IL-6, and arterial blood gas analysis.
Of the 19 patients in this group, 13 (68.4%) had significantly improved symptoms of COVID-19 (5 patients were discharged directly and 8 patients were transferred after improvement). One case was invalid, 1 case was exacerbated, and 4 deaths (21.1%) all critical cases were observed. The lymphocyte count, CRP, lactic acid, oxygenation index, FIB and IL-6 levels were significantly different in the improved group.
Tocilizumab treatment is effective against IL-6 in COVID-19 patients, but it does not completely inhibit the inflammation and cytokine storm in all patients with COVID-19. In the clinical treatment of patients, attention should be paid to the timing of drug administration and other adjuvant treatments.
In this study, we found that most of the patients with COVID-19 had reduced IL-6 levels and inflammation indices following treatment with tocilizumab, however, the therapeutic effect of tocilizumab is not so good in elderly critical patients. Therefore, we should administer tocilizumab as much as possible before patients develop serious secondary complications. Otherwise, if the best opportunity of treatment is missed, the clinical effect will be limited.
Manuscript source: Unsolicited manuscript
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bello BL, Kahrilas PJ, Yoshio S S-Editor: Wang JL L-Editor: Webster JR P-Editor: Xing YX
| 1. | Lipsitch M, Swerdlow DL, Finelli L. Defining the Epidemiology of Covid-19 - Studies Needed. N Engl J Med. 2020;382:1194-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 832] [Cited by in RCA: 676] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 2. | Hu Y, Sun J, Dai Z, Deng H, Li X, Huang Q, Wu Y, Sun L, Xu Y. Prevalence and severity of corona virus disease 2019 (COVID-19): A systematic review and meta-analysis. J Clin Virol. 2020;127:104371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 471] [Cited by in RCA: 387] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 3. | Choudhury R, Barrett CD, Moore HB, Moore EE, McIntyre RC, Moore PK, Talmor DS, Nydam TL, Yaffe MB. Salvage use of tissue plasminogen activator (tPA) in the setting of acute respiratory distress syndrome (ARDS) due to COVID-19 in the USA: a Markov decision analysis. World J Emerg Surg. 2020;15:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 433] [Article Influence: 86.6] [Reference Citation Analysis (0)] |
| 5. | Chiappelli F, Khakshooy A, Greenberg G. CoViD-19 Immunopathology and Immunotherapy. Bioinformation. 2020;16:219-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 6. | Buonaguro FM, Puzanov I, Ascierto PA. Anti-IL6R role in treatment of COVID-19-related ARDS. J Transl Med. 2020;18:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 7. | Fu B, Xu X, Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med. 2020;18:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 298] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 8. | Yang H, Sun G, Tang F, Peng M, Gao Y, Peng J, Xie H, Zhao Y, Jin Z. Clinical features and outcomes of pregnant women suspected of coronavirus disease 2019. J Infect. 2020;81:e40-e44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 9. | Cure E, Cumhur Cure M. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may be harmful in patients with diabetes during COVID-19 pandemic. Diabetes Metab Syndr. 2020;14:349-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 10. | Li G, He X, Zhang L, Ran Q, Wang J, Xiong A, Wu D, Chen F, Sun J, Chang C. Assessing ACE2 expression patterns in lung tissues in the pathogenesis of COVID-19. J Autoimmun. 2020;112:102463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 218] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 11. | Ceribelli A, Motta F, De Santis M, Ansari AA, Ridgway WM, Gershwin ME, Selmi C. Recommendations for coronavirus infection in rheumatic diseases treated with biologic therapy. J Autoimmun. 2020;109:102442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 12. | Zhang X, Song K, Tong F, Fei M, Guo H, Lu Z, Wang J, Zheng C. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. 2020;4:1307-1310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 181] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 13. | Michot JM, Albiges L, Chaput N, Saada V, Pommeret F, Griscelli F, Balleyguier C, Besse B, Marabelle A, Netzer F, Merad M, Robert C, Barlesi F, Gachot B, Stoclin A. Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report. Ann Oncol. 2020;31:961-964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 248] [Article Influence: 49.6] [Reference Citation Analysis (0)] |