Published online Sep 6, 2020. doi: 10.12998/wjcc.v8.i17.3751
Peer-review started: February 29, 2020
First decision: April 2, 2020
Revised: May 9, 2020
Accepted: August 9, 2020
Article in press: August 9, 2020
Published online: September 6, 2020
Processing time: 188 Days and 1.4 Hours
Pancreatic neuroendocrine tumors (PanNETs) are heterogeneous and indolent; systemic therapy is not essential for every patient with metastatic PanNET. The National Comprehensive Cancer Network guidelines state that delaying treatment is an option for PanNET with distant metastasis, if the patient has stable disease. However, specific factors that influence surveillance were not mentioned. In addition, data regarding the period of active surveillance in patients with metastatic PanNET are lacking.
To specifically determine factors influencing active surveillance in patients with liver metastatic nonfunctioning PanNETs (NF-PanNETs).
Seventy-six patients with liver metastatic NF-PanNETs who received active surveillance from a high-volume institution were enrolled. Time to disease progression (TTP) and time to initiation of systemic therapy were determined.
Thirty-one (40.8%) patients had recurrent liver disease after R0 resection; 45 (59.2%) were diagnosed with liver metastasis. The median follow-up period was 42 mo and 90.7% patients were observed to have disease progression. The median TTP (mTTP) was 10 mo. Multivariate analysis showed that the largest axis of the liver metastasis > 5 mm (P = 0.04), non-resection of the primary tumor (P = 0.024), and T3-4 stage (P = 0.028) were associated with a shorter TTP. The mTTP in patients with no risk factors was 24 mo, which was significantly longer than that in patients with one (10 mo) or more (6 mo) risk factors (P < 0.001). A nomogram with three risk factors showed reasonable calibration, with a C-index of 0.603 (95% confidence interval: 0.47-0.74).
Active surveillance may only be safe for metastatic NF-PanNET patients with favorable risk factors, and other patients progressed rapidly without treatment. Further studies with a larger sample size and a control group are needed.
Core tip: Experience in active surveillance of metastatic pancreatic neuroendocrine tumors (PanNETs) is insufficient in clinical practice. This study includes the experience of active surveillance in 76 patients with liver metastatic nonfunctioning PanNETs (NF-PanNETs) over 20 years. We demonstrated that largest axis of the liver metastasis > 5 mm, non-resection of the primary tumor and T3-4 stage are poor prognostic factors and affect the survival of PanNET patients with liver metastasis. Patients with favorable factors had a time to progressive disease of more than two years without systemic treatment. This subset of PanNET patients with liver metastasis should undergo active surveillance.
- Citation: Gao HL, Wang WQ, Xu HX, Wu CT, Li H, Ni QX, Yu XJ, Liu L. Active surveillance in metastatic pancreatic neuroendocrine tumors: A 20-year single-institutional experience. World J Clin Cases 2020; 8(17): 3751-3762
- URL: https://www.wjgnet.com/2307-8960/full/v8/i17/3751.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i17.3751
Pancreatic neuroendocrine tumors (PanNETs), which account for 31.5% of total gastroenteropancreatic NETs, are the most common form of pancreatic tumors in China[1]. About one-fourth of PanNET patients have metastasis at diagnosis[2], and the liver is the most common metastatic site. The prognosis of patients with metastatic PanNETs is heterogeneous and varies widely, from 10 mo to more than 10 years. Multiple lines of systemic treatment are considered the standard of care in metastatic nonfunctioning PanNETs (NF-PanNETs), namely, somatostatin analogs, chemotherapy[3], sunitinib, and everolimus[4]. However, systemic therapy may not be essential for every patient with metastatic PanNET. To avoid over-treating indolent asymptomatic tumors, the National Comprehensive Cancer Network guidelines for PanNETs state that, for locoregional advanced disease and/or distant metastasis, tumor marker analysis and screening every 3-12 mo is an option if the patient is asymptomatic, has low tumor burden, and has stable disease[5]. However, specific factors that influenced surveillance were not mentioned. In addition, data regarding the period of active surveillance in patients with metastatic PanNET are lacking.
The benefits of active surveillance have been well established in randomized clinical trials of other metastatic malignancies, such as metastatic renal cell carcinoma (RCC)[6], melanoma[7,8], and metastatic prostate cancer[9,10]. In this study, we retrospectively reviewed the clinicopathological data of patients with liver metastatic NF-PanNETs who received active surveillance in our institution over the past 20 years. Additionally, we established a nomogram to predict the suitable characteristics for active surveillance in liver metastatic NF-PanNET patients.
A retrospective review of the NF-PanNET databases of the Pancreatic Oncology Department, Fudan University Shanghai Cancer Center (FUSCC) was performed. 396 PanNET patients with metastasis were evaluated. All patients had histologically confirmed PanNET between 1998 and 2018. Pathology records of all included patients were reviewed by the Pathology Department at the FUSCC. All patients had asymptomatic NF-PanNET with liver metastasis, and patients without treatment were selected. We did not specify any laboratory parameters or comorbidities for eligibility. This research was approved by the hospital ethics committee, and written consent was provided for patient information to be used for research purposes. The study was approved by the FUSCC ethical committee.
Data were retrieved from patient medical records and included patient demographics (age and sex), pathology reports (T stage, N stage, tumor grade, Ki-67 index, and differentiation), and radiographical documentation [computed tomography (CT), magnetic resonance imaging (MRI), and somatostatin receptor scintigraphy (SRS)]. The tumor burden of liver metastasis was measured using the AW VolumeShare 5 system, and the axis of the liver metastasis was measured using MRI screening. Tumor grading was in accordance with the 2017 World Health Organization classification[11]. Tumor TNM staging was based on the 2006 ENETS TNM staging system for patients with PanNETs[12].
Follow-up was performed via outpatient visit or telephone in October 2018. Patients received an enhanced MRI or CT scan every three months. Study investigators assessed progression according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1[13]. The active surveillance time in the study was defined as the time from the start of surveillance to the initiation of systemic therapy or withdrawal of consent. The time to progression (TTP) during surveillance was defined as the interval between the start of surveillance and disease progression as evaluated by RECIST 1.1. Overall survival (OS) was defined as the time of diagnosis to death from any cause or the date of the last follow-up (censor date). The median follow-up time was calculated based on censored observations.
Categorical variables are presented as frequencies and proportions. Categorical data were compared by means of the Chi Square-test. Median OS and TTP were calculated using Kaplan-Meier curves, with subgroups being compared using the log-rank test. Cox proportional hazard models were performed to identify independent predictors of TTP. Univariate analysis was performed for age, sex, resection of primary tumor, T stage, N stage, the largest axis of the liver metastasis, tumor burden of the liver metastasis. Subsequently, all variables with a P value < 0.10 were selected for multivariable analysis. Subanalysis was performed to identify predictors of TTP for the different histopathologic subtypes. A P value < 0.05 was considered statistically significant. Data were analyzed using SPSS 24.0 software (SPSS, Chicago, IL, USA). The nomogram and C-index were calculated using R (version 2.12.2; http://r-project.org).
The study included 76 patients who received active surveillance during the follow-up period. The decision to choose active surveillance in 66 patients was made jointly by the multidisciplinary team, while 10 out 76 patients choose active surveillance because they refused medical treatment due to the financial burden. Patients were initially diagnosed between August 1998 and February 2018. Demographic and baseline characteristics of the 76 included patients are listed in Table 1. Less than half of the patients (40.8%) were diagnosed as stage I-III at the time of surgery, and had recurrent disease during follow-up. In addition, 59.2% of patients were diagnosed with stage IV disease. The median disease-free survival was 31 mo (range: 6-132 mo). Over half of the patients (59.2%) had liver metastasis at the time of diagnosis. All patients had pathological confirmation of liver metastasis by biopsy. All patients had an Eastern Cooperative Oncology Group performance status of 0 to 2 when active surveillance was initiated.
| Number | |
| Age (range, yr) | 51 (18-69) |
| Gender | |
| Male | 41 (53.9) |
| Female | 35 (46.1) |
| T stage | |
| T1 | 5 (6.5) |
| T2 | 24 (31.2) |
| T3 | 25 (32.9) |
| T4 | 11 (14.4) |
| N stage | |
| N0 | 36 (47.4) |
| N1 | 40 (52.6) |
| M stage at diagnosis | |
| M0 | 31 (40.8) |
| M1 | 45 (59.2) |
| Grade of primary tumor | |
| G1 | 13 (17.1) |
| G2 | 63 (82.9) |
| Grade of liver metastasis tumor | |
| G1 | 12 (19.4) |
| G2 | 50 (80.6) |
| Surgery | |
| Resection of primary tumor | 51 (67.1) |
| Non-resection of primary tumor | 25 (32.9) |
| Local treatment of liver metastasis | |
| Yes (resection, TACE, ablation) | 33 (43.4) |
| No local treatment | 43 (56.6) |
| Max diameter of liver metastasis | |
| < 5 mm | 27 (35.5) |
| > 5 mm | 49 (64.5) |
| Mean tumor burden of liver metastasis (range) | 0.5% (0.03%-80%) |
Patients were followed up for a median 42 mo (range: 8-240 mo). The median active surveillance time was 14 mo [95% confidence interval (CI): 11.13–16.87]. Sixty-nine (90.7%) patients met the criteria for RECIST-defined progressive disease (PD) at some point in the study, and the median TTP (mTTP) during surveillance was 10 mo (95%CI: 7.23-12.76). Of the 76 patients, 66 started systemic therapy during the follow-up period. However, 10 patients with PD refused treatment due to personal or financial problems, and 4 of these patients died as a result of disease progression. The median OS for the 10 untreated patients was not reached (range: 10-113 mo). The median OS for the whole cohort was also not reached.
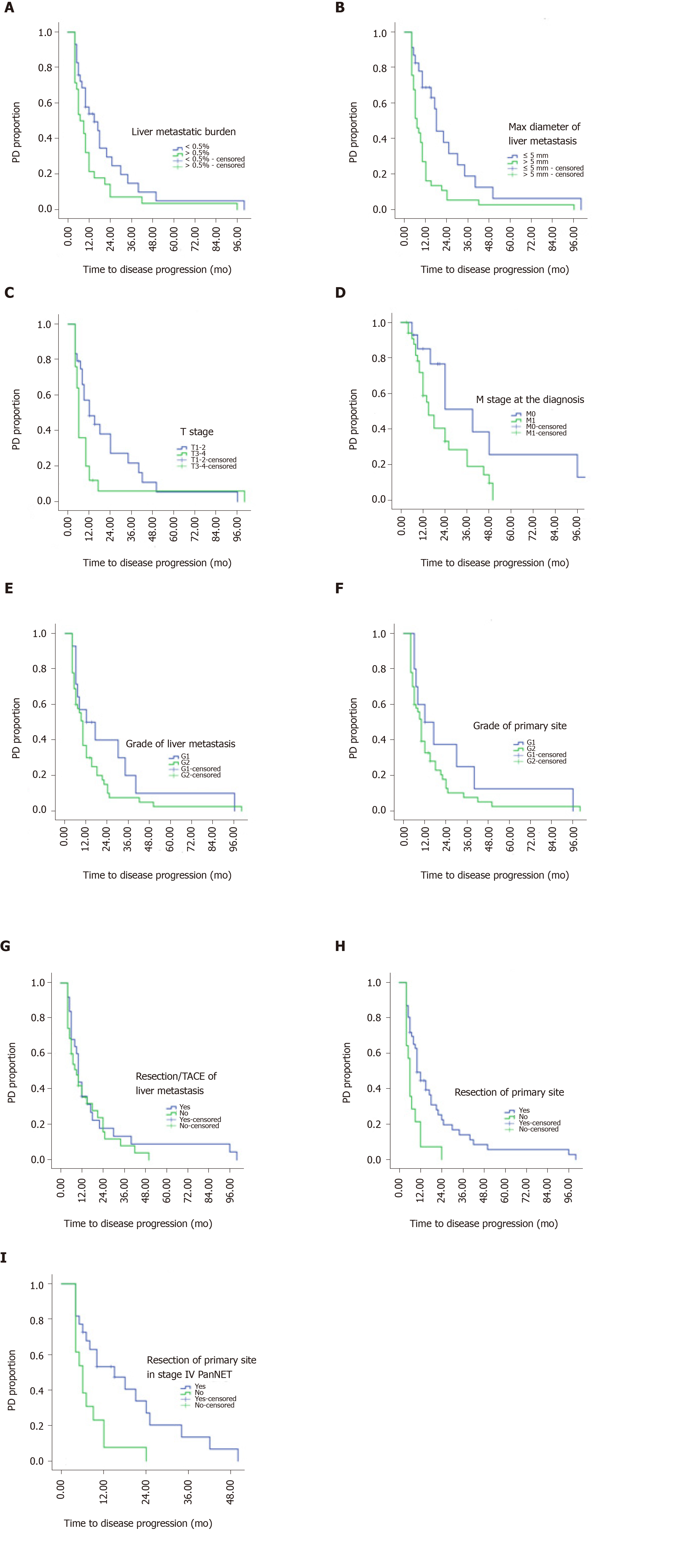
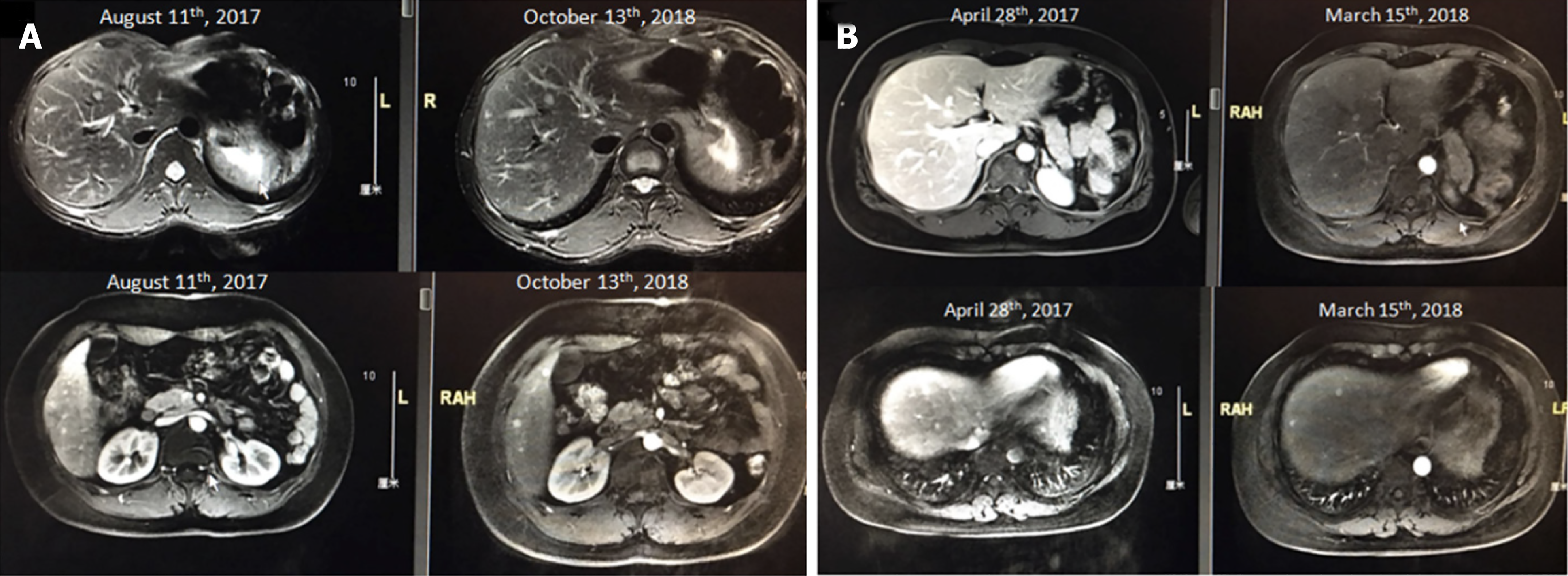
Univariate analysis of baseline prognostic factors revealed that the following risk factors were significantly associated with TTP during surveillance (Figure 1): Resection of the primary tumor (resected vs non-resected: 10 mo vs 6 mo, P = 0.002), and T stage (T1-2 vs T3-4: 12 mo vs 6 mo, P = 0.038). Patients with the largest axis of the liver metastasis ≤ 5 mm had longer mTTP than patients with liver metastasis > 5 mm (18 mo vs 7 mo, P = 0.002), also those with a tumor burden of the liver metastasis < 0.5% had longer mTTP than patients with a tumor burden > 0.5% (15 mo vs 7 mo, P = 0.037). When examining the Ki-67 index of the liver metastases, patients with G1 had a longer TTP than those with G2, but the result was not statistically significant (12 mo vs 10 mo, P = 0.165). In the subgroup with liver metastasis, debulking resection of the primary tumor was also significantly correlated with a longer TTP (15 mo vs 6 mo, P = 0.007), whereas debulking resection (transarterial embolization or ablation) of the liver metastasis showed no relationship with TTP (P > 0.05). The axis of liver metastasis was measured by MRI screening (Figure 2).
In the multivariate analysis, only the largest axis of the liver metastasis [hazard ratio (HR): 2.11, 95%CI: 1.0-4.44; P = 0.049], resection of the primary tumor (HR: 2.11, 95%CI: 1.01-4.39; P = 0.045), and T stage (HR: 2.53, 95%CI: 1.3-4.93; P = 0.006) were independent prognostic factors (Tables 2 and 3).
| 95% Confidence interval | |||
| Univariate analysis | Hazard ratio | Lower | Upper |
| Largest axis of liver metastasis | 2.718 | 1.375 | 5.372 |
| Tumor burden of liver metastasis | 2.262 | 1.064 | 4.81 |
| Resection of primary tumor | 2.112 | 1.015 | 4.394 |
| T stage | 1.888 | 1.208 | 2.952 |
| 95% Confidence interval | |||||||
| Multivariate analysis | B | Standard error | Wald | Significant | Exp(B) | Lower | Upper |
| Largest axis of liver metastasis | 0.810 | 0.395 | 4.206 | 0.040 | 2.247 | 1.037 | 4.872 |
| Grade | 0.614 | 0.384 | 2.559 | 0.110 | 1.848 | 0.871 | 3.924 |
| T stage | 0.793 | 0.362 | 4.810 | 0.028 | 2.211 | 1.088 | 4.492 |
| Resection of primary tumor | 0.888 | 0.394 | 5.076 | 0.024 | 2.431 | 1.123 | 5.266 |
| Age | -0.284 | 0.364 | 0.608 | 0.436 | 0.753 | 0.369 | 1.536 |
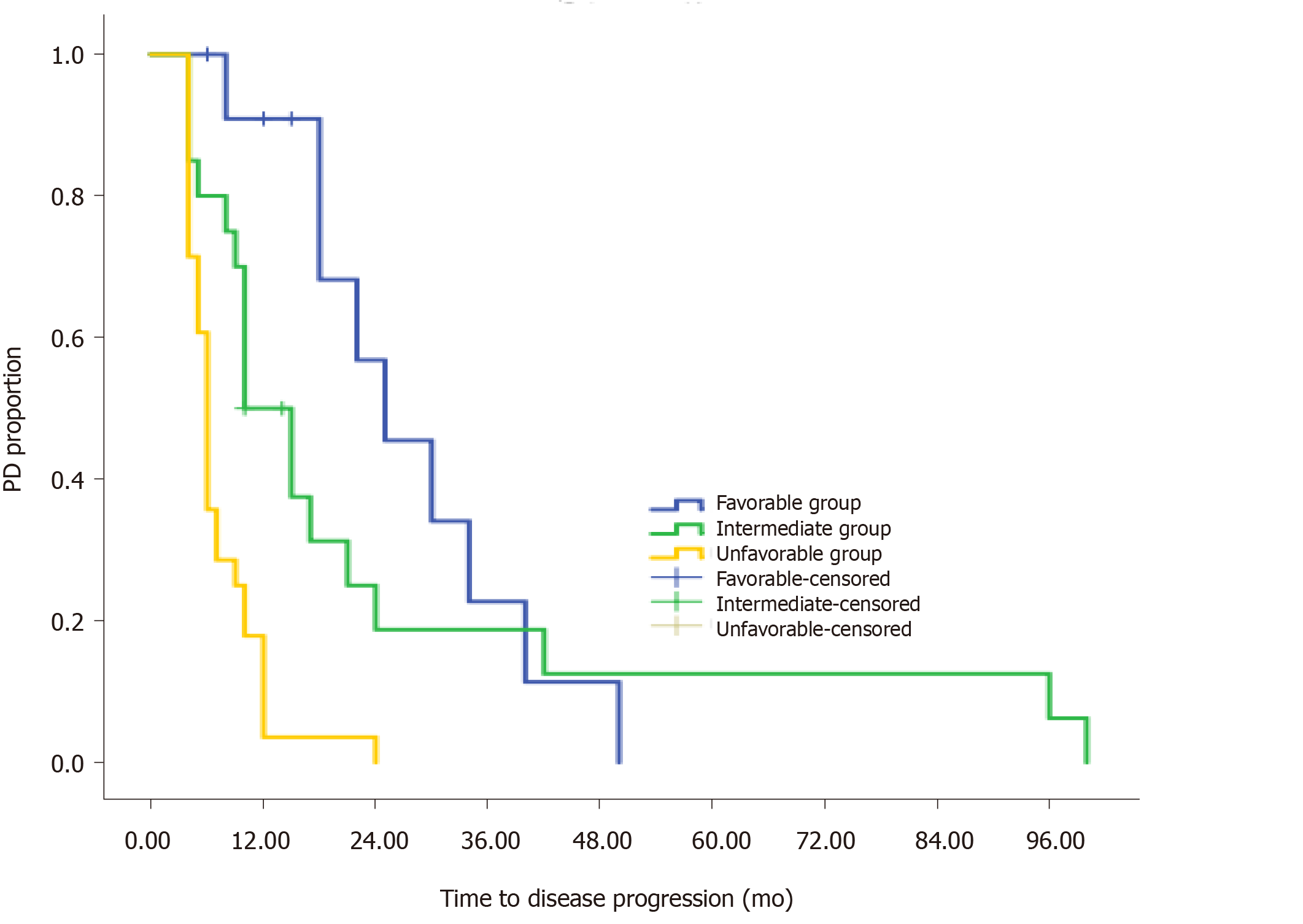
On the basis of the multivariate analysis, we used a recursive partitioning algorithm to separate patients based on the risk factors (largest axis of metastasis > 5 mm, T3-T4, and non-resection of the primary tumor) into a favorable group consisting of patients with no risk factors, an intermediate group consisting of patients with one risk factor, and an unfavorable group consisting of patients with two or three risk factors. The favorable group had an estimated mTTP of 25 mo, whereas the intermediate and unfavorable groups had an estimated mTTP of 10 mo and 6 mo, respectively (Figure 3). The difference was significant (HR: 2.545, 95%CI: 1.65-3.92; P < 0.001).
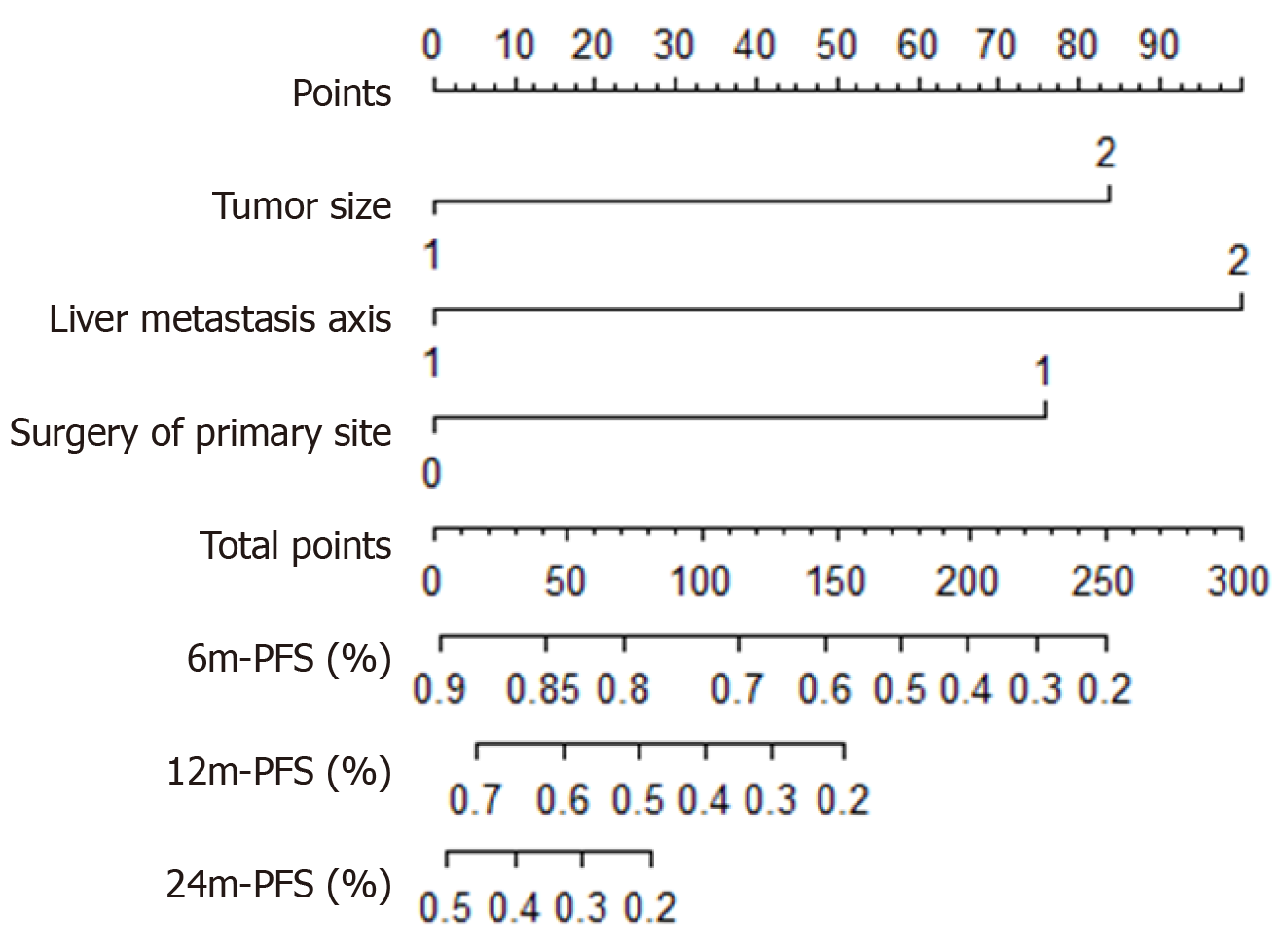
A prognostic nomogram that combined all three independent predictors for TTP during surveillance of metastatic PanNETs is shown in Figure 4. The C-index of the nomogram for predicting TTP was 0.603 (95%CI: 0.47-0.74). Moreover, the calibration plot showed good calibration.
The findings from our study indicated that active surveillance was a viable strategy in some patients with metastatic NF-PanNETs. The median surveillance period was more than 12 mo, and the mTTP during surveillance was 10 mo. For patients with no risk factors, the mTTP was more than 24 mo. Our multivariate analysis and nomogram also showed that patients with fewer adverse prognostic features should be considered for active surveillance.
Metastatic PanNET is a disease characterized by a varied natural history. Many prognostic schemas have been developed, leading to a 5-year OS rate of more than 60%[14]. Stage, grade, and functionality are all independent factors related to the prognosis of PanNETs[15,16], and some gene mutations are also associated with prognosis, such as MEN1, ATRX/DAXX[17], and genes in the mTOR pathway[18], indicating the diverse underlying biology of metastatic NF-PanNETs.
To our knowledge, very little prospective evaluation has been carried out to study surveillance in metastatic NF-PanNETs. However, active surveillance has been used in cases of neuroendocrine neoplasms in other organs. Almost 50% of patients with asymptomatic metastatic pheochromocytomas (PCs) and paragangliomas (PGLs) had stable disease after a year of follow-up[19]. It was concluded that selected PC/PGL patients with slowly progressing tumors could safely use the wait-and-see strategy as an option[20]. In cases of medullary thyroid carcinoma, patients with low disease volume should be considered for a wait-and-see strategy; only those with a large volume and PD are clear candidates for systemic therapy[21]. Treatment strategies for other indolent malignancies also take an active surveillance approach into account. A prospective phase II trial of 52 patients with RCC showed that a subset of patients with metastatic RCC can safely undergo surveillance before starting systemic therapy. Favorable factors include fewer International Metastatic Database Consortium adverse risk factors and fewer organs affected by metastasis[6]. A retrospective series of 58 patients with metastatic RCC noted an initial observation period of 12.4 mo until systemic therapy was started[22]. For patients with follicular lymphoma with low tumor burden, a study examining a watch-and-wait strategy indicated a median time to starting therapy of 31.1 mo; 46% of patients did not need treatment at 3 years, and there was no difference in OS compared to the treatment group[23].
A surveillance-based approach has been investigated in small NF-PanNETs[24]. Surveillance of liver metastatic NF-PanNETs has not been fully evaluated. In the CLARINET trial[25], although 96% of patients had stable disease in the 3 to 6 mo before randomization, mPFS and 24-mo PFS rate were significantly prolonged with lanreotide in comparison with placebo. This result supports that starting SSA therapy before tumor progression may prevent tumor growth. However, our retrospective analysis restaged NF-PanNET patients with liver metastasis and found that, a subset of favorable patients (less 10%) in our cohort, as defined by the largest axis of the liver metastasis < 5 mm, T1–T2, and resection of the primary site, showed a TTP of more than 2 years without systemic therapy. This subpopulation may be considered for active surveillance in clinical management, if the patient’s situation allows. Appropriate selection of patients and adequate monitoring are crucial for this approach. In our study, more than 90% patients with risk factors had PD after 6-10 mo, which made them unsuitable for active surveillance and systemic targeted therapy should be started immediately. The exclusion and inclusion criteria in clinical trials may also need to take these factors into account, as patients with favorable factors may have stable disease without any treatment.
Our study has some limitations. First, it was a retrospective study. We did not have another cohort on which to test the nomogram, and the C-index of the nomogram was not very high. Second, patients in the study were not from a highly selected population. Some patients refused systemic therapy due to financial problems. Furthermore, we did not set a control group of patients with treatment. Nevertheless, prospective assessment of this strategy in selected patients would provide guidance for physicians on how to select appropriate patients for active surveillance.
In conclusion, our study showed that active surveillance might be an approach for metastatic NF-PanNET patients with favorable risk factors, while other patients with metastatic NF-PanNET progressed rapidly without treatment. Additional investigations into the risks and benefits of surveillance are warranted.
Pancreatic neuroendocrine tumors (PanNETs) are heterogeneous and indolent; the National Comprehensive Cancer Network (NCCN) guidelines state that delaying treatment is an option for PanNET with distant metastasis, if the patient has stable disease.
The NCCN did not mention specific factors that influenced surveillance. In addition, data regarding the period of active surveillance in patients with metastatic PanNET are lacking.
To describe the factors influencing active surveillance in patients with liver metastatic nonfunctioning PanNETs (NF-PanNETs).
Clinicopathological characteristics of patients with liver metastatic NF-PanNETs who received active surveillance were collected, and the median time to disease progression (TTP) was measured.
The median TTP (mTTP) was 10 mo in 76 patients with liver metastatic NF-PanNETs. Multivariate analysis showed that the largest axis of the liver metastasis > 5 mm (P = 0.04), non-resection of the primary tumor (P = 0.024), and T3-T4 stage (P = 0.028) were associated with a shorter TTP. The mTTP in patients with no risk factors was 24 mo, which was significantly longer than that in patients with one (10 mo) or more (6 mo) risk factors (P < 0.001). A nomogram with three risk factors showed reasonable calibration, with a C-index of 0.603 (95%CI: 0.47–0.74).
Patients with favorable factors had a time to progressive disease of more than two years without systemic treatment; further studies with a larger sample size and a control are required.
Active surveillance may only be safe for metastatic NF-PanNET patients with favorable risk factors, and other patients progressed rapidly without treatment.
We thank Professor Sheng-Jian Zhang, from the Radiology Department of FUSCC, for CT/MRI imaging support, and Ze-Zhou Wang, from the Cancer Prevent Department of FUSCC, for statistics review.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Link A, Perrotti S S-Editor: Liu M L-Editor: Webster JR P-Editor: Wang LL
| 1. | Fan JH, Zhang YQ, Shi SS, Chen YJ, Yuan XH, Jiang LM, Wang SM, Ma L, He YT, Feng CY, Sun XB, Liu Q, Deloso K, Chi Y, Qiao YL. A nation-wide retrospective epidemiological study of gastroenteropancreatic neuroendocrine neoplasms in china. Oncotarget. 2017;8:71699-71708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Luo G, Javed A, Strosberg JR, Jin K, Zhang Y, Liu C, Xu J, Soares K, Weiss MJ, Zheng L, Wolfgang CL, Cives M, Wong J, Wang W, Sun J, Shao C, Wang W, Tan H, Li J, Ni Q, Shen L, Chen M, He J, Chen J, Yu X. Modified Staging Classification for Pancreatic Neuroendocrine Tumors on the Basis of the American Joint Committee on Cancer and European Neuroendocrine Tumor Society Systems. J Clin Oncol. 2017;35:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 3. | Pavel M, Valle JW, Eriksson B, Rinke A, Caplin M, Chen J, Costa F, Falkerby J, Fazio N, Gorbounova V, de Herder W, Kulke M, Lombard-Bohas C, O'Connor J, Sorbye H, Garcia-Carbonero R; Antibes Consensus Conference Participants; Antibes Consensus Conference participants. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasms: Systemic Therapy - Biotherapy and Novel Targeted Agents. Neuroendocrinology. 2017;105:266-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 4. | Garcia-Carbonero R, Rinke A, Valle JW, Fazio N, Caplin M, Gorbounova V, O Connor J, Eriksson B, Sorbye H, Kulke M, Chen J, Falkerby J, Costa F, de Herder W, Lombard-Bohas C, Pavel M; Antibes Consensus Conference participants. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasms. Systemic Therapy 2: Chemotherapy. Neuroendocrinology. 2017;105:281-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 5. | National Comprehensive Cancer Network (NCCN) Neuroendocrine and Adrenal Tumors. NCCN Clinical Practice Guidelines in Oncology. NCCN Guidelines Version 4. 2018. Available from: https://www.nccn.org/professionals/physician_gls/Default.aspx. |
| 6. | Rini BI, Dorff TB, Elson P, Rodriguez CS, Shepard D, Wood L, Humbert J, Pyle L, Wong YN, Finke JH, Rayman PA, Larkin JM, Garcia JA, Plimack ER. Active surveillance in metastatic renal-cell carcinoma: a prospective, phase 2 trial. Lancet Oncol. 2016;17:1317-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 195] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 7. | Burmeister BH, Henderson MA, Ainslie J, Fisher R, Di Iulio J, Smithers BM, Hong A, Shannon K, Scolyer RA, Carruthers S, Coventry BJ, Babington S, Duprat J, Hoekstra HJ, Thompson JF. Adjuvant radiotherapy versus observation alone for patients at risk of lymph-node field relapse after therapeutic lymphadenectomy for melanoma: a randomised trial. Lancet Oncol. 2012;13:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 8. | Faries MB, Thompson JF, Cochran AJ, Andtbacka RH, Mozzillo N, Zager JS, Jahkola T, Bowles TL, Testori A, Beitsch PD, Hoekstra HJ, Moncrieff M, Ingvar C, Wouters MWJM, Sabel MS, Levine EA, Agnese D, Henderson M, Dummer R, Rossi CR, Neves RI, Trocha SD, Wright F, Byrd DR, Matter M, Hsueh E, MacKenzie-Ross A, Johnson DB, Terheyden P, Berger AC, Huston TL, Wayne JD, Smithers BM, Neuman HB, Schneebaum S, Gershenwald JE, Ariyan CE, Desai DC, Jacobs L, McMasters KM, Gesierich A, Hersey P, Bines SD, Kane JM, Barth RJ, McKinnon G, Farma JM, Schultz E, Vidal-Sicart S, Hoefer RA, Lewis JM, Scheri R, Kelley MC, Nieweg OE, Noyes RD, Hoon DSB, Wang HJ, Elashoff DA, Elashoff RM. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N Engl J Med. 2017;376:2211-2222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 1000] [Article Influence: 125.0] [Reference Citation Analysis (0)] |
| 9. | Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, Lambert B, Delrue L, Bultijnck R, Claeys T, Goetghebeur E, Villeirs G, De Man K, Ameye F, Billiet I, Joniau S, Vanhaverbeke F, De Meerleer G. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol. 2018;36:446-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 670] [Cited by in RCA: 963] [Article Influence: 120.4] [Reference Citation Analysis (0)] |
| 10. | Decaestecker K, De Meerleer G, Ameye F, Fonteyne V, Lambert B, Joniau S, Delrue L, Billiet I, Duthoy W, Junius S, Huysse W, Lumen N, Ost P. Surveillance or metastasis-directed Therapy for OligoMetastatic Prostate cancer recurrence (STOMP): study protocol for a randomized phase II trial. BMC Cancer. 2014;14:671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 11. | Lloyd RV, Osamura RY, Klöppel G, Rosai J. WHO Classification of Tumours of Endocrine Organs. 4th ed. IARC WHO Classification of Tumours, 2017; 10. . |
| 12. | Rindi G. The ENETS guidelines: the new TNM classification system. Tumori. 2010;96:806-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12751] [Cited by in RCA: 13076] [Article Influence: 523.0] [Reference Citation Analysis (0)] |
| 14. | Gao H, Liu L, Wang W, Xu H, Jin K, Wu C, Qi Z, Zhang S, Liu C, Xu J, Ni Q, Yu X. Novel recurrence risk stratification of resected pancreatic neuroendocrine tumor. Cancer Lett. 2018;412:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Panzuto F, Boninsegna L, Fazio N, Campana D, Pia Brizzi M, Capurso G, Scarpa A, De Braud F, Dogliotti L, Tomassetti P, Delle Fave G, Falconi M. Metastatic and locally advanced pancreatic endocrine carcinomas: analysis of factors associated with disease progression. J Clin Oncol. 2011;29:2372-2377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 16. | Ellison TA, Wolfgang CL, Shi C, Cameron JL, Murakami P, Mun LJ, Singhi AD, Cornish TC, Olino K, Meriden Z, Choti M, Diaz LA, Pawlik TM, Schulick RD, Hruban RH, Edil BH. A single institution's 26-year experience with nonfunctional pancreatic neuroendocrine tumors: a validation of current staging systems and a new prognostic nomogram. Ann Surg. 2014;259:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 17. | Marinoni I, Kurrer AS, Vassella E, Dettmer M, Rudolph T, Banz V, Hunger F, Pasquinelli S, Speel EJ, Perren A. Loss of DAXX and ATRX are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors. Gastroenterology. 2014;146:453-60.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 332] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 18. | Scarpa A, Chang DK, Nones K, Corbo V, Patch AM, Bailey P, Lawlor RT, Johns AL, Miller DK, Mafficini A, Rusev B, Scardoni M, Antonello D, Barbi S, Sikora KO, Cingarlini S, Vicentini C, McKay S, Quinn MC, Bruxner TJ, Christ AN, Harliwong I, Idrisoglu S, McLean S, Nourse C, Nourbakhsh E, Wilson PJ, Anderson MJ, Fink JL, Newell F, Waddell N, Holmes O, Kazakoff SH, Leonard C, Wood S, Xu Q, Nagaraj SH, Amato E, Dalai I, Bersani S, Cataldo I, Dei Tos AP, Capelli P, Davì MV, Landoni L, Malpaga A, Miotto M, Whitehall VL, Leggett BA, Harris JL, Harris J, Jones MD, Humphris J, Chantrill LA, Chin V, Nagrial AM, Pajic M, Scarlett CJ, Pinho A, Rooman I, Toon C, Wu J, Pinese M, Cowley M, Barbour A, Mawson A, Humphrey ES, Colvin EK, Chou A, Lovell JA, Jamieson NB, Duthie F, Gingras MC, Fisher WE, Dagg RA, Lau LM, Lee M, Pickett HA, Reddel RR, Samra JS, Kench JG, Merrett ND, Epari K, Nguyen NQ, Zeps N, Falconi M, Simbolo M, Butturini G, Van Buren G, Partelli S, Fassan M; Australian Pancreatic Cancer Genome Initiative, Khanna KK, Gill AJ, Wheeler DA, Gibbs RA, Musgrove EA, Bassi C, Tortora G, Pederzoli P, Pearson JV, Waddell N, Biankin AV, Grimmond SM. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature. 2017;543:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 681] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 19. | Hescot S, Leboulleux S, Amar L, Vezzosi D, Borget I, Bournaud-Salinas C, de la Fouchardiere C, Libé R, Do Cao C, Niccoli P, Tabarin A, Raingeard I, Chougnet C, Giraud S, Gimenez-Roqueplo AP, Young J, Borson-Chazot F, Bertherat J, Wemeau JL, Bertagna X, Plouin PF, Schlumberger M, Baudin E; French group of Endocrine and Adrenal tumors (Groupe des Tumeurs Endocrines-REseau NAtional des Tumeurs ENdocrines and COrtico-MEdullo Tumeurs Endocrines networks). One-year progression-free survival of therapy-naive patients with malignant pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2013;98:4006-4012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Baudin E, Habra MA, Deschamps F, Cote G, Dumont F, Cabanillas M, Arfi-Roufe J, Berdelou A, Moon B, Al Ghuzlan A, Patel S, Leboulleux S, Jimenez C. Therapy of endocrine disease: treatment of malignant pheochromocytoma and paraganglioma. Eur J Endocrinol. 2014;171:R111-R122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Galofré JC, Santamaría Sandi J, Capdevila J, Navarro González E, Zafón Llopis C, Ramón Y Cajal Asensio T, Gómez Sáez JM, Jiménez-Fonseca P, Riesco Eizaguirre G, Grande E. Consensus on the management of advanced medullary thyroid carcinoma on behalf of the Working Group of Thyroid Cancer of the Spanish Society of Endocrinology (SEEN) and the Spanish Task Force Group for Orphan and Infrequent Tumors (GETHI). Endocrinol Nutr. 2015;62:e37-e46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Park I, Lee JL, Ahn JH, Lee DH, Lee KH, Jeong IG, Song C, Hong B, Hong JH, Ahn H. Active surveillance for metastatic or recurrent renal cell carcinoma. J Cancer Res Clin Oncol. 2014;140:1421-1428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Ardeshna KM, Qian W, Smith P, Braganca N, Lowry L, Patrick P, Warden J, Stevens L, Pocock CF, Miall F, Cunningham D, Davies J, Jack A, Stephens R, Walewski J, Ferhanoglu B, Bradstock K, Linch DC. Rituximab versus a watch-and-wait approach in patients with advanced-stage, asymptomatic, non-bulky follicular lymphoma: an open-label randomised phase 3 trial. Lancet Oncol. 2014;15:424-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 282] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 24. | Rosenberg AM, Friedmann P, Del Rivero J, Libutti SK, Laird AM. Resection versus expectant management of small incidentally discovered nonfunctional pancreatic neuroendocrine tumors. Surgery. 2016;159:302-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 25. | Caplin ME, Pavel M, ĆwikÅ‚a JB, Phan AT, Raderer M, SedláÄková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P; CLARINET Investigators. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1290] [Article Influence: 117.3] [Reference Citation Analysis (0)] |