Published online Sep 6, 2020. doi: 10.12998/wjcc.v8.i17.3718
Peer-review started: June 18, 2020
First decision: July 24, 2020
Revised: July 28, 2020
Accepted: August 13, 2020
Article in press: August 13, 2020
Published online: September 6, 2020
Processing time: 78 Days and 1.8 Hours
Gemcitabine plus nab-paclitaxel (GA) and modified FOLFIRINOX (FFX) have been widely used as standard first-line treatment in pancreatic cancer. However, it is unclear which regimen is more efficacious.
To evaluate a retrospective analysis comparing the efficacy and safety of FFX and GA as first-line chemotherapeutic regimens in patients with metastatic pancreatic cancer.
We retrospectively analyzed and compared outcomes in 101 patients who presented with pancreatic cancer and were treated with either GA (n = 54) or FFX (n = 47). Moreover, we performed subgroup analysis based on the neutrophil/lymphocyte ratio (NLR) and Eastern Cooperative Oncology Group (ECOG) performance status.
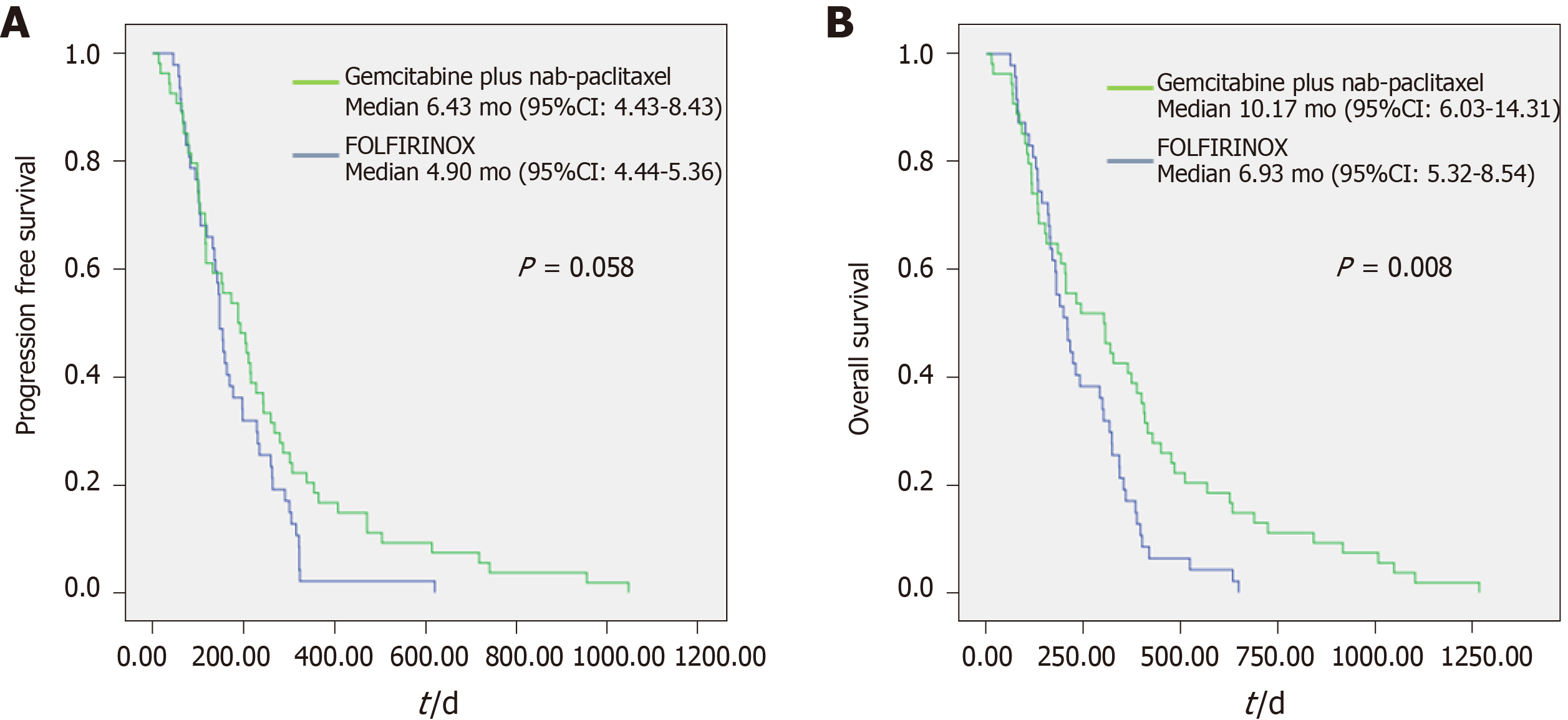
There were no significant differences between two groups in baseline characteristics, except for the ECOG performance status. The median progression-free survival (PFS) (6.43 mo vs 4.90 mo, P = 0.058) was comparable between two groups; however, median overall survival (OS) (10.17 mo vs 6.93 mo, P = 0.008) was longer in patients who received GA regimen. In patients with ECOG 0 (PFS: 8.93 mo vs 5.43 mo, P = 0.002; OS: 16.10 mo vs 6.97 mo, P = 0.000) and those with NLR < 3 (PFS: 8.10 mo vs 6.57 mo, P = 0.008; OS: 12.87 mo vs 9.93 mo, P = 0.002), GA regimen showed higher efficacy.
GA regimen may be recommended to the patients with NLR < 3 or ECOG 0 status although GA and FFX showed comparable efficacy outcomes in patients with metastatic pancreatic cancer.
Core tip: Gemcitabine plus nab-paclitaxel (GA) and modified FOLFIRINOX (FFX) have been widely used as standard first-line treatment in pancreatic cancer. However, it is unclear which regimen is more efficacious. Our retrospective study aims to explore the efficacy and safety of FFX and GA as first-line chemotherapeutic regimens in patients with metastatic pancreatic cancer. Our data showed that comparable result in both regimens. However, GA regimen have better result of median progression free survival and overall survival in patients with ECOG 0 status or neutrophil/lymphocyte ratio < 3.
- Citation: Han SY, Kim DU, Seol YM, Kim S, Lee NK, Hong SB, Seo HI. Comparison of gemcitabine plus nab-paclitaxel and FOLFIRINOX in metastatic pancreatic cancer. World J Clin Cases 2020; 8(17): 3718-3729
- URL: https://www.wjgnet.com/2307-8960/full/v8/i17/3718.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i17.3718
Pancreatic cancer has a poor prognosis, with a 5-year survival rate of less than 10%[1]. Most patients are diagnosed at an advanced stage, and less than one-third of the patients are suitable for surgical resection. Therefore, systemic chemotherapy is an important approach for treating patients at the metastatic stage, including patients with a locally advanced disease.
Recently, many advances have been made in chemotherapy development for pancreatic cancer. Gemcitabine plus nab-paclitaxel (GA) and modified FOLFIRINOX (FFX) therapies have been widely used as standard first-line treatments for pancreatic cancer. The FOLFIRINOX regimen, which consists of 5-fluorouracil (5-FU), leucovorin, oxaliplatin, and irinotecan, was introduced by the PRODIGE4/ACCORD11 trial[2]. In this clinical trial, FOLFIRINOX yielded superior survival rates when compared to gemcitabine monotherapy. Another randomized phase III trial, MPACT, showed that a combination of gemcitabine and nab-paclitaxel yielded a statistically significant survival benefit and response rate when compared with gemcitabine monotherapy[3]. As a result, these two regimens are recommended as the first-line therapy for metastatic pancreatic cancer[4,5]. Although there have been comparative studies on these two chemotherapy regimens, it remains unclear which of the two is more efficacious. In a pivotal trial for each regimen, FFX showed numerically better results than GA in progression-free survival (PFS) (5.5 vs 6.4 mo) and overall survival (OS) (8.5 vs 11.1 mo)[2,3]. However, in these studies' analysis, direct comparisons are difficult to make because of the different baseline characteristics of the patients, which include known prognostic factors. A study conducted among patients living in South Korea revealed that the GA regimen showed better results for peritoneal metastasis among patients aged 65 years or older or having a Charlson comorbidity index of 9 or higher[6]. However, FFX showed better results than GA in studies conducted in Europe and Canada[7,8]. A recently published meta-analysis[9] showed no major benefit of one regimen over the other. Thus, more research is needed to understand whether the efficacies of these two regimens are similar or different depending on the situation.
In this study, we retrospectively reviewed the efficacy and safety of FFX and GA as first-line chemotherapeutic regimens in patients with metastatic pancreatic cancer and evaluated to identify the predictive markers of which chemotherapy regimens were more effective.
We retrospectively reviewed medical records of patients with metastatic pancreatic cancer and included those who received either GA or FFX as first-line chemotherapy between January 2013 and September 2019 at Pusan National University Hospital, Busan, Korea. All patients were histologically diagnosed with ductal adenocarcinoma. This study was performed in accordance with the ethical guidelines of the Helsinki Declaration (revised in 2013), and the study protocol was approved by the Institutional Review Board of Pusan National University, IRB No. H-2002-023-088.
GA therapy consisted of a 30-min intravenous infusion of nab-paclitaxel at a dose of 125 mg/m2, followed by gemcitabine at a dose of 1000 mg/m2 on days 1, 8, and 15, administered every four weeks, as described in the MPACT trial[3]. FFX therapy consisted of a sequence of a 2-h intravenous infusion of oxaliplatin at 85 mg/m2, a 90-min intravenous infusion of irinotecan at 180 mg/m2, a 2-h infusion of leucovorin at 400 mg/m2, an intravenous bolus of 5-FU at 400 mg/m2, and a 46-h continuous infusion of 5-FU at 2400 mg/m2 administered every two weeks[10]. Tumor response was assessed every 10–12 wk using computed tomography and graded according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. All patients who were available had a follow-up period of at least six months (excluding those lost during the follow-up). Patients, who were lost during the follow-up period were analyzed assuming there was a disease progression on the last visit date or death.
The primary outcomes of the study were the PFS and OS in patients who were treated with two regimens. Secondary outcomes were used to assess the safety and feasibility of each regimen and to identify special factors related to prognosis. We performed a Cox regression analysis to identify factors that correlated with PFS and OS. The Eastern Cooperative Oncology Group (ECOG) performance status and the neutrophil/lymphocyte ratio (NLR) were identified as significant factors at a 99% confidence level (see Supplementary 1 and 2). Thus, a subgroup analysis was performed on these factors. These factors were also used to correlate patient situation with the efficacy of each regimen. The NLR and platelet/lymphocyte ratio (PLR) were evaluated at the 1st tumor response to chemotherapy as well.
Statistical analysis was performed using SPSS software (version 22.0, IBM Corp., Armonk, NY, United States). Categorical data were expressed as frequency and percentage, with between-group differences evaluated using the chi-square test. Continuous data were expressed as mean ± SD, with between-group differences evaluated using independent Student’s t-test. Statistical significance was determined at P < 0.05. For comparison, OS and PFS were assessed using Kaplan–Meier survival curves and the log-rank test. For subgroup analysis, the Cox regression test was used to test for variables affecting the PFS and OS at 99% confidence.
The baseline characteristics of the 101 patients are summarized in Table 1. Unless otherwise indicated, the following data were presented with the GA group listed first, followed by the FFX group. Of the total patients, 54 were treated with GA and 47 were treated with FFX. Mean patient age (65.7 ± 7.8 vs 64.4 ± 8.4 years, P = 0.445) and the proportion of males (72.2% vs 66.0%, P = 0.501) were similar between the groups. There were no significant baseline differences between the groups except for the ECOG performance status; patients with ECOG 2 status were present only in the GA group (11.1% vs 0%, P = 0.018). Patients with an ECOG 0 status were at a higher ratio in the FFX group than in the GA group (48.1% vs 51.9%, P = 0.054). In the GA group, number of metastasis ≥ 2 was more common than that of single metastasis when compared with the FFX group (number of metastasis ≥ 2: 88.9% vs 74.5%, P = 0.060). The mean NLR was slightly higher in the GA group (3.72 ± 2.98 vs 3.0 ± 1.87, P = 0.167).
| Variable | GA (n = 54) | FOLFIRINOX (n = 47) | P value |
| Age (yr) | 65.7 ± 7.8 | 64.4 ± 8.4 | 0.445 |
| Male | 39 (72.2) | 31 (66.0) | 0.501 |
| Body weight (kg) | 59.2 ± 9.5 | 58.5 ± 9.9 | 0.716 |
| Height (m) | 1.65 ± 0.08 | 1.63 ± 0.07 | 0.352 |
| Body mass index (kg/m2) | 21.9 ± 3.1 | 22.0 ± 3.5 | 0.852 |
| Psoas muscle area (cm2) | 8.6 ± 2.9 | 8.3 ± 2.1 | 0.639 |
| Number of metastasis ≥ 2 | 48 (88.9) | 35 (74.5) | 0.060 |
| Liver meta | 32 (59.3) | 31 (66.0) | 0.493 |
| Carcinomatosis peritonei | 21 (38.9) | 16 (34.0) | 0.618 |
| Lung meta | 13 (24.1) | 8 (17.0) | 0.389 |
| Primary tumor site | 0.778 | ||
| Head | 25 (46.3) | 25 (53.2) | |
| Body | 14 (25.9) | 8 (17.0) | |
| Tail | 15 (27.8) | 14 (29.8) | |
| ECOG performance status | 0.018a | ||
| 0 or 1 | 48 (88.9) | 47 (100) | |
| 2 | 6 (11.1) | 0 (0) | |
| Diabetes | 22 (40.7) | 23 (48.9) | 0.414 |
| Hypertension | 12 (22.2) | 12 (25.5) | 0.700 |
| Laboratory findings | |||
| White blood cell (/µL) | 6465.6 ± 2743.0 | 9780.0 ± 16931.6 | 0.159 |
| Platelet (103/µL) | 235.9 ± 122.4 | 270.1 ±107.7 | 0.142 |
| Neutrophil/lymphocyte ratio | 3.72 ± 2.98 | 3.0 ± 1.87 | 0.167 |
| Pletelet/lymphocyte ratio | 190.2 ± 120.7 | 159.5 ± 71.0 | 0.129 |
| C-related protein (mg/dL) | 1.49 ± 2.23 | 1.29 ± 2.00 | 0.641 |
| Albumin (g/dL) | 3.92 ± 0.46 | 3.98 ± 0.57 | 0.552 |
| Total bilirubin (mg/dL) | 1.41 ± 3.06 | 1.42 ± 1.60 | 0.988 |
| CA 19-9 (U/mL) | 12881.3 ± 54407.2 | 1307.1 ± 2079.1 | 0.153 |
Table 2 shows the chemotherapy response data for each regimen. When comparing the best response for each regimen, the disease control rate was similar between the groups (83.3% vs 76.6%, P = 0.402). The proportion of patients who took a reduced dose of the regimen was similar (75.9% vs 68.1%, P = 0.385). However, the mean delivery dose of chemotherapy showed a significant difference between the groups (78.9 ± 16.5% vs 87.3 ± 11.6%, P = 0.005). The proportion of patients who received more than 80% of the expected dose was higher in the FFX group (44.4% vs 65.2%, P = 0.038). The median duration of chemotherapy was longer in the GA group (2.33 vs 1.63 mo, P = 0.005). There was no significant difference of the median PFS between the groups (6.43 vs 4.90 mo, P = 0.058); however, median OS was longer in the GA group (10.17 vs 6.93 mo, P = 0.008) (Figure 1). Each group had a similar proportion of patients who received secondary chemotherapy (44.4% vs 40.4%, P = 0.687). In the GA group, tegafur/gimeracil/oteracil was most frequently used as secondary chemotherapy (n = 15, 27.8%), while in the FFX group, gemcitabine-mono was most commonly used as secondary chemotherapy (n = 12, 25.5%).
| GA (n = 54) | FOLFIRINOX (n = 47) | P value | |
| Best response | 0.216 | ||
| PR | 10 (18.5) | 5 (10.6) | |
| SD | 35 (64.8) | 31 (66.0) | |
| PD | 9 (16.7) | 11 (23.4) | |
| DCR (PR + SD) | 45 (83.3) | 36 (76.6) | 0.402 |
| Dose reduction | 41 (75.9) | 32 (68.1) | 0.385 |
| Delivery dose (%) | 78.9 ± 16.5 | 87.3 ± 11.6 | 0.005b |
| Total over 80% dose | 24 (44.4) | 30 (65.2) | 0.038a |
| Duration of chemotherapy (mo) | 2.33 (1.43-3.24) | 1.63 (1.19-2.08) | 0.005b |
| Progression free survival (mo) | 6.43 (4.43-8.43) | 4.90 (4.44-5.36) | 0.058 |
| Overall survival (mo) | 10.17 (6.03-14.31) | 6.93 (5.32-8.54) | 0.008b |
| 2nd Chemotherapy | 24 (44.4) | 19 (40.4) | 0.687 |
| TS-1 | 15 (27.8) | 1 (2.1) | |
| Gemcitabine mono | 0 (0) | 12 (25.5) | |
| GA | 0 (0) | 5 (10.6) | |
| FOLFIRINOX | 3 (5.6) | 0 (0) | |
| Onyvide | 1 (1.9) | 0 (0) | |
| 5-FU base | 5 (9.3) | 1 (2.1) | |
| 2nd chemotherapy PFS (mo) | 3.23 (2.55-3.91) | 2.70 (1.56-3.84) | 0.191 |
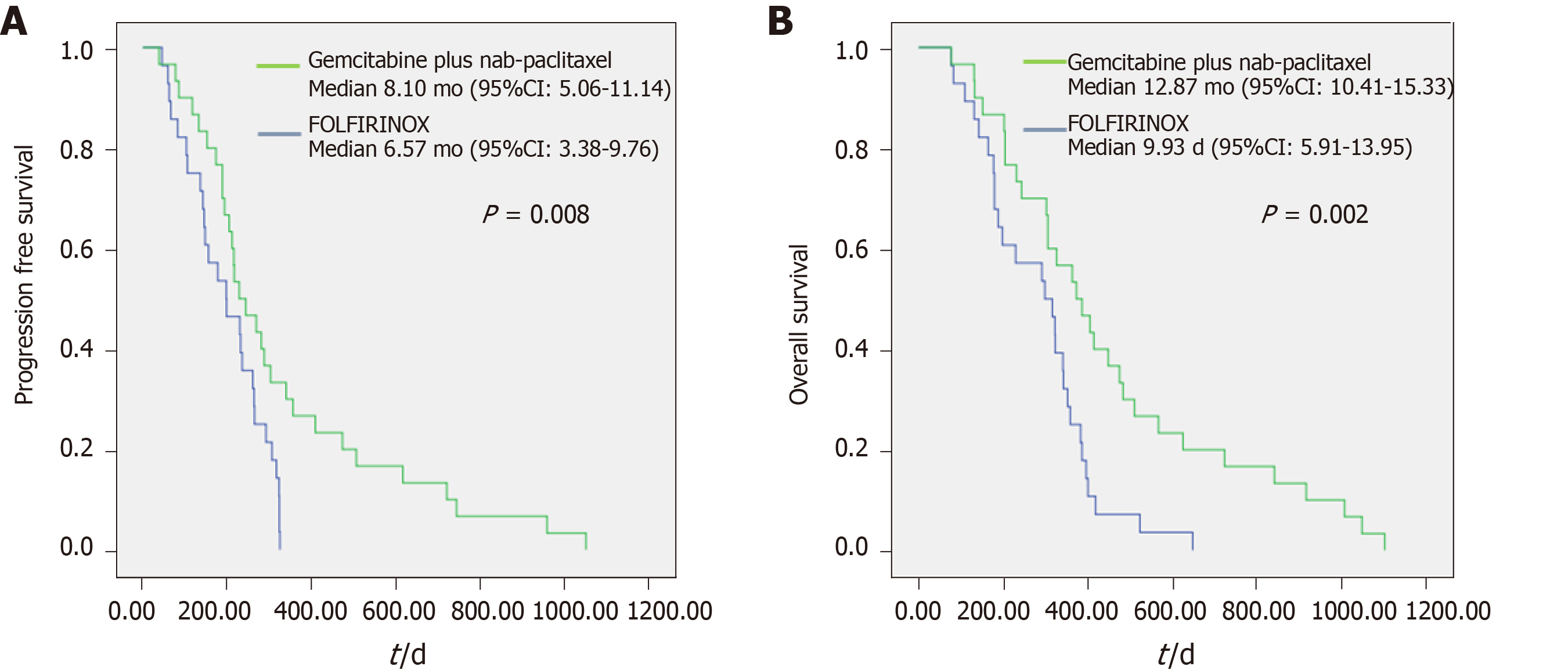
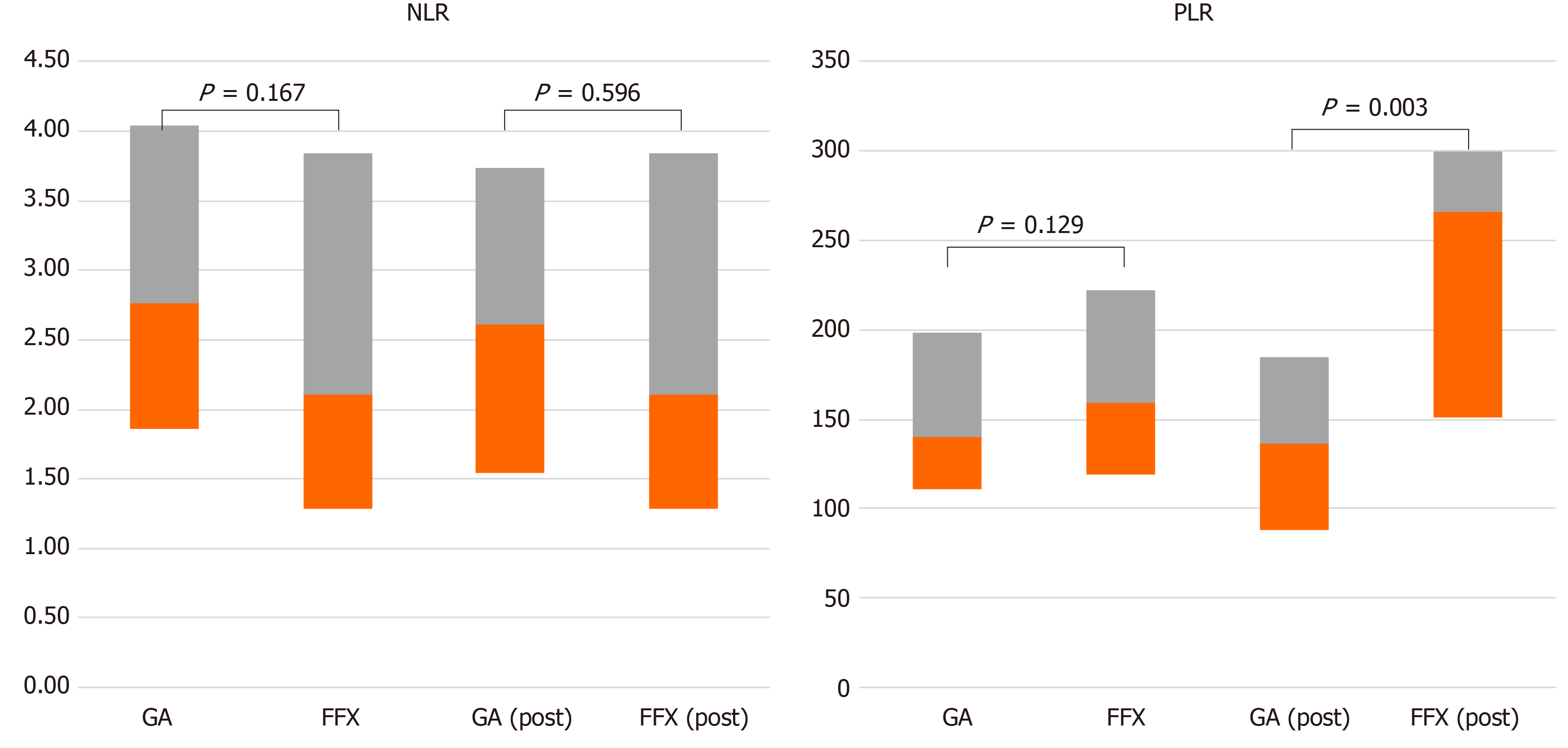
Table 3 shows response comparison depending on the NLR in subgroup analysis. When comparing between NLR < 3 and NLR > 3, the patients in two groups showed significant differences in PFS and OS. The patients in the NLR < 3 group had a longer median PFS (NLR > 3 vs NLR < 3: 3.83 vs 7.60 mo, P = 0.000) and OS (NLR > 3 vs NLR < 3: 4.47 vs 10.87 mo, P = 0.001) than those in the NLR > 3 group. In the NLR < 3 group, comparing the GA and FFX regimens, median PFS (8.10 vs 6.57 mo, P = 0.008) and median OS (12.87 vs 9.93 mo, P = 0.002) showed significant difference between two groups (Figure 2). Furthermore, Figure 3 shows patient responses in the NLR and PLR groups to different chemotherapy regimens. The mean of NLR was not different before and after chemotherapy. However, PLR was different before and after chemotherapy for both regimens. Table 4 and Supplementary Table 3 shows that a decrease in the NLR and CA19-9 after chemotherapy were associated with a good prognosis. CA19-9 decreased after GA chemotherapy, but increased after FFX chemotherapy. The response of the NLR seems to have a greater influence on the prognosis rather than response of CA19-9. Patients with decreased NLR after chemotherapy were associated with high disease control rate, longer PFS, and OS when compared with patients without decreased NLR after chemotherapy. However, change in PLR was not associated with prognosis, which was only influenced by the PLR that was checked before chemotherapy administration (see supplementary Table 4).
| NLR ≥ 3 (n = 43) | NLR < 3 (n = 58) | P value | |||||
| GA (n = 24) | FFX (n = 19) | P value | GA (n = 30) | FFX (n = 28) | P value | ||
| PFS (mo) | 3.83 (3.19-4.47) | 7.60 (6.51-8.69) | 0.000b | ||||
| 3.40 (2.76-4.04) | 4.40 (2.65-6.15) | 0.676 | 8.10 (5.06-11.14) | 6.57 (3.38-9.76) | 0.008b | ||
| OS (mo) | 4.47 (2.80-6.14) | 10.87 (9.22-12.52) | 0.001b | ||||
| 3.87 (2.79-4.95) | 5.33 (3.87-6.80) | 0.648 | 12.87 (10.41-15.33) | 9.93 (5.91-13.95) | 0.002b | ||
| Before chemotherapy > post chemotherapy | Post chemotherapy NLR < 3n = 62 | Post chemotherapy NLR ≥ 3n = 39 | P value | ||
| Low > Low,n = 47 | High > Low,n = 15 | Low > High,n = 11 | High > High,n = 28 | ||
| PFS, median mo (95%CI) | 7.20 (6.07-8.33) | 3.47 (2.81-4.13) | 0.000b | ||
| 7.60 (6.42-8.79) | 5.63 (3.14-8.12) | 5.77 (2.29-9.26) | 3.33 (3.16-3.50) | 0.000b | |
| OS, median mo (95%CI) | 10.87 (9.16-12.58) | 4.47 (3.37-5.57) | 0.002b | ||
| 11.4 (9.93-12.87) | 8.00 (2.45-13.55) | 7.63 (3.10-12.16) | 7.40 (4.49-10.31) | 0.001b | |
| GA regimen | 24 (51.1) | 8 (53.3) | 6 (54.5) | 16 (57.1) | 0.112 |
| DCR | 43 (91.5) | 14 (93.3) | 8 (72.7) | 16 (57.1) | 0.000b |
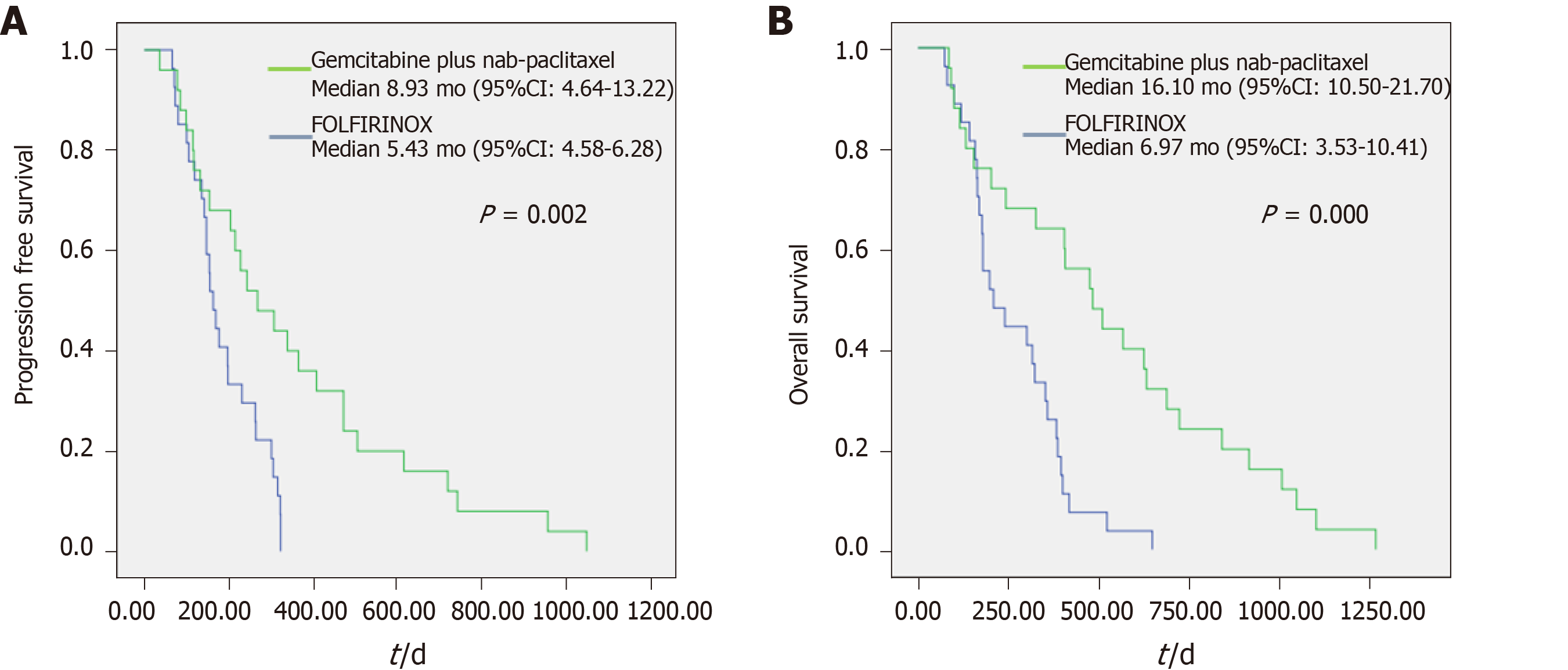
Table 5 shows response comparison depending on the ECOG performance status in subgroup analysis. The patients in three groups had significant differences in median PFS (6.60 vs 3.90 vs 3.27 mo, P = 0.003) and OS (10.77 vs 6.27 vs 4.47 mo, P = 0.000). In the group with ECOG 0 status, median PFS (8.93 vs 5.43 mo, P = 0.002) and median OS (16.10 vs 6.97 mo, P = 0.000) showed significant differences (Figure 4).
| ECOG = 0 (n = 52) | ECOG = 1 (n = 43) | ECOG = 2 (n = 6) | P value | |||||||
| GA (n = 25) | FFX (n = 27) | P value | GA (n = 23) | FFX (n = 20) | P value | GA (n = 6) | FFX (n = 0) | P value | ||
| PFS (mo) | 6.60 (4.28-8.92) | 3.90 (2.46-5.34) | 3.27 (0.00-8.12) | 0.003b | ||||||
| 8.93 (4.64-13.22) | 5.43 (4.58-6.28) | 0.002b | 3.87 (2.03-5.71) | 4.40 (1.84-6.96) | 0.511 | 3.27 (0.00-8.12) | NA | NA | ||
| OS (mo) | 10.77 (6.26-15.28) | 6.27 (4.26-8.28) | 4.47 (0.00-14.00) | 0.000b | ||||||
| 16.10 (10.50-21.70) | 6.97 (3.53-10.41) | 0.000b | 6.10 (3.31-8.89) | 6.27 (2.76-9.78) | 0.674 | 4.47 (0.00-14.00) | NA | NA | ||
Adverse events associated with each chemotherapy regimen are listed in Table 6. For the GA and FFX groups, thromboembolism (5.6% vs 19.1%, P = 0.035), neuropathy (51.9% vs 23.4%, P = 0.000), and nausea (20.4% vs 46.8%, P = 0.008) significantly differed between the groups. Neuropathy was higher in the GA group, with grade 3 or 4 having a significantly higher ratio. Nausea was higher in the FFX group, frequently with grade 1 or 2. There was no difference in admission rates for these adverse events between the groups (33.3% vs 38.3%, P = 0.608). The incidence of colitis and pneumonia also showed no significant difference (20.4% vs 27.7%, P = 0.616).
| Adverse events | GA (n = 54), n (%) | FOLFIRINOX (n = 47), n (%) | P value |
| Admission | 18 (33.3) | 18 (38.3) | 0.608 |
| Thromboembolism | 3 (5.6) | 9 (19.1) | 0.035a |
| Neuropathy (Grade 1,2/3,4) | 28 (51.9) (11/17) | 11 (23.4) (10/1) | 0.000b |
| Neutropenia (Grade 1,2/3,4) | 38 (70.4) (12/26) | 35 (74.5) (10/25) | 0.496 |
| Thrombocytopenia (Grade 1,2/3,4) | 22 (40.7) (13/9) | 17 (36.2) (6/11) | 0.893 |
| Nausea (Grade 1,2/3,4) | 11 (20.4) (9/2) | 22 (46.8) (18/4) | 0.008b |
| Fatigue (Grade 1,2/3,4) | 31 (57.4) (19/12) | 26 (55.3) (17/9) | 0.740 |
| Diarrhea (Grade 1,2/3,4) | 10 (18.5) (7/3) | 12 (25.5) (7/5) | 0.322 |
| Colitis/pneumonia | 11 (20.4) (4/7) | 13 (27.7) (7/6) | 0.616 |
Herein, we showed that the efficacy and safety of the GA regimen were comparable with those of the FFX regimen. NLR > 3 or ECOG status of 1 or 2 were poor prognostic factors for PFS and OS. Patients with an NLR < 3 or ECOG 0 status seemed to benefit more from GA than from FFX. Furthermore, the decrease of NLR after chemotherapy was related to a good prognosis.
Median PFS in the GA and FFX groups were 6.43 and 4.90 mo, respectively; median OS were 10.17 and 6.93 mo, respectively. The disease control rates in the GA and FFX groups were 83.3% and 72.3%, respectively. Compared with pivotal western trial studies, our study showed that the GA regimen yielded numerically better outcome (our result vs pivotal western trial study, PFS: 6.43 vs 5.5 mo; OS: 10.17 vs 8.5 mo) and the FFX regimen showed numerically worse result (PFS: 4.90 vs 6.4 mo; OS: 6.93 vs 11.1 mo). Objective response rates are lower (18.5% for GA and 10.6% for FFX) than those in pivotal western trial studies[2,3]. However, the disease control rate is about 80% similar to that in other studies. Compared to a European study[11] that showed a dose reduction rate of 20.5%, our study shows that of 72.3%. The low objective response rate may be attributed to the higher dose reduction rate than that of other studies.
In a recently published meta-analysis[9] and a systematic review article[12], these two chemotherapy regimens showed similar results. However, when analyzing these studies, the difference between Asian and Western patients may have affected their responses to chemotherapy. In a study conducted among patients living in South Korea, the GA regimen showed better results for peritoneal metastasis among patients of 65 years or older, and a Charlson comorbidity index of 9 or higher[6]. Another study[13] that was published in Korea, reported comparable result for the two regimens; however, the GA group showed longer survival without statistical significance although the patients in the FFX group were approximately 10 years younger than those in the GA group. It is thought that the GA regimen may show better results in situations when the prognostic factors are similar. In another study that analyzed patients living in Japan, the GA regimen showed a higher 1-year survival rate than the FFX regimen[14]. However, studies conducted in Europe and Canada showed better results with FFX than with GA[7,8]. While this difference was not proven experimentally, it could suggest a possible trend, possibly due to genetic differences or physical conditions. In our study, the GA regimen did not prove superior in PFS over FFX; however, in special situations, the GA regimen was more efficacious than the FFX regimen.
One of such situations was an NLR < 3. The NLR is known to be an inflammatory marker and a prognostic factor in patients with cancer[15]. There are several hypotheses suggesting that a high NLR is associated with a poor prognosis in patients with pancreatic cancer. First, myeloid-derived suppressor cells (MDSC)[16,17] have been shown to inhibit the immune response of T cells and NK cells, promote cancer cell growth, and induce distant metastasis of cancer cells[16,18]. As there is no marker to discriminate between PMN-MDSC and neutrophils, an increase in the NLR is thought to be due to an increase in PMN-MDSC, which explains the association with poor prognosis in patients with malignancy. Second, cancer induces the secretion of tumor microenvironment inflammatory factors such as IL-6, IL-17, TNF-alpha, and TGF-beta, which may further induce an increased neutrophil count[19-22]. Third, cancer-related obstructive cholangitis may cause increased neutrophil count. It may be indirectly related to cancer prognosis, but inflammation will make the tumor microenvironment immunosuppressive, resulting in a poor prognosis in patients with cancer[23,24]. Our study also showed that a high NLR was associated a poor prognosis. NLR < 3 was an important prognostic factor for patients who received chemotherapy. Patients with NLR < 3 had a PFS that was four months longer and an OS that was six months longer than those in patients with NLR > 3. The GA regimen had a tendency toward a longer PFS than the FFX regimen in patients with NLR < 3. The variation of NLR after chemotherapy is associated with diseased control rate, PFS and OS. Therefore, evaluating the NLR during routine chemotherapy response assessment will be an effective tool for predicting the prognosis.
Another differential situation was the ECOG 0 status. This scale was developed to assess how a patient’s disease progresses and to determine the appropriate treatment and prognosis[25]. ECOG 0 status was defined as fully active performance without any restriction. ECOG 1 was defined as being restricted in physically strenuous activity. EOCG 2 was defined as being unable to carry out any work activities, only capable of self-care. Our study revealed that the response to the two chemotherapeutic regimens was different in patients with an ECOG 0 status. The exact mechanism is unknown, but the GA regimen was more effective in patients with a better physical condition, similar to that in patients with a low NLR. FFX regimens may also be more burdensome to an Asian patient, who may have a weaker physique than that of a Western patient, as it is difficult to receive second-line chemotherapy. Therefore, the decision made for the first-line chemotherapy may be more important.
We evaluated for other prognostic factors such as PLR and CA19-9. PLR is also known as a representative inflammatory marker[26]. One study in Korea showed that PLR was a prognostic marker in metastatic pancreatic cancer. Therefore, we evaluated the prognostic value in metastatic pancreatic cancer; however, there was no significant relation with prognosis. And CA19-9 is very well known and proven as a prognostic factor in pancreatic cancer. In our study, 8 patients (7.9%) had significantly decreased after chemotherapy. They also had a good prognosis. Change in NLR after chemotherapy may significantly influence the prognosis more than the change in CA19-9.
Comparing adverse events between the GA and FFX regimens, the rate of total adverse events was similar, but some specific adverse events were different. First, thromboembolic events occurred more frequently in patients who received the FFX regimen than in those who received the GA regimen. It occurred in nearly 20% of FFX group patients; therefore, when patients receive FFX chemotherapy, this event should be watched for carefully. Second, neuropathy more frequently occurred in patients receiving the GA regimen, especially grade 3 or 4 neuropathy. It is an irreversible condition; hence, when patients are receiving GA chemotherapy, the physician should monitor for neuropathy. If neuropathy is detected early, nab-paclitaxel should be reduced and drug administration should as conservatively as possible. Third, as nausea commonly occurred with the FFX regimen, physicians should prescribe antiemetic drugs as needed.
Our study had several limitations. First, this study had a single-center, retrospective design and enrolled a relatively low number of patients. Second, some parameters (ECOG performance status) were different between the groups in baseline characteristics. Third, disease progression could not be accurately identified in 18 patients (17.8%) for reasons such as loss during follow-up, death before the disease progressed, or the efficacy of chemotherapy maintained until the evaluation day.
In conclusion, GA and FFX showed comparable efficacy outcomes and safety profiles. Patients who had an NLR below 3 or an ECOG 0 status had a better prognosis than did the other patients. The GA regimen was more efficacious as a first-line treatment in patients who had better prognosis factors such as ECOG 0 or NLR below 3. We suggest that the patients with the LNR below 3 or ECOG 0 status should initially undergo the GA regimen. However, further studies are needed to prove which regimen works better in a wider range of situations.
In pancreatic cancer patients, it is not well-known which chemo regimens are more effective.
It is hypothesized that there is a predictive markers of which chemotherapy regimens.
In this study, the authors aimed to determine which chemo regimen is more efficacious in metastatic pancreatic cancer patients.
The authors performed analysis compare the patient who received gemcitabine plus nab-paclitaxel (GA) and FOLFIRINOX.
There was no significant difference in overall survival between the gemcitabine mono and combination chemotherapy groups. However, patients with Eastern Cooperative Oncology Group (ECOG) 0 or neutrophil/lymphocyte ratio (NLR) < 3 had better result when using GA regimen.
GA chemotherapy may be a better option to manage metastatic pancreatic cancer with ECOG 0 or NLR < 3.
There were some predictive markers of efficacy of chemotherapy regimen in metastatic pancreatic cancer.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Morris DL S-Editor: Yan JP L-Editor: A P-Editor: Xing YX
| 1. | Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11065] [Cited by in RCA: 12186] [Article Influence: 1523.3] [Reference Citation Analysis (3)] |
| 2. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5634] [Article Influence: 402.4] [Reference Citation Analysis (1)] |
| 3. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4883] [Article Influence: 406.9] [Reference Citation Analysis (0)] |
| 4. | Sohal DP, Mangu PB, Khorana AA, Shah MA, Philip PA, O'Reilly EM, Uronis HE, Ramanathan RK, Crane CH, Engebretson A, Ruggiero JT, Copur MS, Lau M, Urba S, Laheru D. Metastatic Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:2784-2796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 234] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 5. | Tempero MA, Malafa MP, Chiorean EG, Czito B, Scaife C, Narang AK, Fountzilas C, Wolpin BM, Al-Hawary M, Asbun H, Behrman SW, Benson AB, Binder E, Cardin DB, Cha C, Chung V, Dillhoff M, Dotan E, Ferrone CR, Fisher G, Hardacre J, Hawkins WG, Ko AH, LoConte N, Lowy AM, Moravek C, Nakakura EK, O'Reilly EM, Obando J, Reddy S, Thayer S, Wolff RA, Burns JL, Zuccarino-Catania G. Pancreatic Adenocarcinoma, Version 1.2019. J Natl Compr Canc Netw. 2019;17:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 271] [Article Influence: 54.2] [Reference Citation Analysis (2)] |
| 6. | Kang J, Hwang I, Yoo C, Kim KP, Jeong JH, Chang HM, Lee SS, Park DH, Song TJ, Seo DW, Lee SK, Kim MH, Hong SM, Shin SH, Hwang DW, Song KB, Lee JH, Kim SC, Ryoo BY. Nab-paclitaxel plus gemcitabine versus FOLFIRINOX as the first-line chemotherapy for patients with metastatic pancreatic cancer: retrospective analysis. Invest New Drugs. 2018;36:732-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 7. | Chan KKW, Guo H, Cheng S, Beca JM, Redmond-Misner R, Isaranuwatchai W, Qiao L, Earle C, Berry SR, Biagi JJ, Welch S, Meyers BM, Mittmann N, Coburn N, Arias J, Schwartz D, Dai WF, Gavura S, McLeod R, Kennedy ED. Real-world outcomes of FOLFIRINOX vs gemcitabine and nab-paclitaxel in advanced pancreatic cancer: A population-based propensity score-weighted analysis. Cancer Med. 2020;9:160-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 8. | Williet N, Saint A, Pointet AL, Tougeron D, Pernot S, Pozet A, Bechade D, Trouilloud I, Lourenco N, Hautefeuille V, Locher C, Desrame J, Artru P, Thirot Bidault A, Le Roy B, Pezet D, Phelip JM, Taieb J. Folfirinox versus gemcitabine/nab-paclitaxel as first-line therapy in patients with metastatic pancreatic cancer: a comparative propensity score study. Therap Adv Gastroenterol. 2019;12:1756284819878660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Pusceddu S, Ghidini M, Torchio M, Corti F, Tomasello G, Niger M, Prinzi N, Nichetti F, Coinu A, Di Bartolomeo M, Cabiddu M, Passalacqua R, de Braud F, Petrelli F. Comparative Effectiveness of Gemcitabine plus Nab-Paclitaxel and FOLFIRINOX in the First-Line Setting of Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (1)] |
| 10. | Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O'Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379:2395-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 1943] [Article Influence: 277.6] [Reference Citation Analysis (0)] |
| 11. | Taieb J, Prager GW, Melisi D, Westphalen CB, D'Esquermes N, Ferreras A, Carrato A, Macarulla T. First-line and second-line treatment of patients with metastatic pancreatic adenocarcinoma in routine clinical practice across Europe: a retrospective, observational chart review study. ESMO Open. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Chiorean EG, Cheung WY, Giordano G, Kim G, Al-Batran SE. Real-world comparative effectiveness of nab-paclitaxel plus gemcitabine versus FOLFIRINOX in advanced pancreatic cancer: a systematic review. Ther Adv Med Oncol. 2019;11:1758835919850367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 13. | Cho IR, Kang H, Jo JH, Lee HS, Chung MJ, Park JY, Park SW, Song SY, An C, Park MS, Bang S. FOLFIRINOX vs gemcitabine/nab-paclitaxel for treatment of metastatic pancreatic cancer: Single-center cohort study. World J Gastrointest Oncol. 2020;12:182-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Tahara J, Shimizu K, Otsuka N, Akao J, Takayama Y, Tokushige K. Gemcitabine plus nab-paclitaxel vs. FOLFIRINOX for patients with advanced pancreatic cancer. Cancer Chemother Pharmacol. 2018;82:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Kim HJ, Lee SY, Kim DS, Kang EJ, Kim JS, Choi YJ, Oh SC, Seo JH, Kim JS. Inflammatory markers as prognostic indicators in pancreatic cancer patients who underwent gemcitabine-based palliative chemotherapy. Korean J Intern Med. 2020;35:171-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | De Veirman K, Van Valckenborgh E, Lahmar Q, Geeraerts X, De Bruyne E, Menu E, Van Riet I, Vanderkerken K, Van Ginderachter JA. Myeloid-derived suppressor cells as therapeutic target in hematological malignancies. Front Oncol. 2014;4:349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 17. | Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5480] [Cited by in RCA: 5329] [Article Influence: 333.1] [Reference Citation Analysis (0)] |
| 18. | Idorn M, Køllgaard T, Kongsted P, Sengeløv L, Thor Straten P. Correlation between frequencies of blood monocytic myeloid-derived suppressor cells, regulatory T cells and negative prognostic markers in patients with castration-resistant metastatic prostate cancer. Cancer Immunol Immunother. 2014;63:1177-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 143] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 19. | Li TJ, Jiang YM, Hu YF, Huang L, Yu J, Zhao LY, Deng HJ, Mou TY, Liu H, Yang Y, Zhang Q, Li GX. Interleukin-17-Producing Neutrophils Link Inflammatory Stimuli to Disease Progression by Promoting Angiogenesis in Gastric Cancer. Clin Cancer Res. 2017;23:1575-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 20. | Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65:8896-8904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 313] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 21. | Wislez M, Rabbe N, Marchal J, Milleron B, Crestani B, Mayaud C, Antoine M, Soler P, Cadranel J. Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: role in tumor progression and death. Cancer Res. 2003;63:1405-1412. [PubMed] |
| 22. | Aoyagi Y, Oda T, Kinoshita T, Nakahashi C, Hasebe T, Ohkohchi N, Ochiai A. Overexpression of TGF-beta by infiltrated granulocytes correlates with the expression of collagen mRNA in pancreatic cancer. Br J Cancer. 2004;91:1316-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, Upadhyay P, Uyeminami DL, Pommier A, Küttner V, Maiorino L, Bautista C, Carmona EM, Gimotty PA, Fearon DT, Chang K, Lyons SK, Pinkerton KE, Trotman LC, Goldberg MS, Yeh JT, Egeblad M. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 979] [Cited by in RCA: 1023] [Article Influence: 146.1] [Reference Citation Analysis (0)] |
| 24. | Cools-Lartigue J, Spicer J, Najmeh S, Ferri L. Neutrophil extracellular traps in cancer progression. Cell Mol Life Sci. 2014;71:4179-4194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 182] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 25. | Sørensen JB, Klee M, Palshof T, Hansen HH. Performance status assessment in cancer patients. An inter-observer variability study. Br J Cancer. 1993;67:773-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 298] [Cited by in RCA: 339] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 26. | Gasparyan AY, Ayvazyan L, Mukanova U, Yessirkepov M, Kitas GD. The Platelet-to-Lymphocyte Ratio as an Inflammatory Marker in Rheumatic Diseases. Ann Lab Med. 2019;39:345-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 356] [Article Influence: 59.3] [Reference Citation Analysis (0)] |