Published online Sep 6, 2020. doi: 10.12998/wjcc.v8.i17.3708
Peer-review started: March 11, 2020
First decision: April 25, 2020
Revised: June 4, 2020
Accepted: August 1, 2020
Article in press: August 1, 2020
Published online: September 6, 2020
Processing time: 177 Days and 0.8 Hours
Recently, stool multiplex polymerase chain reaction (PCR) tests have been developed for identifying diarrhea-causing bacterial pathogens. Furthermore, fecal calprotectin is a well-known effective marker for intestinal mucosal inflammation.
To evaluate the efficacy of stool multiplex PCR and fecal calprotectin in acute infectious diarrhea.
Overall, 400 patients with acute infectious diarrhea were enrolled from Kangdong Sacred Heart Hospital (January 2016 to December 2018). Multiplex PCR detected 7 enteropathogenic bacteria including Salmonella, Campylobacter, Shigella, Escherichia coli O157:H7, Aeromonas, Vibrio, and Clostridium difficile. We reviewed clinical and laboratory findings using stool multiplex PCR.
Stool multiplex PCR test detected considerably more bacterial pathogens than stool culture (49.2% vs 5.2%), with Campylobacter as the most common pathogen (54%). Patients with positive stool PCR showed elevated fecal calprotectin expression compared to patients with negative stool PCR (1124.5 ± 816.9 mg/kg vs 609 ± 713.2 mg/kg, P = 0.001). C-reactive protein (OR = 1.01, 95%CI: 1.001-1.027, P = 0.034) and sigmoidoscopy-detected colitis (OR = 4.76, 95%CI: 1.101-20.551, P = 0.037) were independent factors in stool PCR-based detection of bacterial pathogens. Sensitivity and specificity of calprotectin were evaluated to be 70.5% and 60.9%, respectively (adjusted cut-off value = 388 mg/kg).
Stool multiplex PCR test has increased sensitivity in detecting pathogens than conventional culture, and it is correlated with calprotectin expression. Stool multiplex PCR and calprotectin may be effective in predicting clinical severity of infectious diarrhea.
Core tip: Stool multiplex polymerase chain reaction (PCR) test showed improved sensitivity than conventional culture method in detecting bacterial pathogens in hospitalized patients with acute infectious diarrhea. Patients with positive stool PCR showed higher C-reactive protein expression level and colitis on sigmoidoscopy. Fecal calprotectin was found to correlate with the positivity of stool PCR, and the adjusted cut-off value of calprotectin for detecting bacterial pathogens by stool PCR was found to be 388 mg/kg.
- Citation: Ahn JS, Seo SI, Kim J, Kim T, Kang JG, Kim HS, Shin WG, Jang MK, Kim HY. Efficacy of stool multiplex polymerase chain reaction assay in adult patients with acute infectious diarrhea. World J Clin Cases 2020; 8(17): 3708-3717
- URL: https://www.wjgnet.com/2307-8960/full/v8/i17/3708.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i17.3708
Acute diarrhea causes gastrointestinal disease, which increases the economic burden of the health care system. Annually, it has been reported to account for more than 2 million deaths[1,2]. According to World Health Organization report 2003, the median incidence rate for diarrhea in children less than 5 years was 3.2 episodes per child-year, and there has been no significant change since 1980s[3]. In the United Kingdom, up to 17 million cases and 1 million general practice consultations have been reported with infectious intestinal disease every year[4].
Diarrhea is caused by enteric pathogens such as bacteria, viruses, parasites, and fungi. Bacterial diarrhea is predominantly caused by diarrheagenic Escherichia coli (E. coli), Campylobacter spp., Salmonella spp., Shigella spp., Vibrio spp., Yersinia enterocolitica, and Clostridium difficile (C. difficile)[5,6]. As major diarrheal illnesses are self-limited or viral, and approximately half of them persist for less than a day, microbiological investigation is usually avoidable for patients who are diagnosed within 24 hours after the onset of diarrhea[1]. However, it is essential to identify the diarrhea-causing pathogen when hospitalized patients have persistent symptoms.
Stool culture and microscopic examination methods are conventional among laboratories. However, they are time consuming and often lack sensitivity and specificity. Recently, there has been substantial development of multiplex molecular assays for the detection and identification of diarrhea-causing pathogens[7-19]. Stool multiplex polymerase chain reaction (PCR) assay has been suggested to be highly specific and sensitive compared to conventional methods[10-12,16]. Thus, its efficacy should be evaluated in clinical practice.
Fecal calprotectin is 36.5 kDa calcium- and zinc- binding protein, which accounts for about 60% of cytosolic neutrophil protein[20,21]. Calprotectin has been reported to be expressed in the mucosal layer of epithelial cells and absent in the normal intestinal mucosa. Thus, upregulation in calprotectin expression has been suggested as an effective marker for predicting inflammatory conditions[22-26]. Fecal calprotectin has emerged as an effective marker to quantify mucosal inflammation in inflammatory bowel disease (IBD)[25]. Furthermore, it has been shown as a reliable marker to identify inflammatory causes of chronic diarrhea[27]. In children, calprotectin expression has been observed to increase during bacterial infection based on disease severity[20]. However, the efficacy of fecal calprotectin in acute infectious diarrhea has not been elucidated in adults.
Several studies have investigated the efficacy of stool multiplex PCR assay and calprotectin test in children with acute infectious diarrhea. However, studies on adult patients are limited. In this study, we evaluated the efficacy of stool multiplex PCR test and fecal calprotectin expression in adult patients with acute infectious diarrhea.
Overall, 400 patients with acute infectious diarrhea, who were admitted at Kangdong Sacred Heart Hospital from January 2016 to December 2018, were enrolled in the study and assessed with stool multiplex PCR test. We reviewed clinical and laboratory findings and compared the clinical characteristics with positivity of stool PCR. Acute diarrhea was defined as having three or more loose or watery stools per day, which persisted < 15 d. The exclusion criteria included the following: Patients who were less than 18 years old, diagnosed with chronic diarrhea (> 30 d), inflammatory bowel disease, active gastrointestinal malignancy, or non-infectious causes. The protocol for this study was approved by the institutional review board of Kangdong Sacred Heart Hospital.
Fecal specimens (1-1.5 g) were lysed using Buffer ASL, vortexed for 10-15 s, and centrifuged at 1300 rpm. DNA/RNA were extracted from supernatant using Seegene STARlet IVD and multiplex PCR was performed using Seeplex® Diarrhea-B1 ACE Detection and AllplexTM GI-Bacteria(I) Assay kit (Seegene, Seoul, South Korea)[13]. Multiplex PCR detected bacterial pathogens belonging to Salmonella spp., Campylobacter spp, Shigella spp., E. coli O157:H7, verotoxigenic E. coli (VTEC), Aeromonas spp., Vibrio spp., Yersinia enterocolitica, Clostridium perfringens, and C. difficile toxin B. Sensitivity and specificity of Seeplex detection system were found to be comparable to conventional diagnostic testing algorithm for majority of enteric pathogens[13].
Fecal calprotectin expression was evaluated in 107 patients and assessed for its correlation with positivity of stool PCR. Fecal specimens were collected in black plastic containers and sent to the laboratory in a refrigerated box. Fecal aliquots of approximately 100-500 mg were homogenized in an extraction buffer. Stool sample was then mixed using vortex and incubated for 10 min. The homogenate was transferred to an Eppendorf tube and centrifuged for 5 min at 3000 g. The supernatant was then assessed for calprotectin using EliA Calprotectin Wells (Thermo Fisher Scientific, Sweden), which were coated with monoclonal antibody to calprotectin. Further, calprotectin of patient’s specimen was allowed to bind to pre-coated antibody. Excess components were washed and the calprotectin-conjugate complex was incubated with developing solution. The reaction was stopped and fluorescence in the reaction mixture was measured by Phila250 (Thermo Fisher Scientific)[28]. The cut-off value for positivity was adjusted at 50 mg/kg.
Baseline characteristics were compared using Mann-Whitney U test or χ2 test. Multivariate logistic regression analysis was performed to identify the independent factors that were associated with positivity of stool PCR. Receiver operating characteristic curve (ROC) analysis was performed to determine the cut-off value of calprotectin in detecting bacterial pathogens by stool PCR. P < 0.05 was considered as statistically significant. All statistical analyses were performed using SPSS for Windows (Version 24.0; IBM Corp., Armonk, NY, United States).
A total of 400 patients who were admitted with acute infectious diarrhea and assessed with multiplex stool PCR test were enrolled between January 2016 and December 2018. Mean age was observed to be 50.4 ± 20.4 years and hospitalization time was reported to be 6.1 ± 4.7 d. Initial C-reactive protein (CRP) level was found to be 78.3 ± 82.5 mg/L. Mean body temperature was noted as 37.3 ± 0.9 °C and duration of diarrhea was observed to be 4 ± 4.6 days. Fecal calprotectin expression was examined in 107 (26.7%) patients, and mean fecal calprotectin was evaluated to be 902.9 ± 812 mg/kg (Table 1). A total of 138 patients were reported to undergo sigmoidoscopy and 56.5% of them were diagnosed with colitis.
| Characteristics | Value |
| Age, yr | 50.4 ± 20.4 |
| Male, n (%) | 168 (42) |
| DM/HTN/CKD/CVD [n (%)] | 57 (14.3)/99 (24.8)/8 (2)/18 (4.5) |
| WBC count (× 103/μL) | 9526.9 ± 4803 |
| CRP (mg/L) | 78.3 ± 82.5 |
| Fecal calprotectin (mg/kg) | 902.9 ± 812 |
| Initial body temperature (°C) | 37.3 ± 0.9 |
| Duration of fever (> 38°C), d | 0.58 ± 0.9 |
| Duration of hospitalization, d | 6.1 ± 4.7 |
| Duration of diarrhea, d | 4 ± 4.6 |
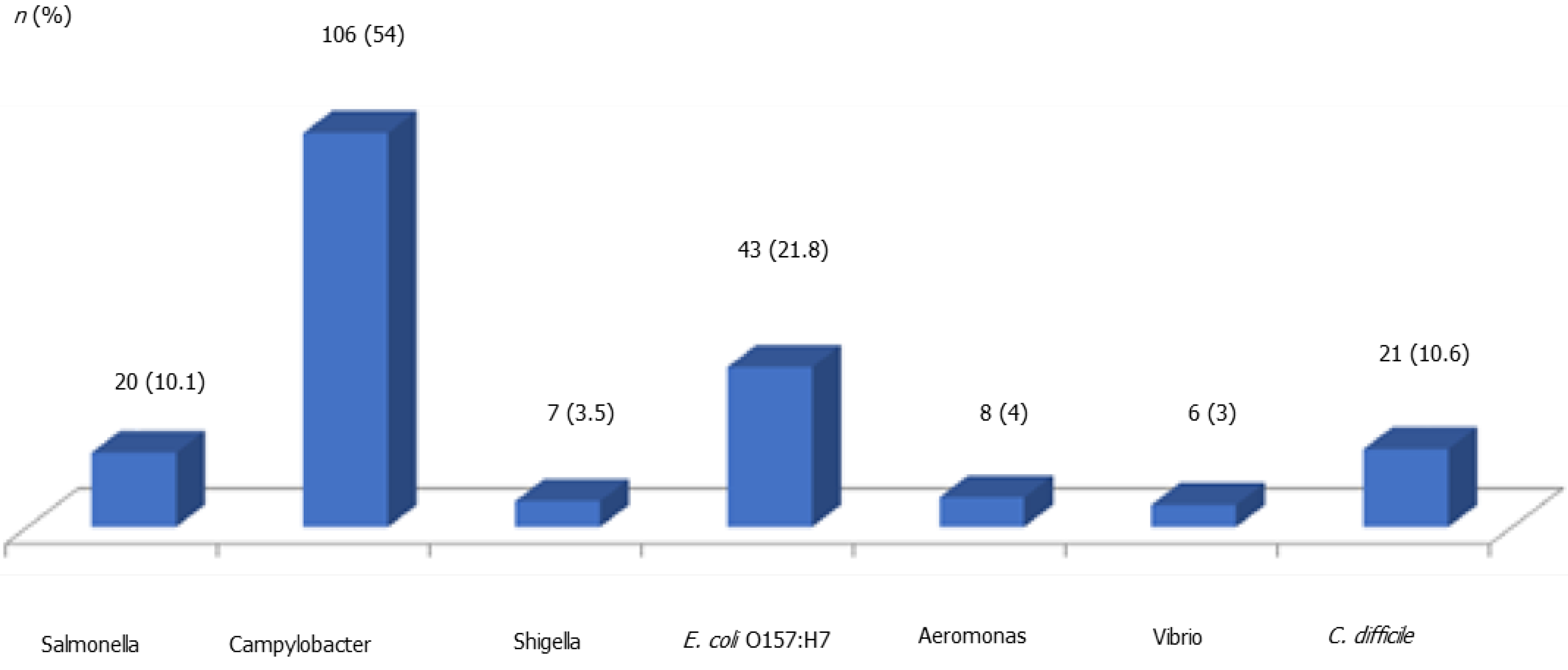
Stool multiplex PCR test detected substantially more bacterial pathogens compared to those detected by stool culture (49.2% vs 5.2%). Stool multiplex PCR assay detected 106 Campylobacter spp., 43 E. coli, 21 Clostridium spp., 20 Salmonella spp., 8 Aeromonas spp., 7 Shigella spp., and 6 Vibrio spp. (Figure 1). Conversely, stool culture detected 8 Salmonella spp., 6 Clostridium spp., 4 Campylobacter spp., and 3 Vibrio spp. (Figure 2). Campylobacter was detected as the most common pathogen in stool PCR (106/197, 54%) while Salmonella was the most common pathogen in stool culture (8/21, 38.1%). From 21 stool culture-identified pathogens, 76.2% (16/21) were also detected by stool multiplex PCR test.
Stool PCR-positive patients were found to have elevated CRP expression (109.8 ± 83.5 mg/L vs 47.7 ± 68.9 mg/L, P = 0.0001), high fever (> 38 °C) [101/197 (51.3%) vs 47/203 (23.2%), P = 0.0001] and upregulated fecal calprotectin expression (1124.5 ± 816.9 mg/kg vs 609 ± 713.2 mg/kg, P = 0.001) compared to that expressed by PCR-negative patients. Additionally, sigmoidoscopy revealed that colitis was more prevalent in PCR-positive group than PCR-negative group [49/65 (75.4%) vs 29/72 (39.7%), P = 0.0001] (Table 2). Multiplex PCR detected 55 enteropathogens in patients with sigmoidoscopy-diagnosed colitis, and 6 of them were found to be double PCR positive. Campylobacter was reported to be the most common pathogen (28/55, 35.9%) detected in patients with diarrhea and colitis.
| Positive stool PCR | Negative stool PCR | P value | |
| Age (yr) | 50.4 ± 20.5 | 50.5 ± 20.3 | 0.97 |
| Male [n (%)] | 76 (38.6) | 92 (45.3) | 0.145 |
| WBC count (103/μL) | 9968.8 ± 5165.1 | 9098.1 ± 4394 | 0.145 |
| CRP (mg/L) | 109.8 ± 83.5 | 47.7 ± 68.9 | 0.0001 |
| Fever > 38°C [n (%)] | 101 (51.3) | 47 (23.2) | 0.0001 |
| Hospital days | 5.9 ± 4.1 | 6.2 ± 5.1 | 0.733 |
| Fever duration (d) | 0.7 ± 0.9 | 0.4 ± 0.9 | 0.0001 |
| Diarrhea duration (d) | 3.8 ± 4.3 | 4.3 ± 4.8 | 0.772 |
| Calprotectin (mg/kg) | 1124.5 ± 816.9 | 609 ± 713.2 | 0.001 |
| Colitis on sigmoidoscopy [n (%)] | 49/65 (75.4) | 29/72 (39.7) | 0.0001 |
To determine the factors predicting positivity of stool PCR, we performed a multivariate logistic regression analysis. As shown in Table 3, fecal calprotectin and body temperature were not found to be associated with stool PCR positivity. The independent factors that predicted detection of bacterial pathogens by stool PCR were observed to be CRP (OR = 1.01, 95%CI: 1.001-1.027, P = 0.034) and sigmoidoscopy-diagnosed colitis (OR = 4.76, 95%CI: 1.101-20.551, P = 0.037).
| P value | OR | 95%CI | |
| CRP | 0.03 | 1.01 | 1.001-1.027 |
| Calprotectin | 0.68 | 1 | 0.999-1.001 |
| Colitis on sigmoidoscopy | 0.03 | 4.75 | 1.101-20.551 |
| Initial body temperature | 0.81 | 1.1 | 0.484-2.498 |
Using ROC analysis, we evaluated the diagnostic efficacy of calprotectin expression in detecting bacterial pathogens by stool PCR. ROC curve revealed an area under curve of 0.699 (95%CI: 0.599-0.798) (Figure 3). Furthermore, sensitivity and specificity of calprotectin were evaluated to be 70.5% and 60.9%, respectively, with an adjusted cut-off value at 388 mg/kg.
Acute diarrheal disease is usually self-limited; however, it can be fatal at times. Thus, it is necessary to predict severity of diarrhea during the early phase of diagnosis. Typically, approximately 2 to 3 d are required to acquire the results of traditional stool culture. Moreover, many bacterial species are not clearly identified by the stool culture method. Therefore, new molecular assays including stool multiplex PCR and calprotectin test may be faster method to assess stool for bacterial infection.
To date, several studies have reported the efficacy of multiplex real-time PCR assays in clinical laboratory settings. However, commercial availability of molecular assays for the identification of enteric pathogens remains limited, and majority of studies only consider stool PCR data. Our study analyzed the clinical characteristics including calprotectin expression level and endoscopic findings based on stool PCR data. Our results showed that patients with stool PCR-detected bacterial pathogens had additional acute clinical characteristics and severe inflammation, which could be detected by sigmoidoscopy.
Previous studies have reported that the detection rate is 20.8% among Korean population and Campylobacter is the most common pathogen to be identified[18]. Our results detected Campylobacter as the most common pathogen in stool PCR (106/197, 54%), with a higher detection rate (49.2%). Thus, our study confirmed that Campylobacter is the most common pathogen detected by stool PCR in hospitalized patients with acute infectious diarrhea, which was not identified by stool culture method. Fatal Campylobacter infections are rare; however, they are more common in severely immunocompromised hosts[6]. Most guidelines recommend that the empiric antimicrobial therapy in adults should consist of either a fluoroquinolone such as ciprofloxacin or azithromycin, depending on the local susceptibility patterns and travel history[2,6]. Several randomized controlled trials have demonstrated a minor but significant benefit from antimicrobial therapy by reducing the duration of symptoms due to Campylobacter gastroenteritis infection[29]. In Asian countries, quinolone resistance can be problematic while treating Campylobacter infections[6]. Therefore, early detection of Campylobacter spp. by stool PCR could be a valuable method for diagnosing acute infectious diarrhea. Further, potential use of stool PCR for detecting known point mutations resulting in bacterial resistance to antibiotics would be a good complementary method for treatment of severe bacterial infection in the future.
In the present study, the independent factors that predicted detection of bacterial pathogens by stool PCR were found to be high CRP and sigmoidoscopy-detected colitis. This implies that bacterial infection causes severe inflammation and can be evaluated from the laboratory finding of CRP expression or endoscopic mucosal inflammation. Thus, physicians can appropriately predict the presence of bacterial pathogens from high CRP expression or endoscopic findings.
Bacterial pathogens can cause invasive diarrhea. Such pathogens have been reported to possess the capability to invade the mucosa of the distal small intestine and colon, as well as stimulate local and systemic inflammatory responses[23]. Fecal calprotectin is a well-known significant marker of intestinal mucosal inflammation, and thus, many researchers have investigated its efficacy. Duman et al[23] evaluated the efficacy of fecal calprotectin in children with acute gastroenteritis. Their study suggested that fecal calprotectin can distinguish bacterial gastroenteritis from viral gastroenteritis and the expression levels are significantly higher in patients with microscopy-positive groups, specific for bacterial gastroenteritis[23]. Our study did not include stool PCR result of viral pathogen, as the sample size of viral pathogen was relatively small.
A prospective multicenter study evaluated fecal calprotectin expression in 2383 adults across Europe with acute bacterial diarrhea[30]. The study revealed that calprotectin had significant sensitivity and specificity to differentiate between noninfectious and bacterial diarrhea[30]. Moreover, sensitivity and specificity of calprotectin were reported to be 83% and 87% with a cut-off value of 14.9 mg/L[30]. Our study showed that the sensitivity and specificity of calprotectin were 70.5% and 60.9%, respectively, with adjusted cut-off value of 388 mg/kg. We assume that the difference might be due to different geographic location (country) and technique of calprotectin assay. In addition, our study included only hospitalized patients, which might have caused the disparity. Our result showed that fecal calprotectin could be elevated in adult infectious diarrhea. It is noteworthy especially in patients with IBD, since monitoring of fecal calprotectin is now routinely performed in IBD. If there is an infectious cause for the diarrhea in IBD patients, the high calprotectin may lead to inappropriate steroid use, it could be harmful.
Our study had some limitations. First, the study design was retrospective and single-centered. The detection of enteric pathogens and magnitude of calprotectin expression could be influenced by the region. Therefore, multicenter prospective study is recommended to validate the efficacy of stool multiplex PCR and fecal calprotectin in adult patients. Second, the sample size for measuring calprotectin was relatively small, which can influence the results. Third, we did not conduct a cost-benefit analysis of stool PCR. Despite these limitations, the current study proves the efficacy of stool multiplex PCR in correlation with fecal calprotectin expression in relatively large-scaled adult patients.
In summary, stool multiplex PCR test showed improved sensitivity than conventional culture method in detecting bacterial pathogens in hospitalized patients with acute infectious diarrhea. Patients with positive stool PCR showed higher CRP expression level and colitis on sigmoidoscopy. Fecal calprotectin was found to correlate with the positivity of stool PCR, and the adjusted cut-off value of calprotectin for detecting bacterial pathogens by stool PCR was found to be 388 mg/kg. Overall, we suggest that stool multiplex PCR and calprotectin test may improve the diagnosis of acute infectious diarrhea with increased sensitivity for a majority of bacterial pathogens.
Stool multiplex polymerase chain reaction (PCR) assay has been suggested to be highly specific and sensitive compared to conventional methods. Thus, its efficacy should be evaluated in clinical practice. Further, the efficacy of fecal calprotectin in acute infectious diarrhea has not been elucidated in adults.
Several studies have investigated the efficacy of stool multiplex PCR assay and calprotectin test in children with acute infectious diarrhea. However, studies on adult patients are limited.
We aimed to evaluate the efficacy of stool multiplex PCR test and fecal calprotectin expression in adult patients with acute infectious diarrhea.
We reviewed clinical and laboratory findings and compared the clinical characteristics according to the positivity of stool PCR. We performed a multivariate logistic regression analysis to determine the factors predicting positivity of stool PCR. Using ROC analysis, we evaluated the diagnostic efficacy of calprotectin expression in detecting bacterial pathogens by stool PCR.
Stool multiplex PCR test detected considerably more bacterial pathogens than stool culture (49.2% vs 5.2%), with Campylobacter as the most common pathogen (54%). C-reactive protein (CRP) (OR = 1.01, 95%CI: 1.001-1.027, P = 0.034) and sigmoidoscopy-detected colitis (OR = 4.76, 95%CI: 1.101-20.551, P = 0.037) were independent factors in stool PCR-based detection of bacterial pathogens. Sensitivity and specificity of calprotectin were evaluated to be 70.5% and 60.9%, respectively (adjusted cut-off value = 388 mg/kg).
Stool multiplex PCR test showed improved sensitivity than conventional culture method in detecting bacterial pathogens in hospitalized patients with acute infectious diarrhea. Patients with positive stool PCR showed higher CRP expression level and colitis on sigmoidoscopy. Fecal calprotectin was found to correlate with the positivity of stool PCR, and the adjusted cut-off value of calprotectin for detecting bacterial pathogens by stool PCR was found to be 388 mg/kg.
We suggest that stool multiplex PCR and calprotectin test may improve the diagnosis of acute infectious diarrhea in adults. Future prospective studies are warranted.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Malnick S, Islek A S-Editor: Ma YJ L-Editor: A P-Editor: Wang LL
| 1. | Thielman NM, Guerrant RL. Clinical practice. Acute infectious diarrhea. N Engl J Med. 2004;350:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 206] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | DuPont HL. Clinical practice. Bacterial diarrhea. N Engl J Med. 2009;361:1560-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003;81:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 4. | Tam CC, Rodrigues LC, Viviani L, Dodds JP, Evans MR, Hunter PR, Gray JJ, Letley LH, Rait G, Tompkins DS, O'Brien SJ; IID2 Study Executive Committee. Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut. 2012;61:69-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 453] [Cited by in RCA: 395] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 5. | DuPont HL. Approach to the patient with infectious colitis. Curr Opin Gastroenterol. 2012;28:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Shane AL, Mody RK, Crump JA, Tarr PI, Steiner TS, Kotloff K, Langley JM, Wanke C, Warren CA, Cheng AC, Cantey J, Pickering LK. 2017 Infectious Diseases Society of America Clinical Practice Guidelines for the Diagnosis and Management of Infectious Diarrhea. Clin Infect Dis. 2017;65:e45-e80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 318] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 7. | Eckbo EJ, Yansouni CP, Pernica JM, Goldfarb DM. New Tools to Test Stool: Managing Travelers' Diarrhea in the Era of Molecular Diagnostics. Infect Dis Clin North Am. 2019;33:197-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Lee S, Park YJ, Lee HK, Kim SY, Kim JY, Lee SY, Yoo JK. Detection of 13 Enteric Bacteria and 5 Viruses Causing Acute Infectious Diarrhea Using Multiplex PCR from Direct Stool Specimens. Ann Clin Microbiol. 2013;16:33. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Sjöling Å, Sadeghipoorjahromi L, Novak D, Tobias J. Detection of major diarrheagenic bacterial pathogens by multiplex PCR panels. Microbiol Res. 2015;172:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Buchan BW, Olson WJ, Pezewski M, Marcon MJ, Novicki T, Uphoff TS, Chandramohan L, Revell P, Ledeboer NA. Clinical evaluation of a real-time PCR assay for identification of Salmonella, Shigella, Campylobacter (Campylobacter jejuni and C. coli), and shiga toxin-producing Escherichia coli isolates in stool specimens. J Clin Microbiol. 2013;51:4001-4007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Harrington SM, Buchan BW, Doern C, Fader R, Ferraro MJ, Pillai DR, Rychert J, Doyle L, Lainesse A, Karchmer T, Mortensen JE. Multicenter evaluation of the BD max enteric bacterial panel PCR assay for rapid detection of Salmonella spp., Shigella spp., Campylobacter spp. (C. jejuni and C. coli), and Shiga toxin 1 and 2 genes. J Clin Microbiol. 2015;53:1639-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Wohlwend N, Tiermann S, Risch L, Risch M, Bodmer T. Evaluation of a Multiplex Real-Time PCR Assay for Detecting Major Bacterial Enteric Pathogens in Fecal Specimens: Intestinal Inflammation and Bacterial Load Are Correlated in Campylobacter Infections. J Clin Microbiol. 2016;54:2262-2266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Coupland LJ, McElarney I, Meader E, Cowley K, Alcock L, Naunton J, Gray J. Simultaneous detection of viral and bacterial enteric pathogens using the Seeplex® Diarrhea ACE detection system. Epidemiol Infect. 2013;141:2111-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Simner PJ, Oethinger M, Stellrecht KA, Pillai DR, Yogev R, Leblond H, Mortensen J. Multisite Evaluation of the BD Max Extended Enteric Bacterial Panel for Detection of Yersinia enterocolitica, Enterotoxigenic Escherichia coli, Vibrio, and Plesiomonas shigelloides from Stool Specimens. J Clin Microbiol. 2017;55:3258-3266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Zboromyrska Y, Hurtado JC, Salvador P, Alvarez-Martínez MJ, Valls ME, Mas J, Marcos MA, Gascón J, Vila J. Aetiology of traveller's diarrhoea: evaluation of a multiplex PCR tool to detect different enteropathogens. Clin Microbiol Infect. 2014;20:O753-O759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Antikainen J, Kantele A, Pakkanen SH, Lääveri T, Riutta J, Vaara M, Kirveskari J. A quantitative polymerase chain reaction assay for rapid detection of 9 pathogens directly from stools of travelers with diarrhea. Clin Gastroenterol Hepatol. 2013;11:1300-1307.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Wiemer D, Loderstaedt U, von Wulffen H, Priesnitz S, Fischer M, Tannich E, Hagen RM. Real-time multiplex PCR for simultaneous detection of Campylobacter jejuni, Salmonella, Shigella and Yersinia species in fecal samples. Int J Med Microbiol. 2011;301:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Cho MC, Noh SA, Kim MN, Kim KM. Direct Application of Multiplex PCR on Stool Specimens for Detection of Enteropathogenic Bacteria. Korean J Clin Microbiol. 2010;13:162. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Schuetz AN. Emerging agents of gastroenteritis: Aeromonas, Plesiomonas, and the diarrheagenic pathotypes of Escherichia coli. Semin Diagn Pathol. 2019;36:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Chen CC, Huang JL, Chang CJ, Kong MS. Fecal calprotectin as a correlative marker in clinical severity of infectious diarrhea and usefulness in evaluating bacterial or viral pathogens in children. J Pediatr Gastroenterol Nutr. 2012;55:541-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Licata A, Randazzo C, Cappello M, Calvaruso V, Butera G, Florena AM, Peralta S, Cammà C, Craxì A. Fecal calprotectin in clinical practice: a noninvasive screening tool for patients with chronic diarrhea. J Clin Gastroenterol. 2012;46:504-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Steinbakk M, Naess-Andresen CF, Lingaas E, Dale I, Brandtzaeg P, Fagerhol MK. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet. 1990;336:763-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 384] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 23. | Duman M, Gencpinar P, Biçmen M, Arslan N, Özden Ö, Üzüm Ö, Çelik D, Sayıner AA, Gülay Z. Fecal calprotectin: can be used to distinguish between bacterial and viral gastroenteritis in children? Am J Emerg Med. 2015;33:1436-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Limburg PJ, Ahlquist DA, Sandborn WJ, Mahoney DW, Devens ME, Harrington JJ, Zinsmeister AR. Fecal calprotectin levels predict colorectal inflammation among patients with chronic diarrhea referred for colonoscopy. Am J Gastroenterol. 2000;95:2831-2837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 126] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Sutherland AD, Gearry RB, Frizelle FA. Review of fecal biomarkers in inflammatory bowel disease. Dis Colon Rectum. 2008;51:1283-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Tibble J, Teahon K, Thjodleifsson B, Roseth A, Sigthorsson G, Bridger S, Foster R, Sherwood R, Fagerhol M, Bjarnason I. A simple method for assessing intestinal inflammation in Crohn's disease. Gut. 2000;47:506-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 351] [Article Influence: 14.0] [Reference Citation Analysis (1)] |
| 27. | Schröder O, Naumann M, Shastri Y, Povse N, Stein J. Prospective evaluation of faecal neutrophil-derived proteins in identifying intestinal inflammation: combination of parameters does not improve diagnostic accuracy of calprotectin. Aliment Pharmacol Ther. 2007;26:1035-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Mindemark M, Larsson A. Ruling out IBD: estimation of the possible economic effects of pre-endoscopic screening with F-calprotectin. Clin Biochem. 2012;45:552-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Ternhag A, Asikainen T, Giesecke J, Ekdahl K. A meta-analysis on the effects of antibiotic treatment on duration of symptoms caused by infection with Campylobacter species. Clin Infect Dis. 2007;44:696-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Shastri YM, Bergis D, Povse N, Schäfer V, Shastri S, Weindel M, Ackermann H, Stein J. Prospective multicenter study evaluating fecal calprotectin in adult acute bacterial diarrhea. Am J Med. 2008;121:1099-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |