Published online Aug 26, 2020. doi: 10.12998/wjcc.v8.i16.3474
Peer-review started: March 20, 2020
First decision: April 29, 2020
Revised: July 5, 2020
Accepted: July 16, 2020
Article in press: July 16, 2020
Published online: August 26, 2020
Processing time: 158 Days and 0.5 Hours
Recent evidence showed that combining endoscopic submucosal dissection (ESD) and laparoscopic sentinel lymph node dissection may avoid unnecessary gastrectomy in treating early mucinous gastric cancer (EMGC) patients with risks of positive lymph node metastasis (pLNM).
To explore the predictive factors for pLNM in EMGC, and to optimize the clinical application of combing ESD and sentinel lymph node dissection in a proper subgroup of patients with EMGC.
Thirty-one patients with EMGC who had undergone gastrectomy with lymph node dissection were consecutively enrolled from January 1988 to December 2016. Univariate and multivariate logistic regression analyses were used to estimate the association between the rates of pLNM and clinicopathological factors, providing odds ratio (OR) with 95% confidence interval. And the association between the number of predictors and the pLNM rate was also investigated.
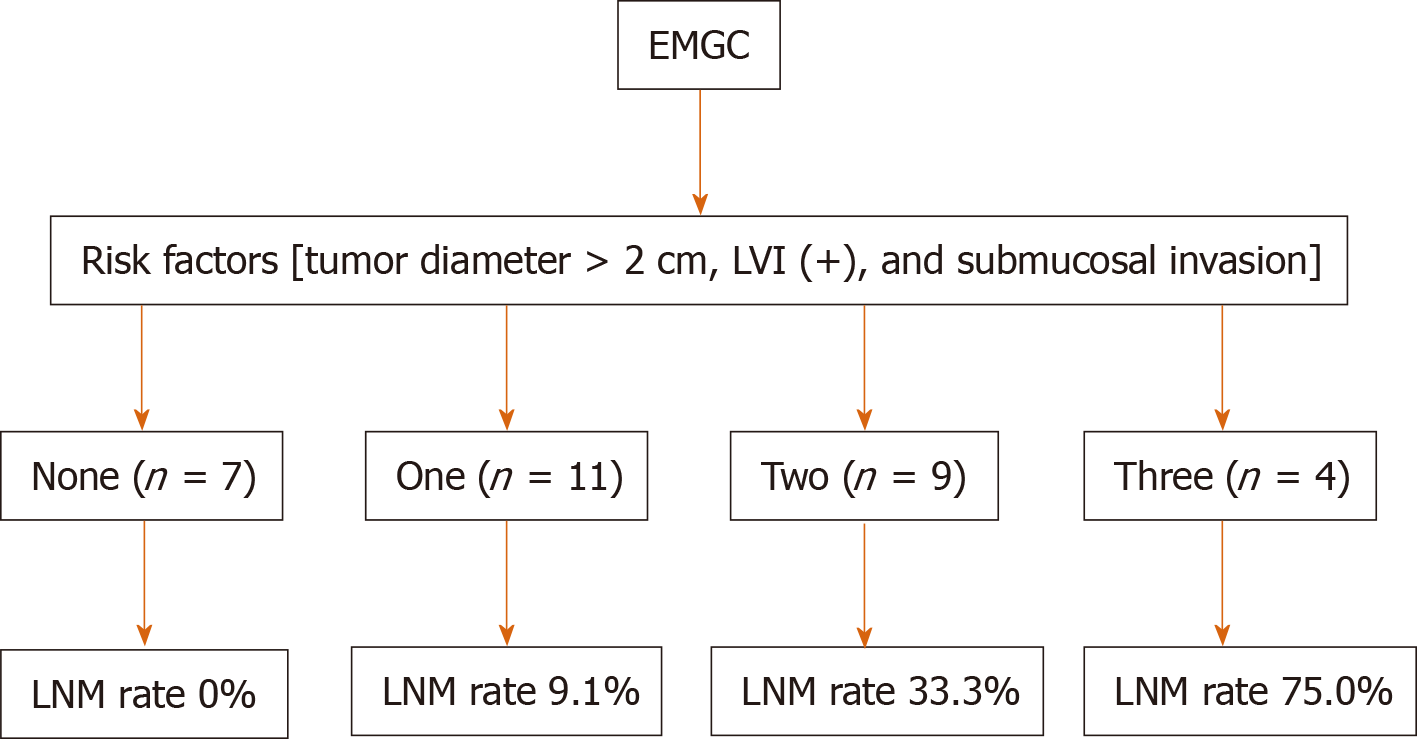
Depth of invasion (OR = 7.342, 1.127-33.256, P = 0.039), tumor diameter (OR = 9.158, 1.348-29.133, P = 0.044), and lymphatic vessel involvement (OR = 27.749, 1.821-33.143, P = 0.019) turned out to be significant and might be the independent risk factors for predicating pLNM in the multivariate analysis. For patients with 1, 2, and 3 risk factors, the pLNM rates were 9.1%, 33.3%, and 75.0%, respectively. pLNM was not detected in seven patients without any of these risk factors.
ESD might serve as a safe and sufficient treatment for intramucosal EMGC if tumor size ≤ 2 cm, and when lymphatic vessel involvement is absent by postoperative histological examination. Combining ESD and sentinel lymph node dissection could be recommended as a safe and effective treatment for EMGC patients with a potential risk of pLNM.
Core tip: A combination of laparoscopic sentinel lymph node dissection (SLND) and endoscopic submucosal dissection (ESD) may avoid unnecessary surgical intervention in early mucinous gastric cancer (EMGC) patients having a potential risk of positive lymph node metastasis (pLNM). ESD allows the complete local resection of the primary tumor, and SLND enables the confirmation of lymph node status. We performed this current retrospective study to identify risk factors that are predictive factor for pLNM in EMGC and to establish a primary criterion and working flowchart to expand the possibility of using ESD and SLND for the treatment of EMGC. The combination of ESD and SLND could be recommended as an effective, minimally invasive treatment for EMGC patients with a high risk of pLNM.
- Citation: Li H, Zhao LL, Zhang XC, Liu DX, Wang GY, Huo ZB, Chen SB. Combination of endoscopic submucosal dissection and laparoscopic sentinel lymph node dissection in early mucinous gastric cancer: Role of lymph node metastasis. World J Clin Cases 2020; 8(16): 3474-3482
- URL: https://www.wjgnet.com/2307-8960/full/v8/i16/3474.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i16.3474
Endoscopic submucosal dissection (ESD) has been increasingly performed in the recent decade all over the world given the clinical evidence and well-accepted benefits[1-5]. ESD is considered a standard treatment for specific early gastric cancer (EGC) with a relatively low lymph node metastasis (LNM) risk, e.g., intramucosal differentiated adenocarcinoma and ≤ 2 cm in diameter with no ulcers[6]. Meanwhile, in undifferentiated EGC with a high risk of pLNM, gastrectomy together with lymphadenectomy is usually performed as the standard surgical procedure[7]. Undifferentiated gastric carcinoma consists of mucinous adenocarcinoma, poorly differentiated adenocarcinoma, and primary signet ring cell carcinoma[6]. However, for approximately 96% of surgical patients with early mucinous gastric cancer (EMGC) confined to the mucosa, no pLNM was observed, suggesting that it might be over-treated for these cases[8]. Therefore, we have proposed new methods to minimize the chance of surgical resection for EMGC. The new technique allows minimally invasive resection of gastric lesions through ESD and laparoscopic sentinel lymph node dissection (SLND)[9-11].
Therefore, we performed this retrospective study to identify risk factors that accounted for pLNM in EMGC and to propose a primary criterion and working flowchart to optimize the indication of applying ESD and SLND in the treatment of EMGC.
The retrospective study enrolled early gastric cancer patients who had undergone gastrectomy with lymphadenectomy in the Department of Oncology, Xingtai People’s Hospital (Xingtai, China) from January 1988 to December 2016.
The inclusion criteria were: (1) Lymphadenectomy beyond D1 dissection; (2) Number of harvested lymph nodes > 16; (3) Pathologically diagnosed with EMGC according to the Japanese Classification of Gastric Carcinoma (JCGC 4th edition)[6,9]; and (4) Enough data for further analyses from the medical records.
After applying the inclusion criteria, a total of 31 patients with pathologically confirmed EMGC were eligible for the final analyses. There were more men than women (21 vs 10) in this study and the mean age was 48 years old (range from 27 to 81).
Lymph nodes were dissected from the en bloc specimens after ESD approach and evaluated by an experienced surgeon according to the classification released by the Japanese Classification of Gastric Carcinoma (JCGC 4th edition)[6,9]. Afterwards, the dissected lymph nodes were sectioned and examined using the hematoxylin and eosin and immunohistochemical staining to collect the histopathologic characteristics. The presence of lymph node metastasis and lymphovascular invasion (LVI) was examined and determined by D2-40 antibody. A series of consistent measuring methods were applied to guarantee the consistency of the treatment and examination results of samples in the past 28 years. Pathological slides were double-checked by another pathologist who was blinded to previous results.
We recorded and collected clinicopathological data according to the JCGC for further analyses[6]. These features included sex (female/male), gastric cancer family history, age, depth of cancer invasion (mucosa/submucosa), macroscopic type, tumor size, lymphatic vessel involvement, presence of ulceration, anatomic location of tumor (lower 1/3, middle 1/3, or upper 1/3 of the stomach), number of tumor sites (single or multitude). Moreover, associations between pLNM and various clinicopathological features were explored.
All statistical analyses in this present study were performed using SPSS software, version 25.0 (IBM Corp, NY, United States). The chi-square test was used to estimate the association between the status of pLNM and clinicopathological characteristics. Furthermore, we used multivariate stepwise logistic regression analysis to determine the independent risk factors for pLNM, providing odds ratios and 95% confidence intervals. A P value < 0.05 was considered statistically significant.
The results of χ2 test for association between pLNM and clinicopathological features are displayed in Table 1. There were trends that submucosal invasion, tumor diameter > 2 cm, and presence of LVI were associated with high risks of pLNM (P < 0.05). Meanwhile, no significant association was observed between pLNM and family history, macroscopic type, presence of ulceration, anatomic location of tumor, number of tumor sites, age, or sex.
| Factor | Lymph node metastasis | |
| Positive number | P value | |
| Age (yr) | 0.839 | |
| < 60 (n = 19) | 4 | |
| ≥ 60 (n = 12) | 3 | 0.850 |
| Sex | ||
| Male (n = 21) | ||
| Female (n = 10) | ||
| Macroscopic type | ||
| I (n = 2) | 0 | |
| II (n = 22) | 6 | |
| III (n = 7) | 1 | |
| Family medical history | 0.904 | |
| Positive (n = 5) | 1 | |
| Negative (n = 26) | 6 | |
| Location | 0.317 | |
| Upper (n = 3) | 1 | |
| Middle (n = 8) | 0 | |
| Lower (n = 20) | 6 | |
| Number of tumors | 0.490 | |
| Single (n = 29) | 7 | |
| Multitude (n = 2) | 0 | |
| Depth of invasion | 0.036 | |
| Mucosa (n = 22) | 2 | |
| Submucosa (n = 9) | 5 | |
| Tumor size in diameter | 0.040 | |
| ≤ 2 cm (n = 25) | 3 | |
| > 2 cm (n = 6) | 4 | |
| Lymphatic vessel involvement | 0.003 | |
| Negative (n = 23) | 1 | |
| Positive (n = 8) | 6 | |
| Ulceration | 0.589 | |
| Negative (n = 25) | 5 | |
| Positive (n = 6) | 2 | |
The univariate analysis from logistic regression showed that three clinicopathological parameters including submucosal invasion, large tumor size, and presence of LVI were significantly associated with pLNM. Moreover, the multivariate analysis confirmed that these three parameters were independent risk factors for pLNM (P < 0.05, Table 2).
| Factor | Hazard ratio | 95%CI | P value |
| Depth of invasion | 7.342 | 1.127-33.256 | 0.039 |
| Mucosa | |||
| Submucosa | |||
| Tumor size | 9.158 | 1.348-29.133 | 0.044 |
| ≤ 2 cm | |||
| > 2 cm | |||
| Lymphatic vessel involvement | 27.749 | 1.821-33.14 | 0.019 |
| Negative | 0 | ||
| Positive |
Seven (22.6%) of 31 patients diagnosed with EMGC had pLNM. The relationship between pLNM and the three independent risk factors (submucosal invasion, lager tumor size, and LVI) was also investigated in EMGC. In EMGC, for patients with 1, 2, and 3 risk factors, pLNM rates were 9.1% (1/11), 33.3% (3/9), and 75.0% (3/4), respectively. For the other seven patients with no risk factors, no pLNM was found (Figure 1).
Conventional gastrectomy with lymphadenectomy has been considered a standard treatment for EGC. However, in recent two decades, mounting studies have demonstrated non-inferior long-term outcomes of ESD compared with surgical gastrectomy[12,13]. The theoretical advantages of ESD over traditional subtotal gastrectomy are less invasiveness, less cost, and better preservation of physiological function[14,15]. Recently, ESD is widely used as curative treatment for differentiated-type EGC that has less risk of LNM[7,16]. For patients with EMGC, major surgery such as gastrectomy with SLND is usually performed even though ESD could also remove the primary gastric lesion en bloc. However, this kind of surgery may eventually turn out to be unnecessary for many EMGC patients, because about 96% of EMGC patients who had undergone gastrectomy were actually at a low risk of LNM. If the primary gastric tumor could be removed en bloc and lymph node metastasis status could be pathologically determined prior to surgery, unnecessary surgical intervention might be avoided. Combining SLND and ESD may avoid unnecessary surgical intervention in EMGC patients with a low risk of pLNM.
The prediction model or proper working flowchart to predict the status of pLNM could optimize treatment approach for EGC. In order to achieve this aim, we try to develop a possible working flowchart of expanding indication of ESD in EMGC. And we retrospectively analyzed the EMGC cases to determine whether the presence of pLNM could be predicted by clinicopathologic features. Our results suggested that several clinicopathologic features accounted for the presence of pLNM: Submucosal invasion, tumor diameter > 2 cm, and positive LVI. These results focusing on EMGC are in line with some previous researchers’ reports, indicating a significant association of large tumor, positive LVI, and submucosal invasion with high pLNM risk[12,13,17-19].
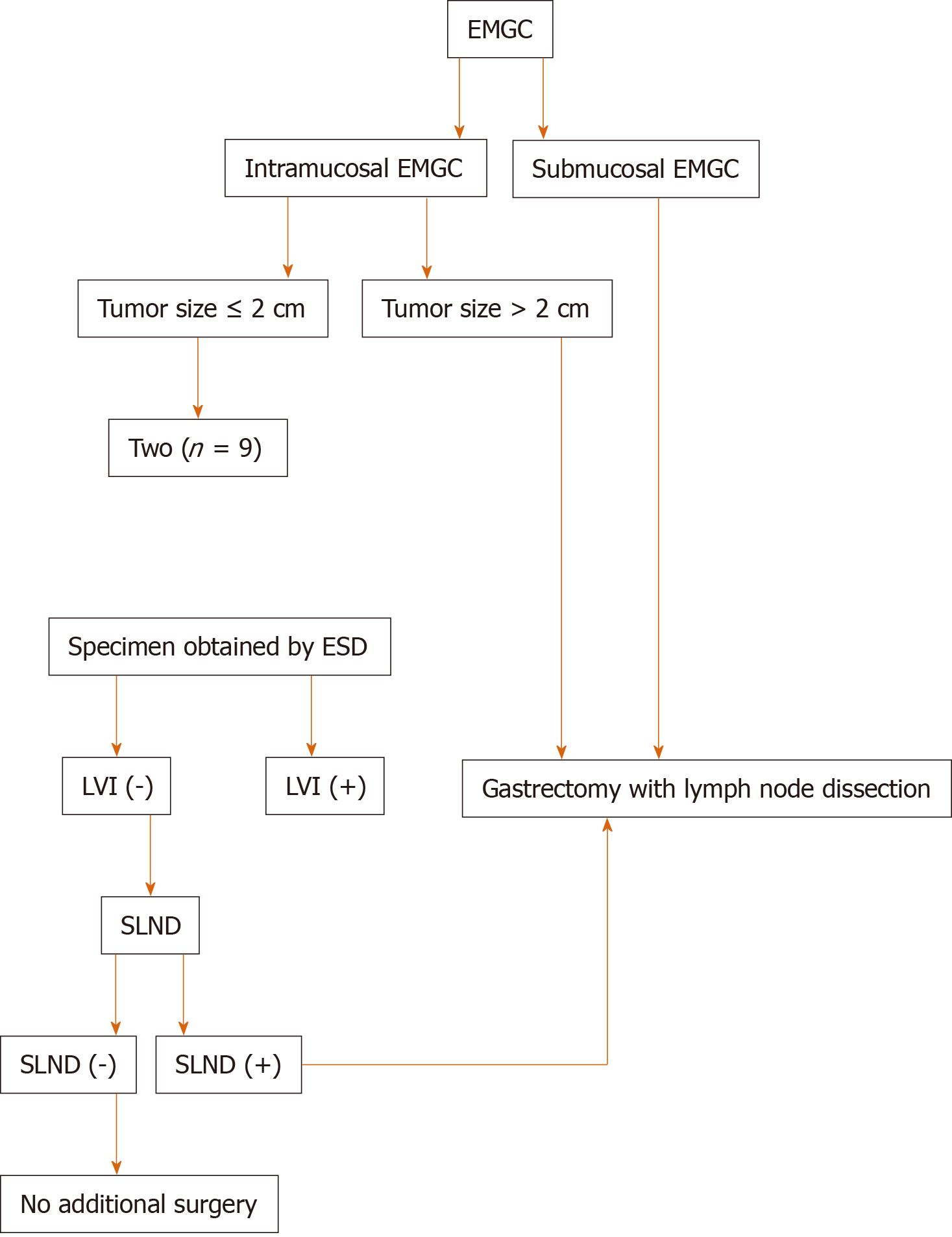
During the research, we tried to divide patients into subgroups among EMGC cases and to see if in one or more subgroups we could rule out the risk of pLNM, i.e., ESD could curably treat these patients. Fortunately, from the results we found that for patients with intramucosal cancer, when tumor size ≤ 2 cm or without LVI, no pLNM had been observed, indicating that for these EMGC cases, ESD could be safely and efficaciously performed while unnecessary gastrectomy might be avoided. On top of that, the association between the three risk factors and pLNM rate was further investigated to optimize the treatment of EMGC. Interestingly, based on current results we could found that there is a strong association between pLNM risk and the number of the three clinicopathologic factors. When there were 1, 2, and 3 risk factors, the rates of pLNM were 9.1% (1/11), 33.3% (3/9), and 75.0% (3/4), respectively. To achieve a better curative outcome, conventional gastrectomy with enough lymphadenectomy are needed in these patients with risk factors.
Combining ESD and SLND might avoid unnecessary gastrectomy in EMGC patients. ESD could ensure en bloc resection of the primary tumor while SLND could confirm the lymph node metastatic status[19,20]. A sentinel node is defined as the lymph node that directly receives lymphatic drainage from the primary tumor[21]. Thus, the sentinel node might be the first few lymph nodes affected by LNM. In order to detect the sentinel node by laparoscopic surgery, four trocar ports were prepared under general anesthesia. Gastric endoscope was then inserted through the patient’s mouth to confirm the presence and location of cancer lesions. To navigate the sentinel lymph nodes, indo-cyanine green dye was injected submucosally around the cancer lesion. Next, stained lymph nodes were identified in the laparoscopic view, and basic dissection was performed. The dissected sentinel lymph nodes were then immediately sent for intra-operative frozen section evaluation. If SLND reveals positive LNM, the patient was then converted to gastrectomy with conventional lymph node dissection at this point[22-24].
We must acknowledge several limitations in the present study. First, it was a single institutional cohort study with limited population, which might lead to information and selection bias. And the evolving standard of clinical treatment and technology in the past decades might also lead to bias. Therefore, more large-scale prospective studies are needed to verify our findings. As the study results suggest, we developed a novel working flowchart for treatment of patients with EMGC (Figure 2). ESD may be a sufficient treatment for intramucosal EMGC if tumor size ≤ 2 cm, and when LVI is absent upon postoperative histological examination. ESD allowed the complete removal of the primary gastric cancer, and SLND confirmed lymph node status in these patients. If intra-operative SLND reveals positive LNM or specimens from ESD indicates the presence of LVI, it is better to perform conventional gastrectomy with lymphadenectomy. In conclusion, combining ESD and SLND could be recommended as a safe and effective treatment for EMGC patients with a high risk of pLNM.
Endoscopic submucosal dissection (ESD) has become a standard treatment for tumors meeting the specific criteria characteristic of very low lymph node metastasis (LNM) risk: Intramucosal differentiated adenocarcinoma and ≤ 2 cm in size with no ulcers. Meanwhile, in undifferentiated early gastric cancer with a high risk of LNM, gastrectomy with lymph node dissection is usually performed as the standard surgical procedure. For undifferentiated gastric cancer, it consists of mucinous adenocarcinoma, poorly differentiated adenocarcinoma, and primary signet ring cell carcinoma. However, for approximately 96% of surgical patients with early mucinous gastric cancer (EMGC) confined to the mucosa, no LNM was observed, suggesting that it might be over-treated for these cases. Therefore, we have proposed new methods to minimize gastric resection for EMGC. The new technique allows minimally invasive resection of gastric lesions through ESD and laparoscopic sentinel lymph node dissection (SLND).
We attempted to identify a subgroup of EMGC patients in whom the risk of LNM can be ruled out and treated them by ESD and SLND, which may serve as a breakthrough treatment for EMGC.
We carried out this retrospective study to determine the clinicopathological factors that are predictive of LNM in EMGC. Furthermore, we established a simple criterion to expand the possibility of using ESD and SLND for the treatment of EMGC.
The association between the clinicopathological factors and the presence of LNM was retrospectively analyzed by univariate and multivariate logistic regression analyses. Odds ratios (OR) with 95% confidence intervals (CIs) were calculated. We further examined the relationship between the positive number of the three significant predictive factors and the LNM rate.
Depth of invasion (OR = 7.342, 95%CI: 1.127-33.256, P = 0.039), the tumor diameter (OR = 9.158, 95%CI: 1.348-29.133, P = 0.044), and lymphatic vessel involvement (OR = 27.749, 95%CI: 1.821-33.143, P = 0.019) were found to be significant and independent risk factors for LNM by multivariate analysis. For patients with one, two, and three of the risk factors, the LNM rates were 9.1%, 33.3%, and 75.0% respectively. LNM were not found in seven patients without one or more of the three risk factors.
ESD might be a sufficient treatment for intramucosal EMGC if tumor size ≤ 2 cm, and when LVI is absent upon postoperative histological examination. The combination of ESD and SLND could be recommended as an effective, minimally invasive treatment for EMGC patients having a potential risk of LNM.
The minimalization of therapeutic invasiveness in order to preserve quality of life is a major topic in the management of early gastric cancer. One of the critical factors in choosing minimally invasive surgery for EMGC would be the precise prediction of whether the patient has LNM or not. Therefore, in the future, the combination of ESD and SLND could be recommended as an effective, minimally invasive treatment for EMGC patients having a potential risk of LNM.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tanabe S S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Jeon HK, Lee SJ, Kim GH, Park DY, Lee BE, Song GA. Endoscopic submucosal dissection for undifferentiated-type early gastric cancer: short- and long-term outcomes. Surg Endosc. 2018;32:1963-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Choi MK, Kim GH, Park DY, Song GA, Kim DU, Ryu DY, Lee BE, Cheong JH, Cho M. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center experience. Surg Endosc. 2013;27:4250-4258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Zhu ZL, Shi HP, Beeharry MK, Feng TN, Yan M, Yuan F, Zheng-Gang Zhu, Zhang BY, Wu W. Expanding the indication of endoscopic submucosal dissection for undifferentiated early gastric cancer is safe or not? Asian J Surg. 2020;43:526-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Bang CS, Baik GH, Shin IS, Kim JB, Suk KT, Yoon JH, Kim YS, Kim DJ, Shin WG, Kim KH, Kim HY, Lim H, Kang HS, Kim JH, Kim JB, Jung SW, Kae SH, Jang HJ, Choi MH. Endoscopic submucosal dissection for early gastric cancer with undifferentiated-type histology: A meta-analysis. World J Gastroenterol. 2015;21:6032-6043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Fukunaga S, Nagami Y, Shiba M, Ominami M, Tanigawa T, Yamagami H, Tanaka H, Muguruma K, Watanabe T, Tominaga K, Fujiwara Y, Ohira M, Hirakawa K, Arakawa T. Long-term prognosis of expanded-indication differentiated-type early gastric cancer treated with endoscopic submucosal dissection or surgery using propensity score analysis. Gastrointest Endosc. 2017;85:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1910] [Article Influence: 238.8] [Reference Citation Analysis (1)] |
| 7. | Son T, Kwon IG, Hyung WJ. Minimally invasive surgery for gastric cancer treatment: current status and future perspectives. Gut Liver. 2014;8:229-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1325] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 9. | Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 495] [Article Influence: 27.5] [Reference Citation Analysis (1)] |
| 10. | Ryu SJ, Kim BW, Kim BG, Kim JH, Kim JS, Kim JI, Park JM, Oh JH, Kim TH, Kim JJ, Park SM, Park CH, Song KY, Lee JH, Kim SG, Kim DJ, Kim W. Endoscopic submucosal dissection versus surgical resection for early gastric cancer: a retrospective multicenter study on immediate and long-term outcome over 5 years. Surg Endosc. 2016;30:5283-5289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Kitagawa Y, Takeuchi H, Takagi Y, Natsugoe S, Terashima M, Murakami N, Fujimura T, Tsujimoto H, Hayashi H, Yoshimizu N, Takagane A, Mohri Y, Nabeshima K, Uenosono Y, Kinami S, Sakamoto J, Morita S, Aikou T, Miwa K, Kitajima M. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol. 2013;31:3704-3710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 12. | Tanabe S, Hirabayashi S, Oda I, Ono H, Nashimoto A, Isobe Y, Miyashiro I, Tsujitani S, Seto Y, Fukagawa T, Nunobe S, Furukawa H, Kodera Y, Kaminishi M, Katai H. Gastric cancer treated by endoscopic submucosal dissection or endoscopic mucosal resection in Japan from 2004 through 2006: JGCA nationwide registry conducted in 2013. Gastric Cancer. 2017;20:834-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Fujimoto A, Goto O, Nishizawa T, Ochiai Y, Horii J, Maehata T, Akimoto T, Kinoshita S, Sagara S, Sasaki M, Uraoka T, Yahagi N. Gastric ESD may be useful as accurate staging and decision of future therapeutic strategy. Endosc Int Open. 2017;5:E90-E95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, Miki A, Ohyama S, Seto Y. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 336] [Article Influence: 18.7] [Reference Citation Analysis (2)] |
| 15. | Tsujimoto H, Yaguchi Y, Kumano I, Takahata R, Ono S, Hase K. Successful gastric submucosal tumor resection using laparoscopic and endoscopic cooperative surgery. World J Surg. 2012;36:327-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Hyung WJ, Cheong JH, Kim J, Chen J, Choi SH, Noh SH. Application of minimally invasive treatment for early gastric cancer. J Surg Oncol. 2004;85:181-5; discussion 186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Fujii H, Ishii E, Tochitani S, Nakaji S, Hirata N, Kusanagi H, Narita M. Lymph node metastasis after endoscopic submucosal dissection of a differentiated gastric cancer confined to the mucosa with an ulcer smaller than 30 mm. Dig Endosc. 2015;27:159-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Min BH, Kim ER, Kim KM, Park CK, Lee JH, Rhee PL, Kim JJ. Surveillance strategy based on the incidence and patterns of recurrence after curative endoscopic submucosal dissection for early gastric cancer. Endoscopy. 2015;47:784-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Natsugoe S, Arigami T, Uenosono Y, Yanagita S. Novel surgical approach based on the sentinel node concept in patients with early gastric cancer. Ann Gastroenterol Surg. 2017;1:180-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Aikou T, Higashi H, Natsugoe S, Hokita S, Baba M, Tako S. Can sentinel node navigation surgery reduce the extent of lymph node dissection in gastric cancer? Ann Surg Oncol. 2001;8:90S-93S. [PubMed] |
| 21. | Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, Foshag LJ, Cochran AJ. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3238] [Cited by in RCA: 2931] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 22. | Cho WY, Kim YJ, Cho JY, Bok GH, Jin SY, Lee TH, Kim HG, Kim JO, Lee JS. Hybrid natural orifice transluminal endoscopic surgery: endoscopic full-thickness resection of early gastric cancer and laparoscopic regional lymph node dissection--14 human cases. Endoscopy. 2011;43:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Ko WJ, Kim YM, Yoo IK, Cho JY. Clinical outcomes of minimally invasive treatment for early gastric cancer in patients beyond the indications of endoscopic submucosal dissection. Surg Endosc. 2018;32:3798-3805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Abe N, Takeuchi H, Ohki A, Yanagida O, Masaki T, Mori T, Sugiyama M. Long-term outcomes of combination of endoscopic submucosal dissection and laparoscopic lymph node dissection without gastrectomy for early gastric cancer patients who have a potential risk of lymph node metastasis. Gastrointest Endosc. 2011;74:792-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |