Published online May 26, 2020. doi: 10.12998/wjcc.v8.i10.2056
Peer-review started: January 31, 2020
First decision: March 18, 2020
Revised: March 21, 2020
Accepted: April 18, 2020
Article in press: April 18, 2020
Published online: May 26, 2020
Processing time: 116 Days and 5.2 Hours
Fulminant lupus myocarditis is a rare but fatal manifestation of systemic lupus erythematosus. Aggressive immunosuppressive treatments are important in its successful management. However, they can significantly damage the immunity and are associated with a considerable risk of infection development and spread. We present a rare and complicated case of a 20-year-old female diagnosed with fulminant lupus myocarditis accompanied by pneumonia. The patient was successfully treated with plasma exchange (PE) for fulminant lupus myocarditis.
A 20-year-old Chinese woman presented to the Hematology Department complaining of fatigue and knee pain. Blood test showed anemia and thrombocytopenia. On the second day of hospitalization, she was transferred to the ICU due to dyspnea and hypotension. Autoimmune profiles showed hypocomplementemia and positive antinuclear antibodies. Computer tomography showed an enlarged heart and pneumonia. Ultrasound revealed an enlarged heart with a low left ventricular ejection fraction. Fulminant lupus myocarditis with cardiogenic shock was initially considered. Due to the accompanying pneumonia, aggressive immunosuppression was contraindicated. Her cardiac function remained critical after the initial therapy of intravenous immunoglobulin and corticosteroids at a conventional dose, but she responded well to later PE therapy plus corticosteroids administration. The patient fully recovered with normal cardiac function.
This case indicates that PE is a valuable treatment choice without adverse effects of immunosuppression in patients with fulminant lupus myocarditis and coexisting infection.
Core tip: Fulminant lupus myocarditis with cardiogenic shock is rare but life-threatening. Although aggressive immunosuppressive treatment plays an important role in its successful management, it may lead to a considerable risk of infection development and spread. Plasma exchange (PE) can quickly remove antibodies and antigen-antibody complexes from lupus patients without adverse effects of immunosuppression and infection spread. Here, we present a rare and complicated case of a female patient successfully treated with PE for fulminant lupus myocarditis accompanied by pneumonia. This case indicates that PE is a valuable treatment choice without immunosuppression, especially for severe lupus myocarditis patients complicated by infection.
- Citation: Xing ZX, Yu K, Yang H, Liu GY, Chen N, Wang Y, Chen M. Successful use of plasma exchange in fulminant lupus myocarditis coexisting with pneumonia: A case report. World J Clin Cases 2020; 8(10): 2056-2065
- URL: https://www.wjgnet.com/2307-8960/full/v8/i10/2056.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i10.2056
Systemic lupus erythematosus (SLE) is the most prevalent autoimmune disorder with multisystem impairment and heterogeneous clinical presentations, and it typically affects females between puberty and the menopause[1]. Although SLE is known to be associated with an increased risk of cardiac impairment which includes coronary atherosclerosis, valvular heart disease, myocarditis and pericarditis, fulminant lupus myocarditis is an uncommon but serious manifestation of SLE[2]. Lupus myocarditis can be the first manifestation of the disease or occurs during follow-up[3]. The clinical presentations of lupus myocarditis vary greatly from asymptomatic or oligosymptomatic to life-threatening fulminant myocarditis with cardiogenic shock, and the mortality rate is approximately 20%[4]. Therefore, the diagnosis and treatment of severe lupus myocarditis remain challenging.
Aggressive immunosuppressive therapies, such as high-dose pulse corticosteroid therapy and immunosuppressive agents, are the most effective therapies for severe lupus myocarditis and most patients can achieve a satisfactory outcome[5]. However, aggressive immunosuppressive therapies may significantly damage the host immunity and lead to a considerable risk of infection development and spread[6]. Plasma exchange (PE), as an alternative therapy without immunosuppression, has been demonstrated to be safe and effective in treating severe lupus-related complications such as encephalitis, thrombotic thrombocytopenic purpura, antiphospholipid syndrome and nephritis, but it is rarely reported in cardiogenic shock induced by fulminant lupus myocarditis[7]. Infection, especially pneumonia, remains a leading cause of morbidity and mortality among patients with SLE[8,9]. Here, we report the case of a young woman requiring urgent ICU admission with a clinical diagnosis of cardiogenic shock induced by fulminant lupus myocarditis, with coexisting community-acquired pneumonia. Due to the presence of coexisting pneumonia, aggressive immunosuppressive therapies were not administered and PE was performed, which was shown to be safe and effective in improving impaired cardiac function without the risk of worsening the pneumonia. We also performed a review of the PubMed literature, and found no reports on the use of PE in severe lupus patients with associated infection. Thus, we believe that this is the first case of fulminant lupus myocarditis accompanied by pneumonia successfully treated with PE.
A 20-year-old Chinese woman, with anemia and thrombocytopenia, was admitted to the Hematology Department of our hospital due to progressive fatigue.
The patient presented with progressive fatigue three months ago, which had significantly worsened in the previous few days. Additionally, she had experienced intermittent knee pain with morning stiffness of both legs for almost six months. She had not seen a doctor until this hospital visit. She attended the emergency department of our hospital and initial laboratory tests showed anemia and severe thrombocytopenia. She was then admitted to the Hematology Department where further laboratory work-up was performed. On the second day of hospitalization, she was transferred to the ICU due to severe respiratory distress and shock.
The patient had no previous medical history.
The patient did not have a history of smoking, drinking or drug abuse.
On physical examination, the patient was pale, awake, alert, responsive to questions and in acute respiratory distress. There was some skin petechiae, indicating a bleeding tendency, but there was no skin rash, oral ulcers, alopecia or enlarged lymph nodes. Her heart rate was 140 bpm, blood pressure was 112/70 mmHg with norepinephrine continuously pumped (0.8 μg/kg/min), respiratory rate was 42 breaths/min, and temperature was 37.6 °C. The oxygen saturation remained at 80% on room air and increased to 94% on a high-flow nasal cannula with FiO2 of 40%. These findings suggested severe circulatory shock and respiratory failure. Heart auscultation showed low heart sounds without murmurs, and there were crackles over both lung fields, indicating heart failure associated with pulmonary edema or pneumonia. Her abdomen was soft and not tender, and the liver and spleen were not palpable. She had joint line tenderness in both knees and mild edema in both lower extremities.
The initial laboratory tests are shown in Table 1. Blood tests revealed mild leukocytosis 10.71 × 109/L with moderate anemia (hemoglobin 71 g/L) and severe thrombocytopenia (platelet count 33 × 109/L). Alanine aminotransferase (98 IU/L) and aspartate aminotransferase (301 IU/L) were increased, which may have been attributed to liver congestion induced by heart failure. Creatinine (1.54 mg/dL) was slightly elevated, indicating mild acute renal damage. Activated partial thromboplastin time and prothrombin time were roughly normal. Cardiac damage markers, including myohemoglobin (864.3 ng/mL) and hypersensitive troponin T (142.9 ng/L), were increased. Her plasma N-terminal pro-B-type natriuretic peptide level (> 35000 pg/mL) was significantly high. Autoimmune profiles showed hypocomplementemia with C3 of 36 mg/dL and C4 of 13.7 mg/dL, positive antinuclear antibodies with a titer 1:1000 (speckled nuclear pattern), positive SS-A antibodies (+++), positive SS-B antibodies (++) and positive Ro-52 antibodies (+++). An arterial blood gas on admission to ICU revealed a pH of 7.46, PaCO2 of 21 mmHg, PaO2 of 66 mmHg, lactate of 5.6 mmol/L, and HCO3- of 18 mmol/L with FiO2 of 40% on a high-flow nasal cannula, indicating respiratory failure and circulatory shock. An admission electrocardiogram showed sinus tachycardia with low voltage. Respiratory and blood samples were sent for culture, without positive results. Other laboratory investigations revealed normal thyroid function and urine analysis.
| Variables | Results | Normal range |
| White blood cells | 10.71 × 109/L | 3.5-9.5 × 109/L |
| Red blood cells | 3.29 × 1012/L | 3.8-5.1 × 1012/L |
| Hemoglobin | 71 g/L | 115-150 g/L |
| Platelets | 33 × 109 /L | 100-300 × 109/L |
| Alanine aminotransferase | 98 IU/L | 7-40 IU/L |
| Aspartate aminotransferase | 301 IU/L | 13-35 IU/L |
| Total bilirubin | 0.65 mg/dL | 0.29-1.2 mg/dL |
| Creatinine | 1.54 mg/dL | 0.34-1.02 mg/dL |
| Prothrombin time | 12.1 s | 9-14 s |
| Activated partial thromboplastin time | 43 s | 20-40 s |
| Myohemoglobin | 864.30 ng/mL | 25-58 ng/mL |
| Hypersensitive troponin T | 142.90 ng/L | < 14 ng/L |
| NT-proBNP | > 35000 pg/mL | < 125 pg/mL |
| Complement 3 | 36 mg/dL | 79-152 mg/dL |
| Complement 4 | 13.7 mg/dL | 16-38 mg/dL |
| ANA (1:100) | +++ | Negative |
| ANA (1:320) | ++ | Negative |
| ANA (1:1000) | + | Negative |
| Anti-SS-A antibody | +++ | Negative |
| Anti-SS-B antibody | ++ | Negative |
| Anti-Ro-52 antibody | +++ | Negative |
| Anti-Smith antibody | Negative | Negative |
| Anti-SCL-70 antibody | Negative | Negative |
| Anti-Jo-1 antibody | Negative | Negative |
| Anti-CENP-B antibody | Negative | Negative |
| Anti-double-stranded DNA antibody | Negative | Negative |
| Rheumatoid factor | < 20 IU/mL | < 20 IU/mL |
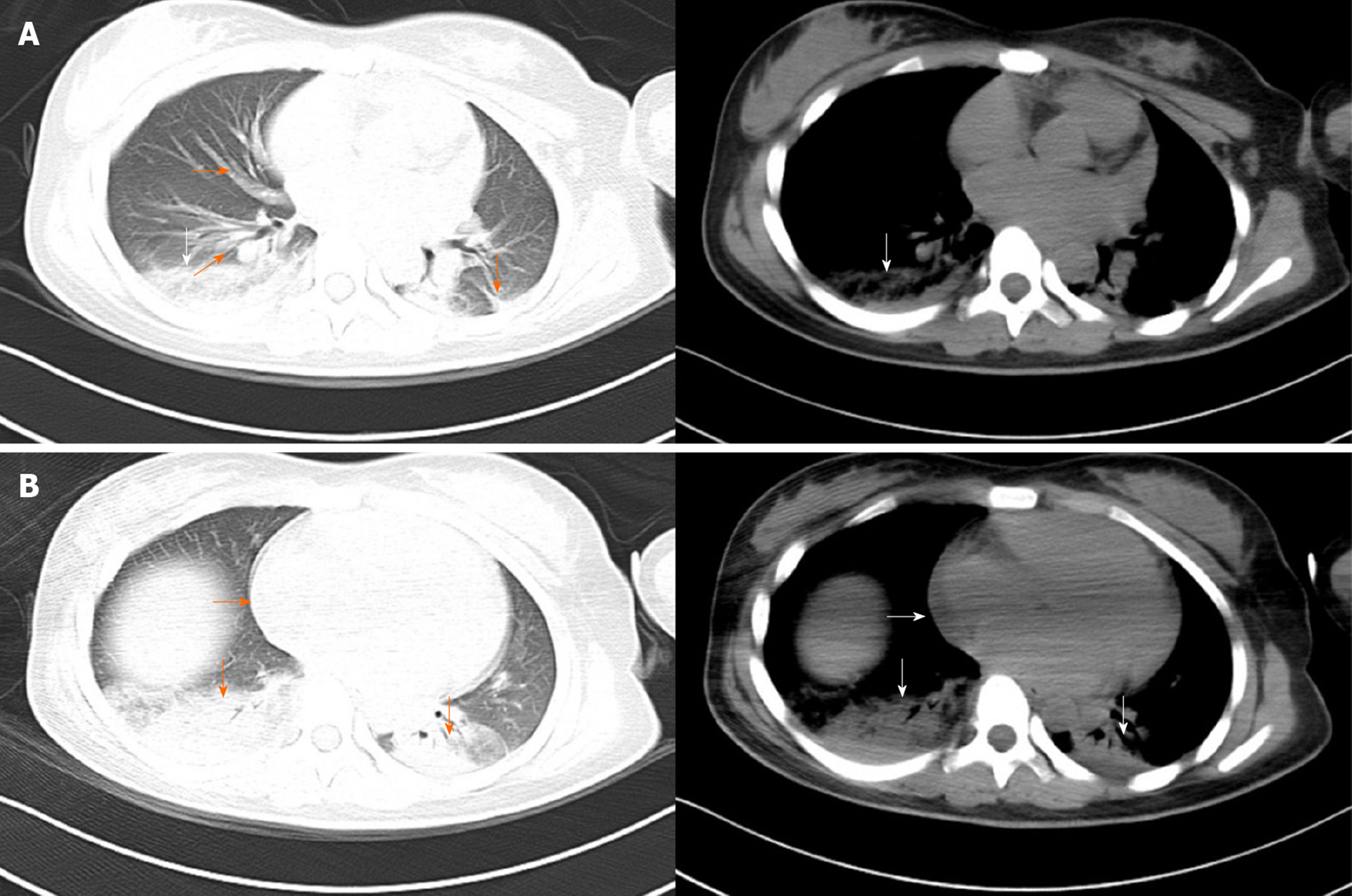
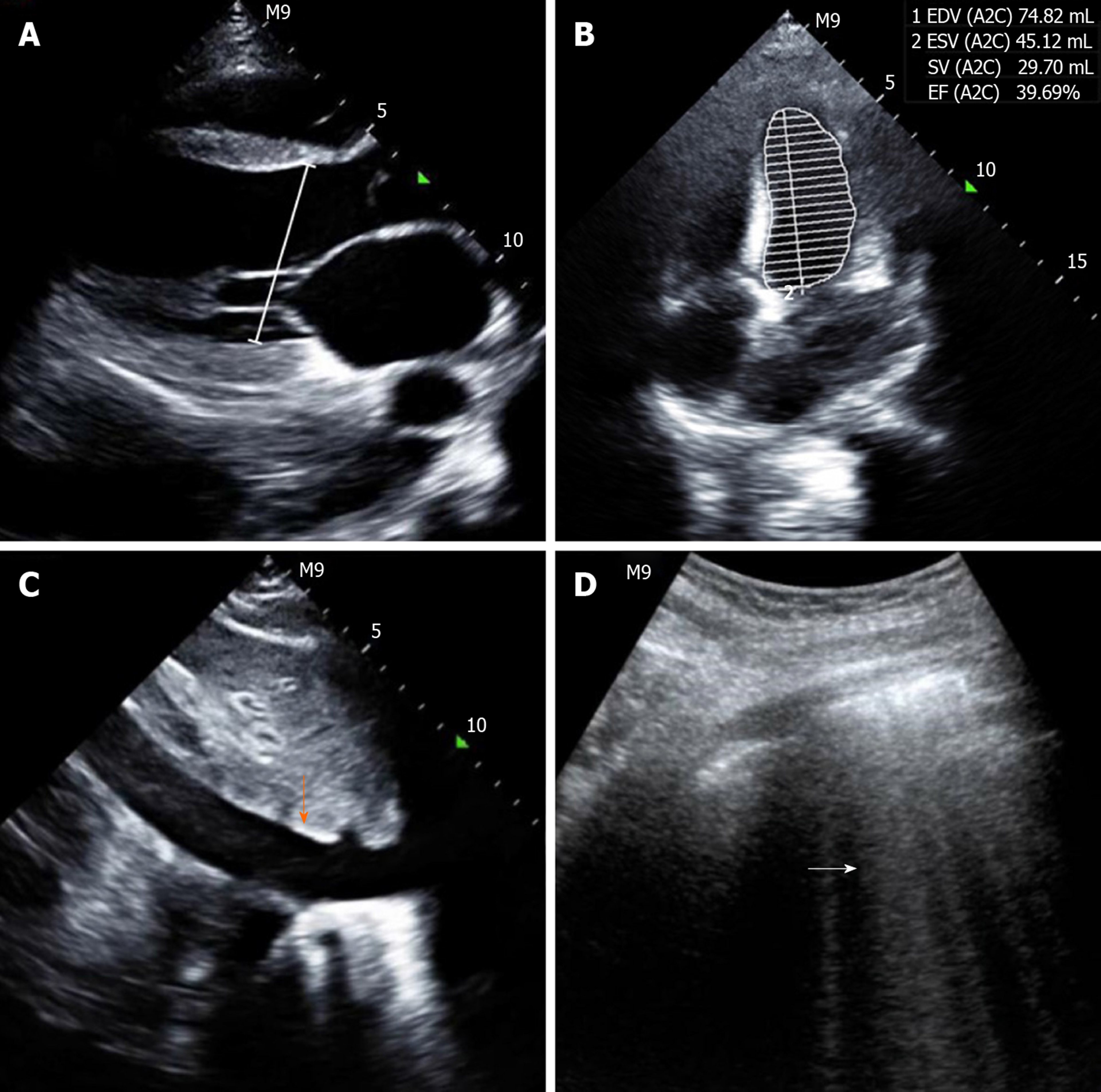
Computed tomography of the chest showed an enlarged heart, increased pulmonary vascular diameter, ground-glass attenuation and interlobar fissure effusion, which suggested heart failure and pulmonary interstitial edema (Figure 1). Additionally, consolidation was found in both lower lobes in the lung, indicating pneumonia (Figure 1). Point-of-care ultrasound revealed an enlarged left ventricle (50 mm) (Figure 2A), global hypokinesia of the left ventricle and significant systolic impairment with a low left ventricular ejection fraction of 39.69% (Figure 2B), a distended inferior vena cava (22 mm) with loss of respiratory variation (Figure 2C) and diffuse B lines in both lung fields (Figure 2D). Based on these imaging findings, cardiogenic shock, pulmonary edema and pneumonia were confirmed.
Viral myocarditis, one of the most common causes of cardiogenic shock in young people, was considered in the primary differential diagnosis. However, the patient had no previous medical history of upper respiratory tract infection and further virological serum tests, such as influenza A and B, enterovirus, adenovirus and cytomegalovirus, were negative. Therefore, viral myocarditis was excluded as the cause in this case. SS-A antibodies and SS-B antibodies were positive; thus, primary Sjogren’s syndrome was considered. However, the patient did not have a dry mouth or dry eyes, and further Schirmer paper-strip tear tests were normal, with 12 mm/5 min and 13 mm/5 min for both eyes. Based on the 2016 American College of Rheumatology and European League Against Rheumatism classification criteria for primary Sjogren’s syndrome[10], this was unlikely to be the cause in this case.
According to the 2012 Systemic Lupus International Collaborating Clinics (SLICC) classification criteria[11], lupus is a serologic diagnosis associated with a clinical diagnosis. The SLICC criteria require at least one clinical and at least one immunologic criterion for a total of four. The patient had thrombocytopenia, knee pain with morning stiffness, positive antinuclear antibodies, hypocomplementemia, severe heart failure and consolidation in the lungs with leukocytosis; thus, the final diagnosis in this case was cardiogenic shock induced by fulminant lupus myocarditis with coexisting community-acquired pneumonia.
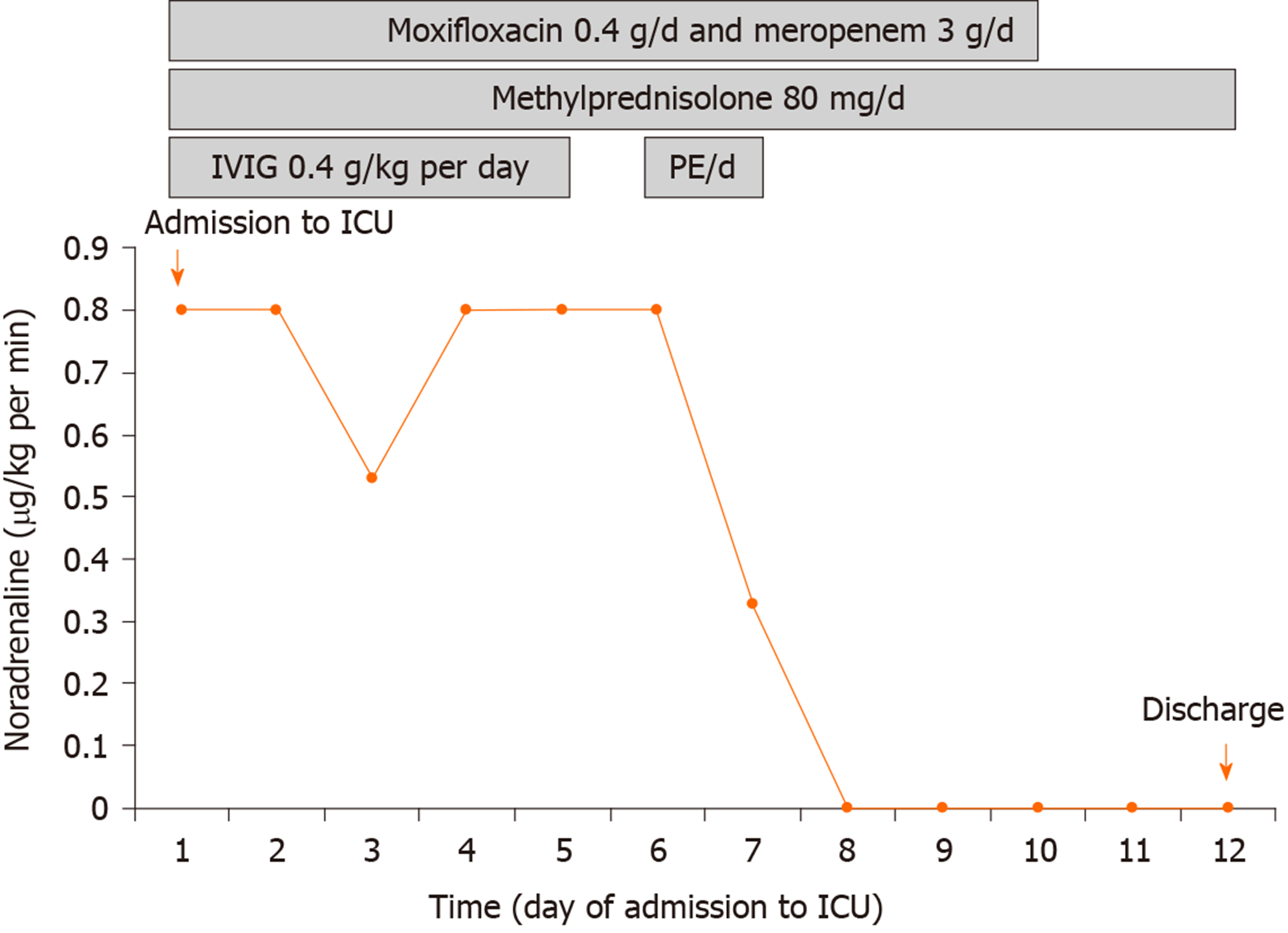
Figure 3 shows the core treatment procedures and clinical course. Given the life-threatening lupus myocarditis which can lead to cardiogenic shock and pulmonary edema, a vasopressor (norepinephrine) and diuretics were administered immediately. Due to the accompanying community-acquired pneumonia, high-dose corticosteroids and immunosuppressants were not used. To treat lupus myocarditis, intravenous immunoglobulin (IVIG) (0.4 g/kg/d) and methylprednisolone (80 mg/d) were administered for five consecutive days, resulting in complete recovery of platelet count, but cardiac function did not improve with the initial treatment. On the basis of methylprednisolone administered at a conventional dose, the patient was treated with PE of fresh frozen plasma (2000 mL/d) twice, and vasopressors were successfully discontinued within 48 h after initiating PE therapy. In addition, the patient received 10 days of intravenous broad-spectrum antibiotic therapy (moxifloxacin and meropenem), which resolved her pneumonia.
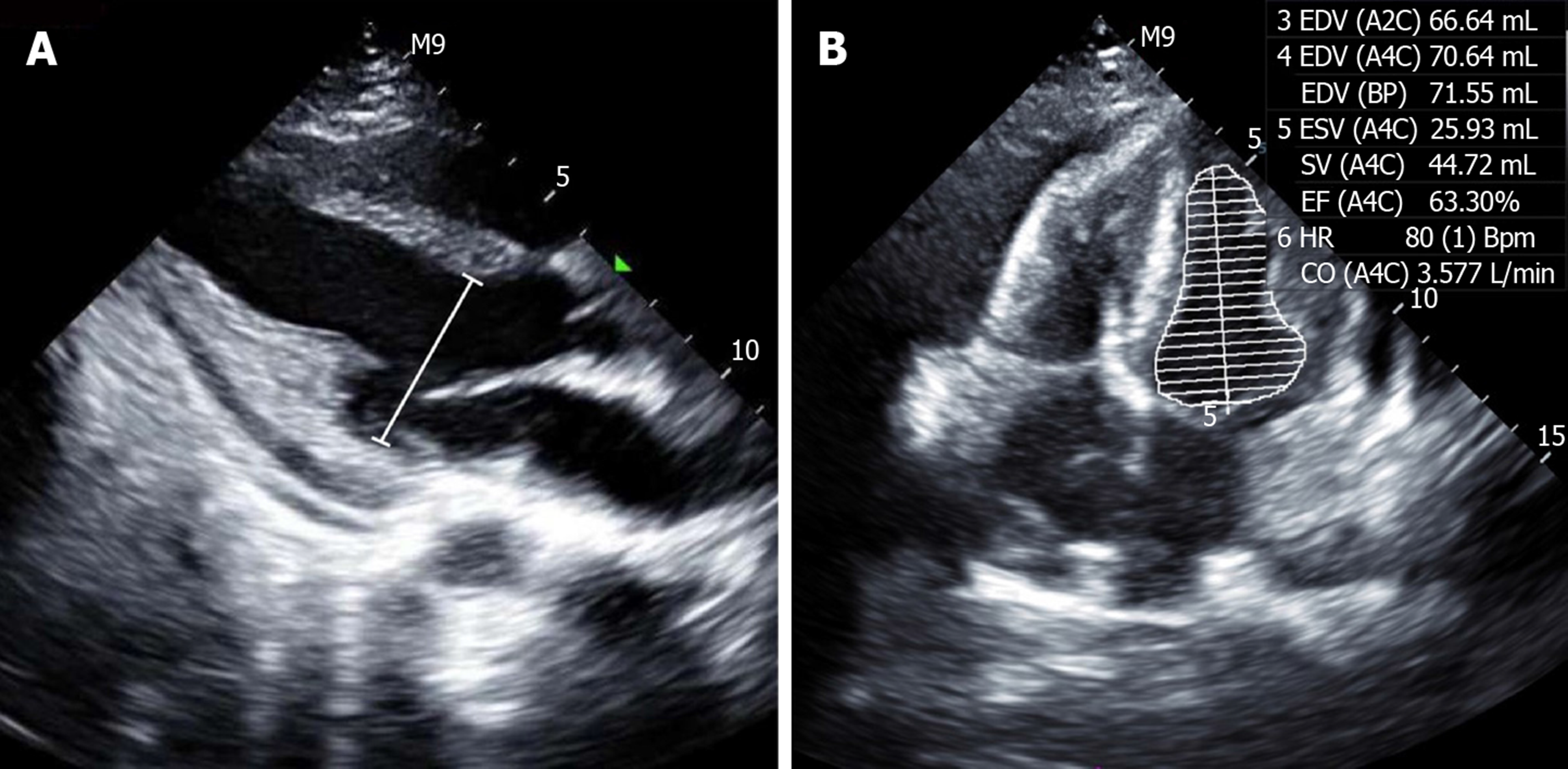
On the tenth day after admission to the ICU, repeated transthoracic echocardiogram showed a normal left ventricular size and function with a left ventricular ejection fraction of 63.3% (Figure 4). The patient remained asymptomatic, and on the twelfth day of admission, the patient was discharged from hospital followed by outpatient clinical follow-up on oral prednisone of 50 mg/d and hydroxychloroquine of 200 mg/d. The patient and her parents were satisfied with this treatment. At ten months after discharge, the patient was followed up in the outpatient department where a rheumatologist assessed the responsiveness to treatment, the activity of SLE, and adverse drug effects. Follow-up evaluations were approximately normal, including blood cell counts, hemoglobin, serum creatinine, urine analysis, bone density screening, ocular screening, electrocardiogram and echocardiography. The patient had no adverse drug reactions or infection caused by corticosteroids, and she gradually reduced and then stopped oral prednisone, and she remained healthy without recurrence of cardiac dysfunction, anemia, thrombocytopenia, fatigue and knee pain. Additionally, there were no manifestations of tumors or tuberculosis. Therefore, SLE was the final cause of cardiogenic shock.
Cardiogenic shock is defined as a condition with low cardiac output caused by a cardiac disorder resulting in both clinical and biochemical manifestations of inadequate tissue perfusion[12]. Fulminant lupus myocarditis is a rare cause of cardiogenic shock and common causes include myocardial infarction, viral myocarditis, Takotsubo cardiomyopathy, hypertrophic cardiomyopathy and idiopathic dilated cardiomyopathy[13]. Myocardial infarction, with characteristics of ST-segment and T-wave on specific leads on the electrocardiogram, remains the most common cause of cardiogenic shock in the middle-aged and elderly population. Viral myocarditis can mimic lupus myocarditis with elevated serum markers of cardiac injury, low cardiac output and similar electrocardiogram manifestations. A definitive diagnosis relies on endomyocardial biopsy which is the gold standard for diagnosis. However, in medical practice, physicians diagnose viral myocarditis based on a combination of clinical features, laboratory analyses, and imaging findings[14]. Similarly, the diagnosis of lupus myocarditis depends largely on clinical suspicion and echocardiographic findings rather than biopsy[3,15]. In our case, clinical presentation, abnormal autoimmune profiles, significantly elevated troponin, global hypokinesia on echocardiogram, electrocardiogram with low voltage and normal viral assays led to the diagnosis of fulminant lupus myocarditis, rather than viral myocarditis causing the cardiogenic shock.
Fulminant lupus myocarditis is a rare but potentially fatal manifestation of SLE, which is a heterogeneous autoimmune disease and may involve almost all organs such as the skin, joints, the central nervous system, the kidneys, the heart and even the gut[16]. SLE usually displays a variable clinical course with insidious onset, making early diagnosis challenging[17]. Therefore, the patient in the current report was previously misdiagnosed with blood disease due to anemia and thrombocytopenia. Additionally, patients with fulminant lupus myocarditis may present with fatigue, fever, dyspnea and hypotension, mimicking pneumonia and septic shock that increases the difficulty of definitive diagnosis[18].
Currently, there are only classification criteria for SLE, but no gold diagnostic standard, thus hampering the definitive diagnosis. The 2012 SLICC criteria, with sensitivity of 97% and specificity of 84%[19], have facilitated the diagnosis. Nevertheless, classification criteria are often misused as diagnostic criteria, which may affect early diagnosis and lead to more misdiagnosed cases[20]. Several conditions, especially disseminated tuberculosis and lymphoma, have clinical and laboratory features that can masquerade those present in SLE[21,22]. Therefore, lupus, tuberculosis, lympho-proliferative disorders, as well as other autoimmune diseases, are often difficult to identify and are easily misdiagnosed. Clinicians should diagnose SLE on the basis of a complete medical history as well as on the adequate constellation of clinical or laboratory findings, after excluding other diseases[20]. Patients with an initial diagnosis of lupus should be followed up for a period to exclude latent tuberculosis and lymphoma. In our case, the ten-month follow-up period showed no tuberculosis or lymphoma; thus, the final diagnosis was fulminant lupus myocarditis.
As myocarditis is not considered in the standard classification criteria for SLE and severe lupus myocarditis is an infrequent manifestation of SLE, current treatment strategies are based on isolated case reports rather than randomized clinical trials[18,23,24]. Several case reports and series have demonstrated a favorable response to aggressive immunosuppressive therapies which mainly include high-dose pulse corticosteroids, methylprednisolone 0.5-1 g for 3-5 d[5,25,26], alone or with immunosuppressive agents, such as cyclophosphamide, azathioprine and mycophenolate mofetil[27,28]. Reports have also shown some benefit from IVIG[29,30]. Moreover, it is reported in one case that IVIG can be used in lupus myocarditis coexisting with infective endocarditis, for which immunosuppressive drugs were contraindicated[31]. In some severe cases of cardiogenic shock, mechanical circulatory support, such as extracorporeal membrane oxygenation, is required[32]. In recent years, biologic therapy, such as rituximab, has been demonstrated to be effective in refractory lupus myocarditis patients[33].
The use of immunosuppressive therapy in SLE carries significant risks of infection. The effect of these drugs on infection is also dose dependent, thus high-dose corticosteroids may create a more significant risk of infection[34]. On the one hand, dysfunction of the innate immune system increases the risk of infection in patients with SLE; on the other hand, patients with SLE may have an increased risk of infection that is augmented by immunosuppressive therapies[9]. Infection, especially pneumonia, is the main cause of mortality in patients with SLE[8]. Considering the adverse effect of aggressive immunosuppressive therapies in worsening or spreading the confirmed or potential infection, this is a dilemma for clinicians when confronting severe lupus myocarditis which may coexist with infection or should be differentiated from infection.
PE is a therapeutic procedure in which the patient’s blood is passed through a device which separates the plasma from other components of blood. The plasma is removed and replaced with fresh frozen plasma[35]. This can remove putative pathogenic autoantibodies and circulating immune complexes from the blood of patients with SLE[36]. PE does not attenuate host immunity and adverse events of PE are uncommon. The indications for PE are acute life-threatening manifestations and severe therapy-resistant manifestations, such as diffuse alveolar hemorrhage, neurolupus, thrombotic thrombocytopenic purpura, catastrophic antiphospholipid syndrome and refractory SLE renal disease, and in pregnancy[36]. Moreover, a small multicenter trial indicated that PE could be considered as a treatment option for refractory lupus nephritis in the setting of a high risk of infection[7]. PE is considered effective and safe in treating these severe complications of SLE, but few cases of PE in lupus myocarditis have been reported to date[7,24,35,37].
Immunosuppression is the cornerstone of therapeutic interventions for SLE[38]. Aggressive immunosuppressive therapies, especially high-dose pulse corticosteroids, are the most effective therapies for severe lupus myocarditis. However, our patient did not receive high-dose pulse corticosteroid therapy due to the adverse effect of immunosuppression which may have aggravated her community-acquired pneumonia. Corticosteroids at a conventional dose and IVIG were prescribed for five days as the initial treatment. As her impaired cardiac function did not improve with this initial therapy, high dose vasopressors were used. PE, a valuable option for treating severe SLE complications without immunosuppression, was performed with administration of corticosteroids at a conventional dose for two days and the patient discontinued vasopressors within 48 h.
Following treatment with corticosteroids, PE therapy was performed and reversed her impaired cardiac function. The limitation of our case report is that the improvement in impaired cardiac function could not be attributed exclusively to PE, as the patient had received IVIG therapy and corticosteroids before undergoing PE therapy. However, there is no doubt that PE can be used as a valuable therapeutic option when infection coexists in severe SLE patients. Our case has demonstrated that PE therapy can be used as an alternative treatment to aggressive immunosuppressive therapies in the background of infection. There are limited clinical data on the management of severe SLE accompanied by infection[7,31,39]. We suggest that the indications for PE in SLE should include acute life-threatening SLE with the comorbidity of infection.
The early diagnosis and optimized treatment for severe lupus myocarditis remain challenging for clinicians. The diagnosis of lupus myocarditis should be considered in young people with acute onset of cardiogenic shock accompanying other organ involvement. Detailed medical history, careful examination and thorough laboratory work-up can aid early diagnosis. Moreover, this case indicates that PE is a good choice without immunosuppression for severe SLE patients with coexisting infection.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rothschild BM S-Editor: Wang J L-Editor: Webster JR E-Editor: Liu JH
| 1. | Kaul A, Gordon C, Crow MK, Touma Z, Urowitz MB, van Vollenhoven R, Ruiz-Irastorza G, Hughes G. Systemic lupus erythematosus. Nat Rev Dis Primers. 2016;2:16039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 809] [Article Influence: 89.9] [Reference Citation Analysis (0)] |
| 2. | Tselios K, Urowitz MB. Cardiovascular and Pulmonary Manifestations of Systemic Lupus Erythematosus. Curr Rheumatol Rev. 2017;13:206-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | Thomas G, Cohen Aubart F, Chiche L, Haroche J, Hié M, Hervier B, Costedoat-Chalumeau N, Mazodier K, Ebbo M, Cluzel P, Cordel N, Ribes D, Chastre J, Schleinitz N, Veit V, Piette JC, Harlé JR, Combes A, Amoura Z. Lupus Myocarditis: Initial Presentation and Longterm Outcomes in a Multicentric Series of 29 Patients. J Rheumatol. 2017;44:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Tanwani J, Tselios K, Gladman DD, Su J, Urowitz MB. Lupus myocarditis: a single center experience and a comparative analysis of observational cohort studies. Lupus. 2018;27:1296-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Zhang L, Zhu YL, Li MT, Gao N, You X, Wu QJ, Su JM, Shen M, Zhao LD, Liu JJ, Zhang FC, Zhao Y, Zeng XF. Lupus Myocarditis: A Case-Control Study from China. Chin Med J (Engl). 2015;128:2588-2594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Carbone J, del Pozo N, Gallego A, Sarmiento E. Immunological risk factors for infection after immunosuppressive and biologic therapies. Expert Rev Anti Infect Ther. 2011;9:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Kronbichler A, Brezina B, Quintana LF, Jayne DR. Efficacy of plasma exchange and immunoadsorption in systemic lupus erythematosus and antiphospholipid syndrome: A systematic review. Autoimmun Rev. 2016;15:38-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 8. | García-Guevara G, Ríos-Corzo R, Díaz-Mora A, López-López M, Hernández-Flores J, Fragoso-Loyo H, Ávila-Vázquez J, Pulido-Ramírez AL, Carrillo-Maravilla E, Jakez-Ocampo J, Sifuentes-Osornio J, Llorente L, Atisha-Fregoso Y. Pneumonia in patients with systemic lupus erythematosus: Epidemiology, microbiology and outcomes. Lupus. 2018;27:1953-1959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Doaty S, Agrawal H, Bauer E, Furst DE. Infection and Lupus: Which Causes Which? Curr Rheumatol Rep. 2016;18:13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, Rasmussen A, Scofield H, Vitali C, Bowman SJ, Mariette X; International Sjögren's Syndrome Criteria Working Group. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren's Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017;69:35-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 1113] [Article Influence: 139.1] [Reference Citation Analysis (0)] |
| 11. | Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O, Sturfelt G, Ramsey-Goldman R, Bae SC, Hanly JG, Sánchez-Guerrero J, Clarke A, Aranow C, Manzi S, Urowitz M, Gladman D, Kalunian K, Costner M, Werth VP, Zoma A, Bernatsky S, Ruiz-Irastorza G, Khamashta MA, Jacobsen S, Buyon JP, Maddison P, Dooley MA, van Vollenhoven RF, Ginzler E, Stoll T, Peschken C, Jorizzo JL, Callen JP, Lim SS, Fessler BJ, Inanc M, Kamen DL, Rahman A, Steinsson K, Franks AG, Sigler L, Hameed S, Fang H, Pham N, Brey R, Weisman MH, McGwin G, Magder LS. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2816] [Cited by in RCA: 3540] [Article Influence: 272.3] [Reference Citation Analysis (0)] |
| 12. | van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H, Washam JB, Cohen MG; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Mission: Lifeline. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation. 2017;136:e232-e268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 1160] [Article Influence: 145.0] [Reference Citation Analysis (0)] |
| 13. | Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation. 2008;117:686-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 558] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 14. | Pollack A, Kontorovich AR, Fuster V, Dec GW. Viral myocarditis--diagnosis, treatment options, and current controversies. Nat Rev Cardiol. 2015;12:670-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 412] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 15. | Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636-2648, 2648a-2648d. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 2274] [Article Influence: 189.5] [Reference Citation Analysis (0)] |
| 16. | Gonzalez A, Wadhwa V, Salomon F, Kaur J, Castro FJ. Lupus enteritis as the only active manifestation of systemic lupus erythematosus: A case report. World J Clin Cases. 2019;7:1315-1322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (2)] |
| 17. | Thong B, Olsen NJ. Systemic lupus erythematosus diagnosis and management. Rheumatology (Oxford). 2017;56:i3-i13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Baquero G, Banchs JE, Naccarelli GV, Gonzalez M, Wolbrette DL. Cardiogenic shock as the initial presentation of systemic lupus erythematosus: a case report and review of the literature. Congest Heart Fail. 2012;18:337-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Hartman EAR, van Royen-Kerkhof A, Jacobs JWG, Welsing PMJ, Fritsch-Stork RDE. Performance of the 2012 Systemic Lupus International Collaborating Clinics classification criteria versus the 1997 American College of Rheumatology classification criteria in adult and juvenile systemic lupus erythematosus. A systematic review and meta-analysis. Autoimmun Rev. 2018;17:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 20. | Larosa M, Iaccarino L, Gatto M, Punzi L, Doria A. Advances in the diagnosis and classification of systemic lupus erythematosus. Expert Rev Clin Immunol. 2016;12:1309-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Li JC, Fong W, Wijaya L, Leung YY. Disseminated tuberculosis masquerading as a presentation of systemic lupus erythematosus. Int J Rheum Dis. 2018;21:352-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Chasset F, Richez C, Martin T, Belot A, Korganow AS, Arnaud L. Rare diseases that mimic Systemic Lupus Erythematosus (Lupus mimickers). Joint Bone Spine. 2019;86:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Fava A, Petri M. Systemic lupus erythematosus: Diagnosis and clinical management. J Autoimmun. 2019;96:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 417] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 24. | Durrance RJ, Movahedian M, Haile W, Teller K, Pinsker R. Systemic Lupus Erythematosus Presenting as Myopericarditis with Acute Heart Failure: A Case Report and Literature Review. Case Rep Rheumatol. 2019;2019:6173276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Rebelato JB, Silveira CFDSMPD, Valadão TFC, Reis FM, Bazan R, Bazan SGZ. Myocarditis with Cardiogenic Shock as the First Manifestation of Systemic Lupus Erythematosus. Arq Bras Cardiol. 2018;111:864-866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Gurjar M, Singhal S, Poddar B, Baronia AK, Azim A. Acute cardiogenic shock in a girl with systemic lupus erythematosus. Indian J Crit Care Med. 2010;14:209-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Du Toit R, Herbst PG, van Rensburg A, du Plessis LM, Reuter H, Doubell AF. Clinical features and outcome of lupus myocarditis in the Western Cape, South Africa. Lupus. 2017;26:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Al-Nokhatha SA, Khogali HI, Al Shehhi MA, Jassim IT. Myocarditis as a lupus challenge: two case reports. J Med Case Rep. 2019;13:343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Micheloud D, Calderón M, Caparrros M, D'Cruz DP. Intravenous immunoglobulin therapy in severe lupus myocarditis: good outcome in three patients. Ann Rheum Dis. 2007;66:986-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Suri V, Varma S, Joshi K, Malhotra P, Kumari S, Jain S. Lupus myocarditis: marked improvement in cardiac function after intravenous immunoglobulin therapy. Rheumatol Int. 2010;30:1503-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Charhon N, Bernard C, Richard JC, Cordel N, Leboucher G, Broussolle C, Sève P. [Off-label use of intravenous immunoglobulin therapy in the treatment of lupus myocarditis: Two case reports and literature review]. Rev Med Interne. 2017;38:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Tariq S, Garg A, Gass A, Aronow WS. Myocarditis due to systemic lupus erythematosus associated with cardiogenic shock. Arch Med Sci. 2018;14:460-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Wang CR, Tsai YS, Li WT. Lupus myocarditis receiving the rituximab therapy-a monocentric retrospective study. Clin Rheumatol. 2018;37:1701-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Kang I, Park SH. Infectious complications in SLE after immunosuppressive therapies. Curr Opin Rheumatol. 2003;15:528-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 35. | Padmanabhan A, Connelly-Smith L, Aqui N, Balogun RA, Klingel R, Meyer E, Pham HP, Schneiderman J, Witt V, Wu Y, Zantek ND, Dunbar NM, Schwartz GEJ. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice - Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Eighth Special Issue. J Clin Apher. 2019;34:171-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 870] [Article Influence: 145.0] [Reference Citation Analysis (0)] |
| 36. | Pagnoux C, Korach JM, Guillevin L. Indications for plasma exchange in systemic lupus erythematosus in 2005. Lupus. 2005;14:871-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Griveas I, Sourgounis A, Visvardis G, Zarifis I, Kyriklidou P, Sakellariou G. Immunoadsorption in lupus myocarditis. Ther Apher Dial. 2004;8:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Jordan N, D'Cruz D. Current and emerging treatment options in the management of lupus. Immunotargets Ther. 2016;5:9-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Durcan L, O'Dwyer T, Petri M. Management strategies and future directions for systemic lupus erythematosus in adults. Lancet. 2019;393:2332-2343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 375] [Article Influence: 62.5] [Reference Citation Analysis (0)] |