Published online May 26, 2020. doi: 10.12998/wjcc.v8.i10.2050
Peer-review started: December 31, 2019
First decision: March 5, 2020
Revised: April 1, 2020
Accepted: April 24, 2020
Article in press: April 24, 2020
Published online: May 26, 2020
Processing time: 146 Days and 2.7 Hours
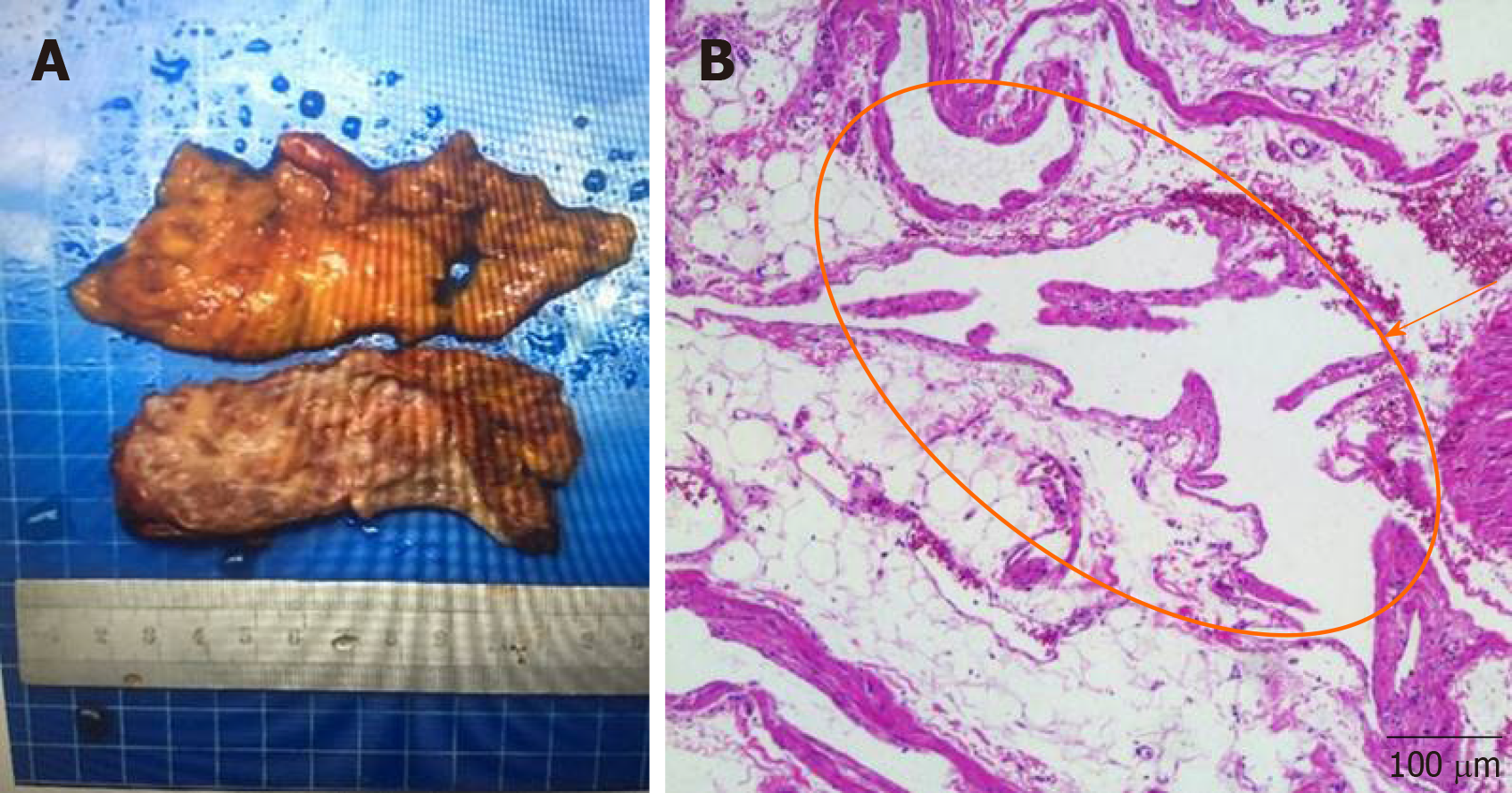
A vascular tumor is a benign tumor with unique clinical and pathological features. Perirenal vascular tumor is extremely rare and has not yet been reported. Clinically, it manifests as soreness and swelling. Color ultrasound and renal angiography illustrated the perirenal mass, which was closely connected with the kidney and the surrounding tissues and organs. Histology showed extensive embedded perirenal fat, and thin-walled vascular tissue displayed a pink stain due to red blood cells.
Herein, a case of robot-assisted retroperitoneal laparoscopic excision of a perirenal vascular tumor is reported. Analysis of the clinical, biological, and histological features of the perirenal vascular tumor can provide an in-depth understanding of the disease, which provides a theoretical and practical basis for better diagnosis and treatment.
This study contributes to a practical basis for the diagnosis and treatment of perirenal hemangiom.
Core tip: Perirenal vascular tumor is an extremely rare disease. We summarized the clinical manifestations, imaging features, and pathological characteristics of a perirenal vascular tumor in a clinical case, and successfully removed the tumor using robotic surgery without complications. This provides a practical model for the diagnosis and treatment of this disease.
- Citation: Zhang C, Fu B, Xu S, Zhou XC, Cheng XF, Fu WQ, Wang GX. Robot-assisted retroperitoneal laparoscopic excision of perirenal vascular tumor: A case report. World J Clin Cases 2020; 8(10): 2050-2055
- URL: https://www.wjgnet.com/2307-8960/full/v8/i10/2050.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i10.2050
Vascular tumors are rare benign cystic tumors that commonly occur in the mouth and face. Originating from the mesenchymal tissue, vascular tumors are mostly microvascular lymphatic malformations caused by dysplasia in the embryonic stage or residual tissue, resulting in the obstruction of fluid reflux, expansion, and protrusion, and are classified as hemangiomas and lymphangiomas. These tumors are mostly benign, and no malignant or invasive characteristics have been observed[1]. The majority of vascular tumors have a slow growth rate, and the size of the tumor increases with age. Typically, they do not regress spontaneously. The most common complications include spontaneous or traumatic hemorrhage, rupture, and infection[2]. According to trauma or surgical history, they are classified into primary and secondary vascular tumors[3]. Primary vascular tumors constitute the most commonly reported type in the clinic. Vascular tumors are common in children and adolescents with a reported hemolymphangioma incidence of 1.2%-2.8% in newborns. The incidence before the age of 2 years was > 90%, while 60% of newborns showed symptoms at birth[4,5]. Lesions can be single, multiple, or diffused, occurring in any part of the body, especially in the loose connective tissue.
Herein, we report the case of a primary perirenal vascular tumor, which is a rare occurrence in the clinic. Only 2 cases of retroperitoneal angiocarcinoma were reported previously[6,7]. Such cases are often subjected to surgical excision in the clinic[8,9]. Currently, experience in the diagnosis and treatment of perirenal vascular tumors is lacking. Herein, we report a case of perirenal vascular tumor from clinical, imaging, and pathological aspects, which could provide elaborate information regarding the study and treatment of vascular tumors.
A 38-year-old female was admitted to the Urological Department of the First Affiliated Hospital of Nanchang University after 37 d of soreness and swelling in the left waist.
The patient initially showed symptoms on November 13, 2018, which were not relieved with the change in body posture. The patient experienced radiating pain, nausea, vomiting, frequent urination, urgent urination, and gross hematuria. She underwent an abdominal color ultrasound at the Jiangxi Provincial Breast Hospital (ultrasound No. 9000022433). The compression type change of the cystic mass in the left middle upper abdominal region and the left side of the abdominal aorta seemed to be connected with the left kidney. On November 27, double renal computed tomography (CT) angiography was performed and routine blood and biochemical examinations were conducted on December 20 at the First Affiliated Hospital of Nanchang University. Subsequently, robot-assisted retroperitoneal laparoscopic excision was performed to excise the perirenal vascular mass on December 25 under general anesthesia.
She was healthy without any previous history of surgery, hypertension, or diabetes.
Physical examination indicated no pressing or percussion pain in both kidneys.
Routine blood and biochemical examinations to assess tumor markers showed no obvious abnormalities (Table 1).
| Laboratory examination | |||
| Hemoglobin | 134 g/L | Globulin | 24.7 g/L |
| Red blood cells | 4.64 × 1012/L | Alanine aminotransferase | 11.0 U/L |
| White blood cells | 5.21 × 109/L | Aspartate aminotransferase | 18.0 U/L |
| Neutrophilic granulocytes | 2.56 × 109/L | Alpha fetoprotein | 1.36 ng/mL |
| Lymphocytes | 2.28 × 109/L | Carcinoembryonic antigen | 0.60 ng/mL |
| Blood platelets | 316 × 109/L | Carbohydrate antigen 12-5 | 41.63 U/mL |
| Urea | 3.9 mmol/L | Carbohydrate antigen 15-3 | 12.30 U/mL |
| Cr | 52.4 μmol/L | Carbohydrate antigen 19-9 | 10.54 U/mL |
| Na | 138.0 mmol/L | Carbohydrate antigen 724 | 7.19 U/mL |
| K | 4.0 mmol/L | Prothrombin time | 9.9 s |
| Albumin | 45.3 g/L | Thrombin time | 17.7 s |
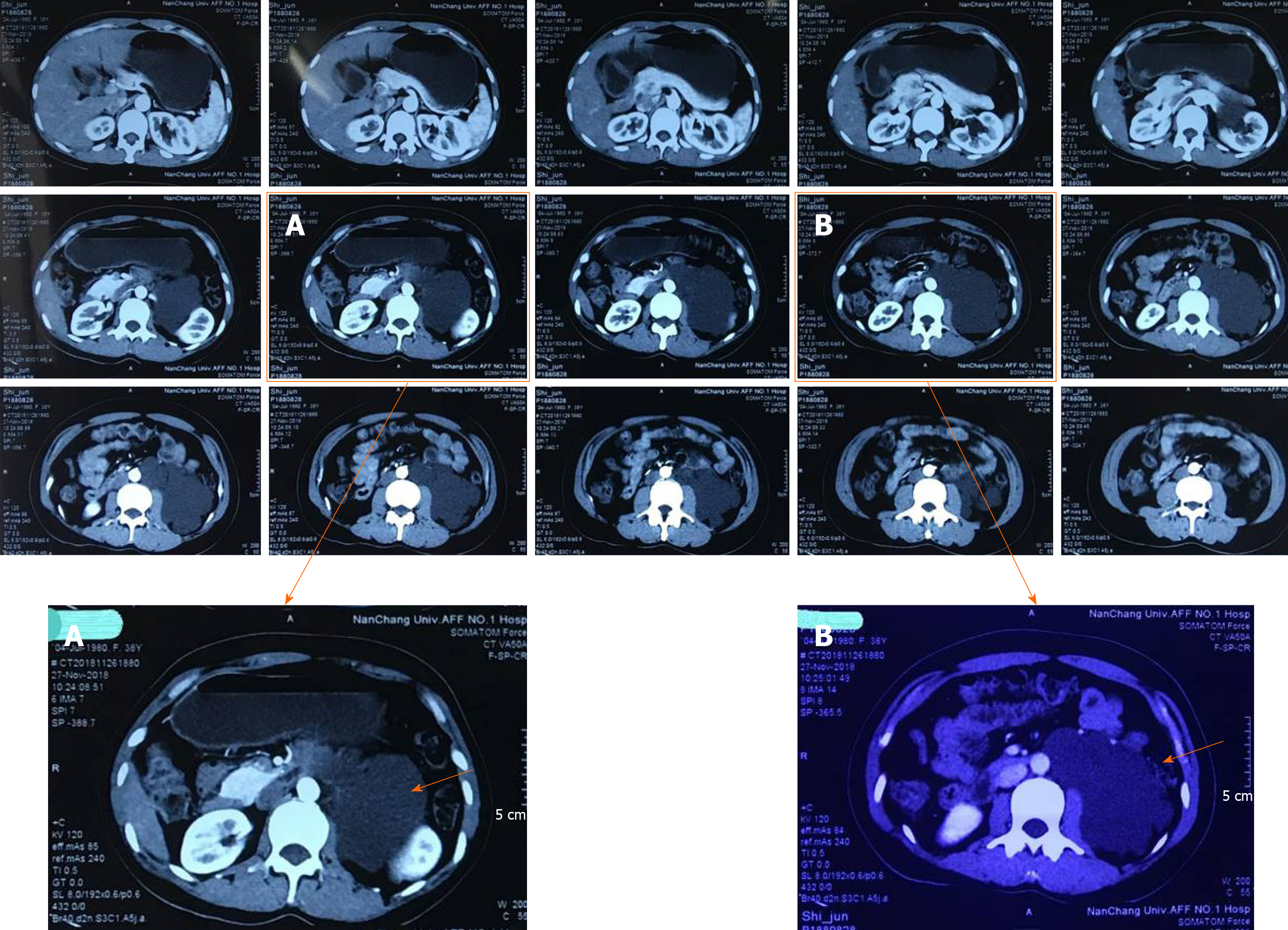
Imaging illustrated a low-density cystic shadow in the left kidney hilus with a lacy edge and a maximum size of approximately 7.7 cm × 10.1 cm (Figure 1A). The density was uniform, the surroundings were compressed, the adjacent left upper ureter was compressed, and left hydronephrosis showed dilation (Figure 1B); this was considered to be a lymphangioma.
Preoperative diagnosis was a left perirenal mass. Pathological diagnosis was a perirenal vascular tumor.
As the patient’s left perirenal mass was huge and closely related to the left kidney, the upper segment of the left ureter, and the left abdominal aorta, the Da Vinci robotic surgical system was selected as it is characterized by a wide operating range, high operating accuracy, and a flexible manipulator operation. Intraoperatively, the mass showed irregular cystic growth close to the surrounding organs and tissues, and was approximately 10 cm × 8 cm in size (Figure 1). Using the flexible mechanical arm of the Da Vinci surgical robotic system, caution was taken to avoid damage to the left kidney, left ureter, abdominal aorta, and peritoneum. The tumor was cystic in the field of view, a small cut was made, the clear cystic fluid was aspirated, and the tumor was completely removed. The wound was repeatedly rinsed with distilled water, and sufficient hemostasis was achieved. After the specimen was excised, a drainage tube was placed beside the left kidney, and the incision was sutured to complete the operation. Intraoperative blood loss was 30 mL, and postoperative routine blood and biochemical results were normal. The drainage tube in the left kidney was removed after 3 d, and the patient was discharged 6 d after surgery. Postoperative pathology showed a perirenal vascular tumor (Figure 2).
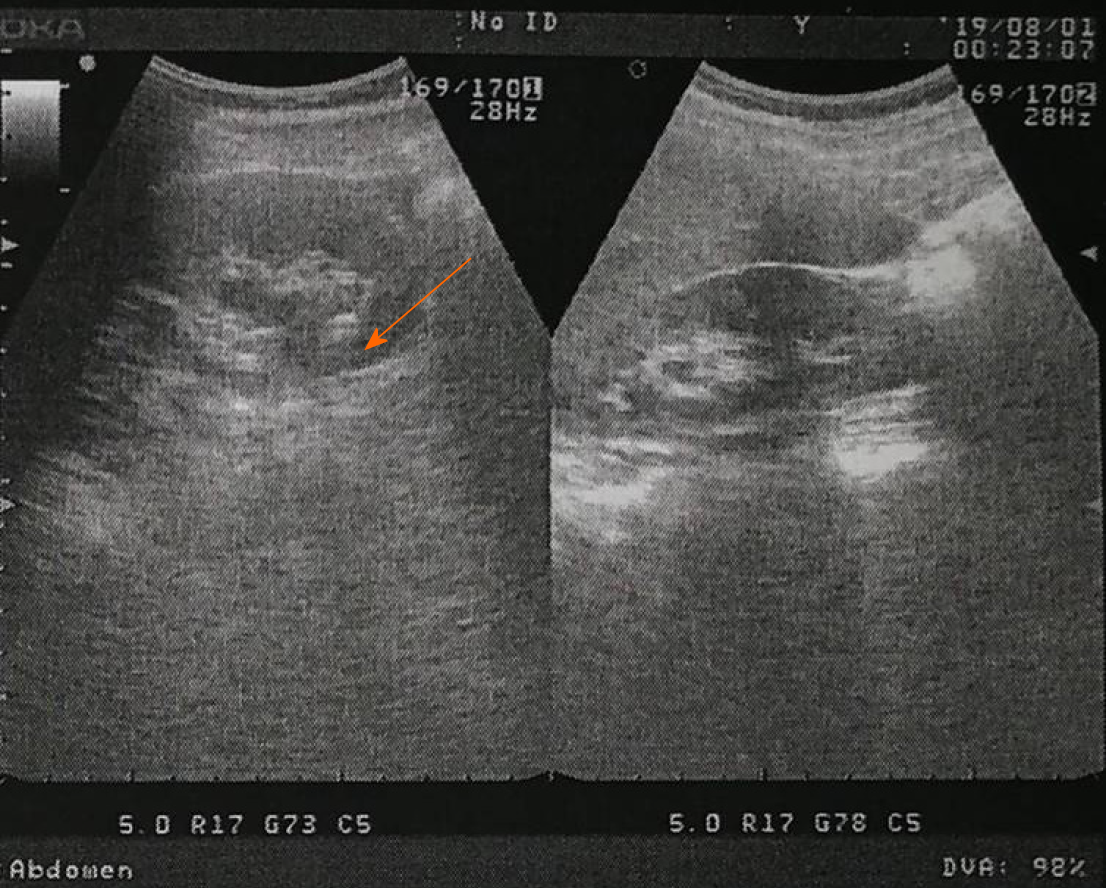
Reexamination was performed at 7 mo postoperatively. Color Doppler ultrasound was performed at the Nanchang Kangqiao Health Physical Examination Center on July 30, 2019, which illustrated a crystal in the left kidney (Figure 3).
In the early stage, vascular tumors are usually asymptomatic; however, most are detected due to their enlarged size leading to compression in the affected site. Diagnosis revealed clearly defined hypoechoic cystic masses by B-ultrasound in a honeycomb or septum formation. CT scan showed a relatively low-density uniform lesion. In the vascular tumor mainly with lymph, CT showed a clear boundary without obvious enhancement. In the vascular tumor mainly with vessels, CT displayed an unclear boundary, and an enhancement in the arterial stage of different degrees was observed. With regard to treatment, surgery is the first option for almost all vascular tumors to prevent or relieve the symptoms of tumor compression.
Our patient with a perirenal vascular tumor is extremely rare. Due to enlargement of the tumor and compression on adjacent organs, the corresponding symptoms were an abdominal mass, soreness, and swelling at the waist. The putative mechanisms are as follows: (1) Congenital malformation of the vascular system; (2) Mutation or deletion of gene expression in the body; and (3) Related to the pathogenesis of previous renal diseases, but the specific mechanism requires further investigation. As the disease is rare, preoperative diagnosis is difficult. Currently, ultrasound and CT are mainly used to determine the range of the mass and its correlation with adjacent organs[10]. If the mass is rich in blood supply, further identification by magnetic resonance imaging is required. In this case, color ultrasound and abdominal CT were the first options for the diagnosis and localization of the perirenal vascular tumor. During imaging, perirenal vascular tumors have pathological features of benign tumors. Therefore, only a few cases have been reported, and different manifestations are essential to further clarify the imaging features of the disease.
Perirenal vascular tumors are benign lesions which develop slowly. If the tumor is small, and the patient does not present obvious clinical symptoms, conservative treatment, such as anti-infective agents can be administered, and when renal function is normal, this is followed by regular observation. If the tumor volume is large, and the renal parenchyma is compressed, hydronephrosis, renal hypertension, and other complications are actively treated by surgery[11]. Surgical excision is the most effective treatment for perirenal vascular tumors. The principle of surgery is to completely excise the lesion and to maximally retain normal renal tissue. Nonetheless, regular reexamination and follow-up are recommended despite the benign nature of vascular tumors.
Since the first Da Vinci robotic surgery in 1988, robotic surgery has been rapidly promoted and applied[12]. It is a favored method in urology due to the small incision, accuracy, and flexible and a stable intraoperative procedure[13]. A recent study reported that the Da Vinci robot could effectively reduce the incidence of perioperative complications in urology and improve the safety and feasibility of surgery[14,15]. When the tumor is large and the base is wide, the Da Vinci robot can expose a wider visual field than traditional surgery, overcoming the disadvantages of traditional deep surgery where the tumor and anatomical space cannot be observed from multiple angles. The lesion can be removed more precisely and steadily, avoiding the difference between the handwork and feel in traditional surgery. This technique greatly reduces surgical trauma and hastens postoperative recovery[16]. The Da Vinci robot results in excellent 3D vision with a flexible and super artificial manual operating system and a novel surgical mode, cooperating with increasingly updated artificial intelligence to continuously promote the development of surgery.
A perirenal vascular tumor is a rare disease. We summarized the clinical manifestations, ultrasound, CT imaging, and pathological features of a perirenal vascular tumor, and recommended surgical excision. The present study provides a theoretical and practical basis for the recognition and diagnosis of perirenal vascular tumors.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mogulkoc R, Tsoulfas G S-Editor: Wang YQ L-Editor: Webster JR E-Editor: Qi LL
| 1. | Chen YA, Li HN, Wang RC, Hung SW, Chiu KY. Malignant Glomus Tumor of the Kidney: A Case Report and Review of the Literature. Clin Genitourin Cancer. 2017;15:e151-e153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Omiyale AO, Carton J. Clinical and Pathologic Features of Primary Angiosarcoma of the Kidney. Curr Urol Rep. 2018;19:4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Figueroa RM, Lopez GJ, Servin TE, Esquinca MH, Gómez-Pedraza A. Pancreatic hemolymphangioma. JOP. 2014;15:399-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 4. | Fang YF, Qiu LF, Du Y, Jiang ZN, Gao M. Small intestinal hemolymphangioma with bleeding: a case report. World J Gastroenterol. 2012;18:2145-2146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Zhang X, Sheng X, Liu F, Jian R, Li L, Luo R. Hemolymphangioma of the chest wall: A rare case report. Oncol Lett. 2012;3:816-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Filston HC. Hemangiomas, cystic hygromas, and teratomas of the head and neck. Semin Pediatr Surg. 1994;3:147-159. [PubMed] |
| 7. | Toyoki Y, Hakamada K, Narumi S, Nara M, Kudoh D, Ishido K, Sasaki M. A case of invasive hemolymphangioma of the pancreas. World J Gastroenterol. 2008;14:2932-2934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Chen Q, Xia J. A giant hemolymphangioma of the pancreas: A case report and literature review. Medicine (Baltimore). 2018;97:e12599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Zhang DY, Lu Z, Ma X, Wang QY, Sun WL, Wu W, Cui PY. Multiple Hemolymphangioma of the Visceral Organs: A Case Report and Review of the Literature. Medicine (Baltimore). 2015;94:e1126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Kosmidis I, Vlachou M, Koutroufinis A, Filiopoulos K. Hemolymphangioma of the lower extremities in children: two case reports. J Orthop Surg Res. 2010;5:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Bigot P, Bouvier A, Panayotopoulos P, Aubé C, Azzouzi AR. Partial nephrectomy after selective embolization of tumor vessels in a hybrid operating room: A new approach of zero ischemia in renal surgery. J Surg Oncol. 2016;113:135-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Autorino R, Kaouk JH, Stolzenburg JU, Gill IS, Mottrie A, Tewari A, Cadeddu JA. Current status and future directions of robotic single-site surgery: a systematic review. Eur Urol. 2013;63:266-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Novara G, Catto JW, Wilson T, Annerstedt M, Chan K, Murphy DG, Motttrie A, Peabody JO, Skinner EC, Wiklund PN, Guru KA, Yuh B. Systematic review and cumulative analysis of perioperative outcomes and complications after robot-assisted radical cystectomy. Eur Urol. 2015;67:376-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 327] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 14. | Agarwal DK, Sharma V, Toussi A, Viers BR, Tollefson MK, Gettman MT, Frank I. Initial Experience with da Vinci Single-port Robot-assisted Radical Prostatectomies. Eur Urol. 2020;77:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 15. | Dobbs RW, Halgrimson WR, Madueke I, Vigneswaran HT, Wilson JO, Crivellaro S. Single-port robot-assisted laparoscopic radical prostatectomy: initial experience and technique with the da Vinci® SP platform. BJU Int. 2019;124:1022-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Rogers CG, Laungani R, Bhandari A, Krane LS, Eun D, Patel MN, Boris R, Shrivastava A, Menon M. Maximizing console surgeon independence during robot-assisted renal surgery by using the Fourth Arm and TilePro. J Endourol. 2009;23:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |